Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), may lead to thyroid disorders, including both thyrotoxicosis and suppression of thyroid function. The aim of the present study was to assess the post-COVID-19 effects on thyroid function in patients without history of thyroid disease after complete recovery from mild-to-moderate COVID-19. Thyroid function tests [thyroid-stimulating hormone (TSH), free thyroxine (fT4), antithyroid antibodies] were performed on 113 patients (median age of 43.0 years; 31.0% male) two months after initial SARS-CoV-2 infection. TSH and fT4 were determined again one month later in this observational, prospective study. Thyroid dysfunction was registered in 61.1% of the patients (78.3% subclinical hypothyroidism, 13% subclinical hyperthyroidism and 8.7% overt hypothyroidism) two months after COVID-19. Moderate rather than mild manifestation of COVID-19 was significantly associated with a higher risk of thyroid dysfunction (OR 5.33; 95% CI: 1.70–16.69, p = 0.002), presence of thyroglobulin antibodies and need for levothyroxine therapy. At the follow-up, the subclinical hypothyroidism persisted in 28.3% of the subjects. Moreover, the TSH level was significantly reduced in comparison to the second month after the initial COVID-19 infection in all the patients (p < 0.001), but not in those with subclinical hypothyroidism and without hormone replacement therapy. Our findings indicate that COVID-19 could have long-term, negative effects on thyroid function. Therefore, thyroid function testing should be included in the follow-up algorithm of COVID-19 survivors.
Introduction
Viral infections are a major environmental factor implicated in the pathophysiology of different thyroid dysfunctions. For example, the aetiology of autoimmune thyroid diseases, as well as subacute thyroiditis (SAT), also known as De Quervain’s thyroiditis, has been related to viral infections [Citation1]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19). With the beginning of the COVID-19 pandemic, there has been accumulating evidence of the short- and long-term adverse effects of COVID-19 on human health. Along with life-threatening damage to the respiratory, cardiovascular, coagulation and nervous system, SARS-CoV-2 infection could lead to transient, but also long-term abnormalities in thyroid function [Citation2]. However, the link between COVID-19 and changes in thyroid function and hormone levels is still unclear. One of the prevailing hypotheses is based on the so-called “low T3 syndrome”, or Euthyroid Sick Syndrome (ESS). EES is characterized by low total triiodothyronine and free triiodothyronine (fT3) levels with low or normal free thyroxine (fT4) and thyroid-stimulating hormone (TSH) [Citation3]. Significantly lower serum concentrations of fT3 have been shown to correlate with COVID-19 severity and to predict all-cause mortality of patients with severe COVID-19 [Citation4,Citation5]. Direct viral destruction of the thyroid gland parenchyma is another main mechanism possibly accounting for thyroid function perturbations in COVID-19 patients. Two known entry points for SARS-CoV-2, angiotensin-converting enzyme 2 (ACE-2) and transmembrane serine protease 2 (TMPRSS2), are highly expressed in thyroid follicular cells [Citation6]. Noteworthy, the mechanism of action of different medications used in the COVID-19 treatment, like anticoagulants, platelet antiaggregant and corticosteroids, may also affect thyroid hormone levels [Citation7,Citation8]. Nevertheless, recent clinical evidence has been discrepant, indicating both thyrotoxicosis and suppression of thyroid function [Citation9]. In addition, the available studies mainly examine the changes in the thyroid function during the active phase of COVID-19 or include patients admitted to intensive care units, whereas SARS-CoV-2 infection mostly causes mild to moderate symptoms [Citation10]. Therefore, the aim of this study was to assess the post-COVID-19 effects on thyroid function in patients without a history of thyroid disease after a complete recovery from mild-to-moderate COVID-19.
Subjects and methods
Ethics statement
The study was approved by the Ethical Review Board of “Dr. Shterev” Hospital, Sofia, Bulgaria (reference number 01/2022) and was conducted in accordance with the Declaration of Helsinki. All participants signed informed consent forms.
Study participants and setting
This observational prospective study involved 113 patients and was conducted between 1st January 2021 and 1st June 2021. All the patients (78 women and 35 men) visited the Endocrinology Consulting Room of “Dr. Shterev” Hospital, Sofia, Bulgaria, two months after initial SARS-CoV-2 infection.
