Abstract
Inflammatory response and oxidative stress state have been largely described in diabetes and depression separately but not in combination. We aimed to explore the involvement of diabetes and unpredictable chronic mild stress (UCMS) separately or in combination on inflammatory response and oxidative stress. Diabetes, UCMS or combined rat models were used. Proinflammatory cytokines, Interleukin 6 (IL-6) and Tumor necrosis factor alpha (TNF-α) were assessed by real time quantitative polymerase chain reaction (q-PCR) and Enzyme-Linked Immunosorbent Assay (ELISA). Superoxide dismutase (SOD) and catalase (CAT) were determined by colorimetric assays. In the diabetes group, IL-6 mRNA expression increased by 55% and 34% (p < 0.05), respectively, in liver and kidney. UCMS alone or in combination with diabetes increased the mRNA of IL-6, respectively, by 85% and 78% in the liver and by 28% and 63% in the kidney (p < 0.01 for all). ELISA showed that diabetes and UCMS act in synergy on TNF-α and IL-6 expression. Diabetes and UCMS separately or in combination inhibited significantly (p < 0.01) the activities of the two anti-oxidant enzymes when compared to controls. The malondialdehyde (MDA) level was significantly enhanced in the group with diabetes combined with UCMS compared to UCMS alone in both organs. UCMS enhances the proinflammatory cytokines release and induces oxidative stress imbalance, and diabetes comorbidity with depression aggravates the inflammatory response and lipid peroxidation. These observations can be useful to better understand depression-induced organ damage.
Introduction
Diabetes currently represents a major challenge in developed and developing countries. Thus, about 415 million people worldwide now live with diabetes [Citation1]. Diabetes is one of the major risk factors for the development and progression of several health problems including cardiovascular, cerebrovascular complication and renal failure [Citation2,Citation3].
Actually, there is abundant evidence that immunological changes such as altered levels of inflammatory cytokines and chemokines participate in the progression of diabetes [Citation4] and that diabetes is recognized as chronic systemic inflammation [Citation5,Citation6]. Cytokines are classified depending on their cellular origin and characteristics in lymphokines, monokines, chemokines and interleukins, and reported to perform various biological functions [Citation7,Citation8]. Enhanced proinflammatory cytokines produced by infiltrated macrophages, monocytes and T-cells have been reported in cases of diabetic nephropathy [Citation9] and various other disorders [Citation10,Citation11]. The pro-inflammatory cytokine TNF-α is considered as the main regulator in insulin resistance [Citation12]. TNF-α is responsible for impaired endothelium-dependent vasodilation in the coronary microcirculation in experimental models of diabetes in rats and mice [Citation13–15].
Depression and anxiety were ranked by the World Health Organization as the fourth major cause of disability and considered as a very serious medical disease with a lifetime prevalence ranging from approximately 11% in low-income countries to 15% in high-income countries [Citation16] and about 450 million persons suffer from mental disturbances or from disturbances of behavior [Citation17]. In humans, numerous studies suggest that diabetic patients have increased risk for major depressive disorder [Citation18], which alters the instrumental activities of daily life or basic activities of daily living, and may lead work disability [Citation1].
Cytokines are key players in the immune activation which has been described in stress and major depressive disorder. The proposed mechanism is an active cytokine production by glial cells which affect neurotransmitters, especially the monoaminergic systems in the brain [Citation19].
It is currently known that diabetes is associated with an enhanced inflammatory response and that depressive symptoms and social stress activate immune response. However, the effect of comorbidity of diabetes and stress on inflammatory response and oxidative stress remains unclear. The aim of this study was to investigate the involvement of diabetes and unpredictable chronic mild stress (UCMS) separately or in combination in inflammatory response and oxidative stress in rat kidney and liver.