The inclusion criteria were: 1/Patients aged >18 years; 2/No previous history of thyroid disease; 3/Patients not undergoing fertility treatment; 4/Patients who had mild or moderate SARS-CoV-2 infection.
COVID-19 was laboratory-confirmed through a reverse transcription polymerase chain reaction (RT-PCR) test from a nasopharyngeal swab in all the study participants. Patients were consulted by an endocrinologist due to persistent complaints of fatigue, anxiety and changes in body weight. COVID-19 severity was classified according to the “Clinical management of COVID-19: interim guidance” published by the World Health Organization [Citation11]. Anticoagulants (low-molecular-weight heparin) or antiplatelet agents (acetylsalicylic acid) had been used during the treatment of the COVID-19 in all the study subjects. Eighteen patients (13 males and 5 females) had received glucocorticoid therapy. Thyroid function tests, including TSH, fT4, thyroglobulin antibody (TgAb) and thyroid peroxidase antibody (TPOAb), were performed on all the study participants at baseline (two months after COVID-19). TSH was tested again at the follow-up visit one month later in all the study participants, while fT4 was determined in a set of 42 patients.
According to the results of the hormones, patients were divided into four groups: euthyroid (normal thyroid hormone levels), subclinical hypothyroidism, overt hypothyroidism and subclinical hyperthyroidism. No patients with clinical hyperthyroidism were detected. Patients were classified according to the recommendations of the American Thyroid Association for the management of thyroid dysfunction [Citation12] and the Bulgarian society of endocrinology [Citation13]. Regarding antithyroid antibodies, the study participants were classified as antibody positive and antibody negative. Levothyroxine therapy was initiated at a dose between 50 and 75 micrograms in thirteen patients (6 males and 7 females).
Thyroid ultrasonography was also performed on а 2D Medison SonoAce X6 Ultra-sound Machine, South Korea. The European Thyroid Association system for ultra-sound assessment of thyroid nodules and stratification of requirement for fine needle aspiration (FNA) and malignancy (EU-TIRADS) was applied [Citation14].
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters square (kg/m2).
Laboratory analysis
Blood samples were taken from the cubital vein from all the subjects according to the standard procedure of blood collection. The TSH levels were determined by the immune-chemiluminescent method (Roche Cobas 8000, Switzerland), with a reference range for the respective laboratory of 0.27–4.20 mUI/mL. The fT4 levels were determined by the immune-chemiluminescent method (Roche Cobas 8000, Switzerland), with a reference interval for the respective laboratory of 12–22 pmol/L. Antithyroid antibody levels (both TgAb and TPOAb) were determined using the electrochemiluminescent ECLIA method (Roche Cobas 8000, Switzerland). The interassay coefficients of variation were 1.8% for the TSH, 1.6% for the fT4 and <13% for the antithyroid antibodies. The lower limit of detection was 0.005 mIU/mL and 0.5 pmol/L for TSH and fT4, respectively.
Data analysis
Statistical analysis was performed using SPSS software, version 23.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to evaluate whether the distribution of continuous variables was normal. Continuous variables were expressed as mean values with standard deviation (±SD) or as median and interquartile range (IQR). The Independent-samples t-test and the Mann–Whitney U-test were used for variables with a normal or non-normal distribution, respectively. Categorical variables were analyzed by the Chi-square test. Wilcoxon paired rank-sum test was used for comparison of longitudinal data on TSH and fT4 levels. A logistic regression analysis was conducted to evaluate the associations between the studied biomarkers and the outcomes. The results were expressed as odds ratios (OR) with 95% confidence intervals (95% CI). Differences were considered statistically significant at the p < 0.05 level.
Results
The median age of the analyzed patients was 43.0 years, and the median BMI was 29.0 kg/m2. The levels of TSH and fT4 in the whole study cohort were 4.28 ± 2.11 mIU/L and 14.23 [13.01–16.32] pmol/L, respectively. The clinical characteristics of the study participants are summarized in .
Table 1. Characteristics of all study participants (n = 113).