Materials and methods
Ethics
The animal protocols used for this study were approved by the University Animal Care and Use Committee of the University of Gabes, Tunisia (Approval number LNFP/Pro152018) and were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Animals
Male Wistar rats weighting between 174 and 190 g were purchased from the Faculty of Sciences of Gabes, Tunisia. The animals were maintained at the same conditions of humidity (70%), temperature (23 °C) and 12-h light/dark cycle and fed commercial pellet diet and tap water ad libitum. Rats were randomly assigned to specific protocol groups: 1) Control group (CTRL) (n = 10), 2) diabetes group (DM) (n = 9), 3) unpredictable chronic mild stress (UCMS) group (UCMS) (n = 10) and 4) combined diabetes and UCMS group (DM + UCMS) (n = 9).
Diabetes was induced in rats with a single intraperitoneal dose of alloxan (250 mg/kg) from Sigma Chemical Co. (St Louis, MO, USA) dissolved in saline (0.9% NaCl) solution. The blood glucose level was monitored with an electronic glucometer (ACCU-CHEK®, Roche, India) by performing a small puncture at the tail.
Chronic unpredictable mild stress was induced as described by Brooks et al. [Citation20]. Rats were singly housed and subjected to different types of stressors in randomly chosen sequences for 8 h each day, 5 days/week, for 8 weeks: (1) Damp bedding: About 200 mL of water was added to each standard cage; (2) swimming in water at room temperature (25 °C); tilt cages to approximately 45° (without bedding); pairing with another neighboring stressed animal rat; No bedding; and inversion of the light/dark cycle. The rats received one of these stressors per day and the same stressor was not applied for 2 days so that the animals could not predict the occurrence of stimulation [Citation21].
Collection of samples
At the end of the study, rats were sacrificed by decapitation. Blood samples were collected into serum vacutainer bottles and centrifuged at 4200 g for 15 min at 4 °C and stored at − 80 °C for about 2 weeks until analysis. The liver and kidney were removed, washed from blood, weighed and frozen immediately and stored at −80 °C.
Assay of biochemical serum markers
The serum levels of urea, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and C-reactive protein (CRP) were assessed by a clinical chemistry and turbidimetry analyzer (Biosystems SA, Barcelona, Spain) referenced SN834000228 at the Habib Bourguiba University Medical Center, Medenine (Tunisia). All reagents were purchased from Biosystems (Spain).
Protein extraction
The protein extraction method was described in our previous study [Citation22]. Briefly, frozen kidney and liver (100 mg) were homogenized in hydrolysis buffer (0.25 mol/L saccharose, 0.05 mol/L Tris-HCL, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), pH 7.4). After centrifugation at 12000 g for 15 min, the protein concentrations were measured in the supernatants according to Bradford method using Bovine serum albumin as a standard.
Enzyme-linked immunosorbent assay (ELISA)
TNF-α and IL-6 levels in liver and kidney homogenates were determined by the commercial ELISA kit (ab234570, Abcam, USA) and (ab236712, Abcam, USA), respectively, in kidney and liver homogenates according to the manufacturer’s guidelines. The data was recorded and calculated using an iMark microplate reader (Bio-Rad).
RNA extraction and real-time reverse transcription (RT)-PCR analysis of IL-6 and TNF-α
mRNA expression was assessed according to a previous protocol [Citation23]. RNA was extracted from kidney and liver using Trizol Reagent (Invitrogen, Carlsbad, CA) and reverse-transcribed with superscript II transcriptase (Invitrogen, France). Quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was performed for IL-6 and TNF-α with a MyiQ thermal cycler (Bio-Rad Laboratories, Hercules, CA) using the resulting cDNA as template, iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA), and the appropriate set of primers (Invitrogen, France) (). Two-step RT-PCR real-time amplifications were carried out as follows: 3 min at 95 °C followed by 10 s at 95 °C and 45 s at 60 °C. For each sample, PCR was performed in duplicate. The expression levels obtained from the standard curves were normalized against 18S rRNA.
Table 1. Primer sequences used for real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR).
Catalase and superoxide dismutase activities assay
CAT activity was determined as previously described [Citation24]. Equivalent volumes of 500 µg of total protein of liver and kidney homogenates were mixed with 500 µL phosphate buffer solution and served to set zero to the spectrophotometer at 240 nm. The reaction was started by adding 100 µL of 200 mmol/L H2O2 in the previous mix. Absorbance was recorded each 15 s for 2 min. One unit of CAT was defined as the amount of enzyme that decomposes 1.0 µmole of H2O2 per minute at pH 7 and 25 °C. The enzyme activity was calculated using an extinction coefficient of 0.043 L/(mmol cm) and expressed in U/mg protein.