Our analysis showed that two months after SARS-CoV-2 infection, only 38.9% of the observed patients were euthyroid, and the remaining 61.1% had abnormal thyroid function tests. In this group, the most common thyroid dysfunction was subclinical hypothyroidism, followed by subclinical hyperthyroidism and overt hypothyroidism accounting for 78.3%, 13% and 8.7%, respectively. No patients were diagnosed with clinical hyperthyroidism. As already mentioned, levothyroxine therapy was initiated only in 13 (11.5%) patients. Statistically significantly more patients were TgAb positive than negative (p = 0.03), while there was no statistical difference in the TPOAb presence. At the follow-up three months post COVID-19, subclinical hypothyroidism was observed in 32 subjects (28.3%) of the whole study cohort, being the only one persisting thyroid pathology. However, in 3 patients with overt and 3 with subclinical hypothyroidism, the normalization of TSH at the third month was a result of the initiation of levothyroxine therapy. Thus, the total percentage of subjects with persistent hypothyroidism 3 months post COVID-19 was even higher (33.6%).
A significantly higher percentage of the males included in the study experienced moderate COVID-19 compared to women (48.6% vs 14.1% respectively, p < 0.001). However, this analysis was not an objective of our investigation. We sought to assess whether the family history of thyroid disease and the severity of COVID-19 manifestation had an impact on the thyroid status and required treatment two months after the initial SARS-CoV-2 infection. The data are presented in .
Table 2. Effects of the family history of thyroid disease and severity of COVID-19 manifestation on the thyroid status and required treatment two months after the initial SARS-CoV-2 infection.
Significantly more TgAb positive patients were detected in the subgroup with moderate disease course of COVID-19 than in the subjects who had experienced only mild COVID-19 symptoms. Univariate logistic regression analysis demonstrated that moderate severity of COVID-19 was significantly associated with a higher risk of thyroid dysfunction (OR 5.33; 95% CI: 1.70–16.69, p = 0.002). As expected, more severe COVID-19 manifestation was significantly associated with a higher need for glucocorticoid therapy (OR 2.80, 95% CI: 1.70–4.60, p < 0.001). To neutralize the possible unfavourable effect of the glucocorticoid therapy during COVID-19 on the thyroid status, we repeated the univariate logistic regression analysis about the relationship between severity of COVID-19 and future development of thyroid dysfunction excluding the patients on glucocorticoids. Patients with moderate COVID-19 symptoms were still at a significantly higher risk of thyroid disorder (n = 95, OR 8.00; 95% CI: 1.97–65.95, p = 0.039). Moderate severity of COVID-19 was also significantly associated with a higher need for levothyroxine treatment. The necessity for hormone replacement therapy was significantly increased in the patients with subclinical and overt hypothyroidism with moderate COVID-19 than in those with mild COVID-19 disease course (n = 60, OR 10.99, 95% CI: 2.70–44.57, p = 0.001). The moderate COVID-19 disease course resulted only in a tendency for lower fT4 levels, but in nonsignificant difference in TSH concentration. By contrast, the presence of family history of thyroid disease led to no significant differences in the examined parameters except for TPOAb presence and requirement of levothyroxine. Significantly more subjects with family predisposition were TPOAb positive and required hormone replacement therapy compared to their counterparts without family history of thyroid disease ().
During the physical examination, all the patients were without subjective or palpable evidence of neck pain. On ultrasound, the thyroid changes in 58 (51.3%) patients showed nonhomogeneous, moderately hypoechoic structure. Poorly demarcated, hypoechoic areas with poor blood flow were registered in half of them. Thyroid nodules were detected in 26 patients (23.0%). Nodule changes were classified as EU-TIRADS − 2 and 3. No nodules suspected for malignancy were observed. The rest of the patients were with relatively homogeneous, normoechoic structure of the gland.
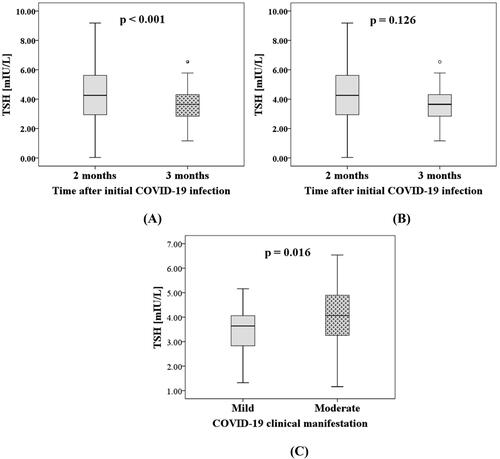
The present study had a prospective design, and the thyroid function was determined again at a follow-up visit three months after the initial COVID-19 infection. The results from the longitudinal comparison of TSH levels at two months (baseline) and at the follow-up one month later, showed a significant decline in TSH levels in the whole group of patients (p < 0.001) as illustrated in . The TSH levels were lower at the third month (4.85 [4.56–5.11] mIU/L) than at the second month (5.73 [4.91–6.53] mIU/L) after COVID-19 when we repeated the analysis only in the subgroup in which the thyroid dysfunction persisted (n = 32, p = 0.008). However, when we excluded the patients on levothyroxine therapy from this subgroup, there was no significant difference (). Interestingly, higher TSH level was observed in the moderate compared to the mild COVID-19 cohort at the follow-up visits one month later (). Moreover, the univariate logistic regression analysis on the results three months after the initial COVID-19 confirmed that moderate COVID-19 manifestation was significantly associated with a higher risk of thyroid dysfunction persistence (OR 3.01, 95% CI: 1.22–7.41, p = 0.028). In the 42 patients with complete sets of fT4, the medians [IQR] of fT4 at baseline (2 months after initial infection) and follow-up were 12.78 [12.03–13.80] pmol/L and 13.81 [12.84–14.50] pmol/L, respectively. Unlike TSH, the level of fT4 was significantly increased at the follow-up compared to the baseline (n = 42, p = 0.014).
Figure 1. Comparative analysis on thyroid-stimulating hormone (TSH) levels: (A) at two months (baseline) and three months after the initial COVID-19 infection in the whole patient cohort (n = 113); (B) at two months (baseline) and three months after the initial COVID-19 infection only in the patients with persistent thyroid dysfunction at the third month and without levothyroxine therapy (n = 24); (C) between patients with mild (n = 85) and moderate manifestation (n = 28) of COVID-19 at the follow-up visit three months after the initial COVID-19.
Discussion
A growing body of evidence has reported severe and complex detrimental effects of COVID-19 not only on the respiratory system, but also on the immune, circulatory, digestive, hepatic, renal, nervous and hematological system [Citation15]. Although the thyroid gland has been depicted as one of the targets for the diverse effects of SARS-CoV-2 infection, the potential relationship between COVID-19 and the thyroid function is poorly understood. Furthermore, most studies are based on data from hospitalized and severely ill patients in the active disease phase. Hence, the major strengths of our study are its prospective design and TSH determination at two and three months after the initial SARS-CoV-2 infection in patients with the most common mild-to-moderate COVID-19 manifestation.
We found that two months post COVID-19 only 38.9% of the patients were euthyroid and the remaining 61.1% had thyroid dysfunction. Lui et al. [Citation16] also explored the association between COVID-19 and thyroid dysfunction in mild-to-moderate COVID-19. Thyroid abnormalities were observed in approximately only 15% of the patients, but the thyroid function was estimated at the time of COVID-19 positive test results [Citation16]. A systematic review of seven studies involving more than 1200 patients with COVID-19 has reported thyroid dysfunction frequency varying between 13% and 64% [Citation17].
The clinical data about thyroid disorders associated with COVID-19 are accumulating, but also rather discrepant, reporting both thyrotoxicosis and suppression of thyroid function. For example, 20% of the 287 patients recruited in the THYRCOV study and hospitalized in a non-intensive care unit were diagnosed with thyrotoxicosis, whereas only 5.2% had hypothyroidism [Citation9]. By contrast, we registered no patients with thyrotoxicosis, 60 (53.1%) with hypothyroidism and only 9 (8.0%) with subclinical hyperthyroidism. Khoo et al. [Citation18] also did not find any patients with thyrotoxicosis, but only 0.6% of their COVID-19 cohort had overt hypothyroidism. However, the distribution of the hypothyroid patients in subclinical and overt subgroups was similar in our and Lania et al.’s study (90% and 10% vs. 86.7% and 13.3%, respectively). Our results differ from the epidemiological data for the prevalence of thyroid dysfunction in Bulgaria, which was reported in two population-based studies conducted in 2006 and 2012 [Citation19,Citation20]. The first study showed an overall prevalence of 3.7% and 6.3% in the explored patient group for hyperthyroidism and hypothyroidism, respectively [Citation19]. According to the data from the second study, subclinical hypothyroidism was observed in 4.5% of women and 2% of men. Hypothyroidism was overt in 3.2% of women and 1.1% of men [Citation20]. In addition, we found that patients with moderate COVID-19 manifestation were at a significantly higher risk of thyroid dysfunction than subjects with mild clinical disease course. Thus, we confirm the positive correlation between thyroid abnormality and clinical severity of COVID-19, previously reported in the literature [Citation4,Citation5,Citation17,Citation21]. Furthermore, the patients with moderate COVID-19 experienced higher necessity not only of glucocorticoid, but also of hormone replacement therapy.
The pathogenetic mechanisms relating COVID-19 to these various thyroid complications are still unclear. It has been revealed that SARS-CoV-2 uses ACE-2 receptors for its cell docking, entry as well as replication. In addition to the lungs, the thyroid and the pituitary glands express ACE-2 receptors. Thus, the hypothalamic–pituitary–thyroid axis may be directly vulnerable to disturbance in patients with COVID-19 [Citation6,Citation18,Citation22]. The destruction of the thyroid parenchyma, which is characteristic of the initial phase of this disease, is associated with the release of thyroid hormones and the manifestation of a transitional phase of thyrotoxicosis. In accordance with this hypothesis, the most common deviation has been transient thyrotoxicosis which could be explained by the monitoring of thyroid function at a relatively early stage of COVID-19 infection [Citation23–26]. However, Feghali et al. [Citation27] documented a clinical case of a 33-year-old female who developed Graves’ disease 8 weeks after COVID-19 infection.
A possible explanation for the difference in our findings might lie in the dynamics of thyroid function alterations after a viral infection. Assuming that the initial deviation of thyroid hormones is based on a destructive process caused by SARS-CoV-2, it is likely that during and soon after infection, most patients would have lower or lower limit TSH, followed by a normalization or increase in its levels after recovery. Thus, the COVID-19 convalescence might continue with a transient phase of thyroid function reduction, which in some patients remains permanent. We monitored the thyroid function two months after SARS-CoV-2 infection. In part of the patients, ultrasound changes with a characteristic of destruction in the thyroid parenchyma were observed. Even though we did not have data on the TSH levels during or immediately after the COVID-19, TSH was above the upper reference limit in more than half of the examined study population at the second month post COVID-19. In addition, patients with moderate COVID-19 disease course tended to have a lower fT4 concentration.
A retrospective-prospective study conducted in a Bosnian outpatient clinic [Citation28] found a significant difference in the number of patients with hypothyroidism and subclinical hypothyroidism in 2020 and 2021 in comparison to 2019, when there was no COVID-19 yet. The average time of diagnosis estimated in that study was 2 months after infection for clinical hypothyroidism and 8 weeks for subclinical hypothyroidism [Citation28], which coincides with the time of the first evaluation of thyroid function after COVID-19 in our study. It has been shown that laboratory indexes in patients with post-COVID-19 subacute thyroiditis returned to normal levels after 1 to 2 months. Nevertheless, two (25%) out of eight patients who were followed-up after a mean of 55 (SD 8) days after hospital discharge were confirmed to have hypothyroidism [Citation23]. In our study, the presence of 9 patients (8.0%) with subclinical hyperthyroidism at the second month might be due to individual recovery time variance. Moreover, total normalization of thyroid indices in all the subjects with subclinical hyperthyroidism was registered at the follow-up one month later.
The hypothesis about thyroid function suppression as a long-term consequence of COVID-19 is further supported by our longitudinal results obtained three months after COVID-19. We registered a significant decrease in TSH levels in the whole study cohort compared to the baseline at the second month. On the contrary, the level of fT4 was significantly increased at the follow-up visit. Noteworthy, the subclinical hypothyroidism persisted in 28.3% of all the study subjects. We could summarize that more than one-third (33.6%) of the patients in our study developed post-COVID-19 subclinical hypothyroidism including also the 6 individuals in whom the normalization of TSH was a result of the initiation of levothyroxine treatment. Several authors have reported a decrease in TSH proportional to the severity of COVID-19 during and soon after infection [Citation18]. Interestingly, higher TSH levels were observed in the moderate compared to the mild COVID-19 cohort at three, but not at two months after COVID-19. In contrast to us, Clarke et al. [Citation29] did not find any alterations in thyroid hormone levels three months after SARS-CoV-2 infection. The lack of a significant decline in TSH levels toward normalization in the cohort with subclinical hypothyroidism but without hormone replacement therapy, underscored the persisting long-term thyroid dysfunction.
Another probable mechanism for the changes in the thyroid function could be the manifestation of the already mentioned Euthyroid Sick Syndrome described by Zou et al. [Citation21] in hospitalized patients with COVID-19 and associated with a more severe disease course and a significant change in inflammatory markers. However, the patients presented in our study had only a mild or moderate course of the disease. Therefore, persistent abnormalities in thyroid function in some of them could not be attributed to ESS during their COVID-19 illness.
On the other hand, the viral infection itself could induce an immunological response associated with an increase in antithyroid antibodies and can be considered a major aetiological factor of autoimmune thyroid disease [Citation16]. Indeed, the novel observation of an increase in antithyroid antibody titers 3 months post COVID-19 has suggested the potential of SARS-CoV-2 to trigger thyroid autoimmunity [Citation30]. Furthermore, a study by Nishihara et al. [Citation31] showed that patients with subacute thyroiditis were moderately positive for TgAb, but not for TPOAb. In agreement with this, significantly more of our patients were TgAb positive. Moreover, we registered statistically significantly more TgAb positive subjects in the subgroup with moderate than in the subgroup with mild manifestations of COVID-19, whereas there was no difference in terms of TPOAb positivity. Hence, the immunological and hormonal parameters of post-COVID patients should be monitored over time.
Another interesting observation is that the COVID-19 occurrence did not provoke higher TgAb positivity or thyroid dysfunction incidence in individuals with a family history of thyroid disease, but higher TPOAb presence. By contrast, a recent study [Citation32] has demonstrated a significant prevalence of subclinical hypothyroidism in COVID-19 infected patients with a family history of thyroid disease, and/or a high level of TPO antibodies. However, most of the participants in that investigation (88.4%) had normal fT3 levels, and there was no significant difference in both fT3 and fT4 levels between the groups with or without a family history of thyroid disease and/or TPO positivity [Citation32]. Of note, the indirect effects on the thyroid gland of immune-mediated post viral inflammatory reaction caused by COVID-19 infection also should not be neglected [Citation33].
The effect of some medications used in COVID-19 treatment should also be taken into account when estimating the COVID-19 impact on the thyroid function. Given the multisystemic involvement during COVID-19 infection, as well as evidence of endothelial dysfunction, inflammation, vascular and thrombotic events, antiplatelet agents, anticoagulants, and corticosteroids are widely used for its treatment. For example, the effects of acetylsalicylic acid include alterations in plasma protein binding, leading to increased free hormone fractions, altered conversion of fT4 to fT3, and correspondingly temporarily suppressed TSH levels. This ultimately results in a reduction in the production of thyroid hormones [Citation7]. Dexamethasone therapy also results in a decrease in endogenous TSH levels due to pituitary axis suppression, as well as in a decrease in active fT3 levels, possibly by blocking conversion processes, plasma protein binding and deiodinase activity [Citation34,Citation35]. Heparin also leads to the displacement of thyroid hormones from their binding proteins. The result is an increase in fT4 and fT3 levels and a slight decrease in TSH levels [Citation36,Citation37]. However, to minimize the possible difference between pharmacologically treated and non-treated subjects, we included only patients treated equally with anticoagulants (low molecular weight heparin) or antiplatelet agents (acetylsalicylic acid). Of note, the association between the COVID-19 severity and the risk for thyroid dysfunction remained significant even after exclusion of all the patients on glucocorticoids. Therefore, the pharmacological therapy is less likely to be the cause for the reported results.
Limitations
The present study should be interpreted by considering several potential limitations. First, although the sample size (n = 113) was relatively bigger or comparable to most of the previous studies in the field [Citation4,Citation21,Citation30], fT4 at the follow-up was measured in only one-third of the patients (n = 42). However, this number of subjects was still sufficient to perform a statistical analysis with adequate power. In addition, our analysis was focussed more on TSH results as a more indicative, reliable, and also cheaper test for thyroid function assessment [Citation38]. Therefore, we did not determine the levels of fT3. Moreover, we did not have data on TSH level during or immediately after the COVID-19, but our longitudinal comparison aimed to delineate the long-term effects of COVID-19 on the thyroid function. Subclinical hypothyroidism may recur after more than three months. Hence, we initially intended to extend the observational period and evaluate the thyroid function in a second, later follow-up, but almost none of the examined patients with thyroid dysfunction attended the Endocrinology Consulting Room for further monitoring.
Conclusions
Our results indicate that COVID-19 might have long-term, negative effects on thyroid function. Both triggering of autoimmune thyroid disease and persistent subclinical hypothyroidism were observed. Therefore, thyroid function testing should be included in the follow-up algorithm even of patients with mild-to-moderate COVID-19 manifestation. However, large-scale prospective trials are warranted to confirm these long-term effects on thyroid function.
Author contributions statement
Conceptualization, V.Y., T.S., and R.S.; methodology, V.Y. and T.S.; software, T.S.; validation, V.Y., T.S., and R.S.; investigation, V.Y.; writing—original draft preparation, V.Y., T.S.; writing—review and editing, V.Y., T.S., and R.S. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This research is supported by the Bulgarian Ministry of Education and Science under the National Program “Young Scientists and Postdoctoral Students – 2”
Data availability statement
The data supporting the findings in this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5.
- Scappaticcio L, Pitoia F, Esposito K, et al. Impact of COVID-19 on the thyroid gland: an update. Rev Endocr Metab Disord. 2021;22(4):803–815.
- Ganesan K, Anastasopoulou C, Wadud K. Euthyroid Sick syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
- Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid. 2021;31(1):8–11.
- Gao W, Guo W, Guo Y, et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. 2021;44(5):1031–1040.
- Li MY, Li L, Zhang Y, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tis-sues. Infect Dis Poverty. 2020;9(1):45.
- Samuels MH, Pillote K, Asher D, et al. Variable effects of nonsteroidal antiinflammatory agents on thyroid test results. J Clin Endocrinol Metab. 2003;88(12):5710–5716.
- Chen W, Tian Y, Li Z, et al. Potential interaction between SARS-CoV-2 and thyroid: a review. Endocrinology. 2021;162:bqab004.
- Lania A, Sandri MT, Cellini M, et al. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020;183(4):381–387.
- Shakiba M, Nazemipour M, Heidarzadeh A, et al. Prevalence of asymptomatic COVID-19 infection using a seroepidemiological survey. Epidemiol Infect. 2020;148:e300.
- World Health Organization. Clinical management of COVID-19: interim guidance. Available online: https://apps.who.int/iris/handle/10665/332196. (Accessed on 30 April 2022)
- Jonklaas J, Bianco AC, Bauer AJ, American Thyroid Association Task Force on Thyroid Hormone Replacement, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751.
- Bulgarian Society of Endocrinology. Recommendations for good practice in Thyroid diseases. Bulgarian Society of Endocrinology: Sofia; 2019. [in Bulgarian].
- Russ G, Bonnema SJ, Erdogan MF, et al. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6(5):225–237.
- Wang W, Su X, Ding Y, et al. Thyroid function abnormalities in COVID-19 patients. Front Endocrinol (Lausanne). 2020;11:623792. )
- Lui DT, Lee CH, Chow WS, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021;106(2):e926–e935.
- Giovanella L, Ruggeri RM, Ovčariček PP, et al. Prevalence of thyroid dysfunction in patients with COVID-19: a systematic review. Clin Transl Imaging. 2021;9(3):233–240.
- Khoo B, Tan T, Clarke SA, et al. Thyroid function before, during, and after COVID-19. J Clin Endocrinol Metab. 2021;106(2):e803–e811.
- Bulgarian Society of Endocrinology. Thyroid dysfunction and cardiovascular risk factors in Bulgarian adults. In Borissova A-M, editor. Epidemiology of endocrine diseases in Bulgaria 2006-2012. Sofia: Paradigma; 2016. p. 277–287. [in Bulgarian]
- Shinkov A, Borissova AM, Vlahov J, et al. Male gender differences in the thyroid ultrasound features, thyroid peroxidase antibodies and thyroid hormone levels: a large population-based study. J Endocrinol Invest. 2014;37(3):269–276.
- Zou R, Wu C, Zhang S, et al. Euthyroid sick syndrome in patients with COVID-19. Front Endocrinol (Lausanne). 2020;11:566439.
- Pal R, Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest. 2020;43(7):1027–1031.
- Muller I, Cannavaro D, Dazzi D, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739–741.
- Brancatella A, Ricci D, Viola N, et al. Subacute thyroiditis after SARS-CoV-2 infection. J Clin Endo-crinol Metab. 2020;105:dgaa276.
- Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with Covid-19 infection. J Endocrinol Invest. 2020;43(8):1173–1174.
- Ruggeri RM, Campennì A, Siracusa M, et al. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones (Athens). 2021;20(1):219–221.
- Feghali K, Atallah J, Norman C. Manifestations of thyroid disease post COVID-19 illness: report of Hashimoto thyroiditis, Graves’ disease, and subacute thyroiditis. J Clin Transl Endocrinol Case Rep. 2021;22:100094.
- Burekovic A, Halilovic D, Sahbaz A. Hypothyroidism and subclinical hypothyroidism as a consequence of COVID-19 infection. Med Arch. 2022;76(1):12–16.
- Clarke SA, Phylactou M, Patel B, et al. Normal adrenal and thyroid function in patients who survive COVID-19 infection. J Clin Endocrinol Metab. 2021;106(8):2208–2220.
- Lui DTW, Lee CH, Chow WS, et al. Insights from a prospective follow-up of thyroid function and autoimmunity among COVID-19 survivors. Endocrinol Metab (Seoul). 2021;36(3):582–589.
- Nishihara E, Amino N, Kudo T, et al. Moderate frequency of anti-thyroglobulin antibodies in the early phase of subacute thyroiditis. Eur Thyroid J. 2019;8(5):268–272.
- Mohammed AH, Yousif AM, Jabbar SA, et al. Assessment of thyroid function in COVID-19 patients. Tabari Biomed Stu Res J. 2021;3(3):8–13.
- Tang Y, Liu J, Zhang D, et al. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708.
- Re RN, Kourides IA, Ridgway EC, et al. The effect of glucocorticoid administration on human pituitary secretion of thyrotropin and prolactin. J Clin Endocrinol Metab. 1976;43(2):338–346.
- Chopra IJ, Williams DE, Orgiazzi J, et al. Opposite effects of dexamethasone on serum concentrations of 3,3',5'-triiodothyronine (reverse T3) and 3,3',5-triiodothyronine (T3). J Clin Endocrinol Metab. 1975;41(5):911–920.
- De Noriega Echevarría I, García-Salido A, Muñoz-Calvo MT, et al. Elevación de los niveles de hormonas tiroideas tras administración de heparina de bajo peso molecular [Elevated thyroid hormone levels following low molecular weight heparin administration]. An Pediatr (Barc). 2017;87(1):50–51.
- Dong BJ. How medications affect thyroid function. West J Med. 2000;172(2):102–106.
- Sheehan MT. Biochemical testing of the thyroid: TSH is the best and, oftentimes, only test needed - a review for primary care. Clin Med Res. 2016;14(2):83–92.