SOD activity was measured in liver and kidney homogenates according to a previous protocol [Citation25]. SOD can induce oxidation of epinephrine to adrenochrome by O2. at alkaline pH. One unit of superoxide dismutase was defined as the amount of extract that inhibits the rate of adrenochrome formation by 50%. Firstly, the spectrophotometer was set to zero by a mix of 640 µL of 0.05 mol/L Na2CO3, pH = 10.2 and 160 µL of epinephrine; secondly, a sample equivalent to 200 µg of total protein was added and the absorbance was recorded each 30 s for 5 min at 480 nm. The results were expressed U/mg protein.
Malondialdehyde (MDA) level measurement
The MDA level was measured according to a previously described method [Citation26]. Samples were mixed with 150 μL of TBS (Tris 50 mmol/L and NaCl 150 mmol/L, pH 7.4) and 250 μL TCA-BHT (20% TCA and BHT 1%). After centrifugation at 10000 g for 10 min, 400 μL of the supernatant was added to HCl 0.6 N and 320 μL Tris-TBA (Tris 26 mmol/L and TBA 120 mmol/L). The mix was incubated at 80 °C for 10 min. The absorbance was measured at 535 nm. Absorbance of TBA-MDA complex was proportional to MDA concentration of lipid peroxide. Results were expressed in mmol of MDA/mg of proteins.
Statistical analysis
Analysis of variance (ANOVA) and pairwise comparisons by Tuckey’s HSD test were done using Statistica software version 13.2 (Statsoft, France). Results are expressed as mean values with standard error of the means (± SEM). Differences were considered statistically significant at p < 0.05, and n values indicate the number of replicates.
Results
Biochemical parameters
The fasting blood glucose levels and biochemical parameters are presented in . We observed that the blood glucose levels in diabetic rats were significantly higher than in the controls (p < 0.01) and reached 240 mg/dL. However, there was no significant change in blood glucose after UCMS induction (p = 0.09).
Table 2. Biochemical serum parameters from control and treated rats.
The biochemical parameters AST and ALT were significantly increased in the diabetes group, respectively, by about 100% and 50% (p < 0.001). However, single UCMS exposure had a non-significant effect on the AST and ALT levels compared to the control group (p = 0.103). On the other hand, in the group of UCMS combined with diabetes there was a significantly higher ALT level as compared with the UCMS group or the diabetes group, by 110% (p < 0.01) and 50% (p < 0.05), respectively.
The serum levels of creatinine and urea were increased significantly in diabetic rats by about 200% and 66%, respectively. Moreover, our results showed that on the one hand single UCMS exposure had a non-significant effect on the two studied parameters, and on the other hand, the combined effect of diabetes and UCMS was similar to the effect diabetes alone.
Our results showed that the level of inflammation protein CRP was increased by 3-fold when compared to controls. The effect of diabetes was amplified by UCMS exposure and increased 5-fold compared to the controls. However, exposure to UCMS alone had no significant effect on the CRP level.
Proinflammatory cytokines expression
The two proinflammatory cytokines were evaluated at the level of mRNA expression in liver and kidney (). The IL-6 mRNA expression evolution was similar in kidney and liver in all used groups. However, the TNF-α m-RNA expression evolution in the liver differed from that observed in the kidney.
Figure 1. Real-time RT-PCR analysis of IL-6 and TNF-α mRNA in liver (A, B) and kidney (C, D) from control and treated rats. Values are presented as ratio fold change of mRNA to control group. Data are mean ± SEM. *p < 0.05, **p < 0.01 versus CTRL; ≠ p < 0.05, ≠ ≠ p < 0.01 versus DM. CTRL: control group, DM: Alloxan-induced diabetic rats, UCMS: unpredictable chronic mild stress group, DM + UCMS: combined group.
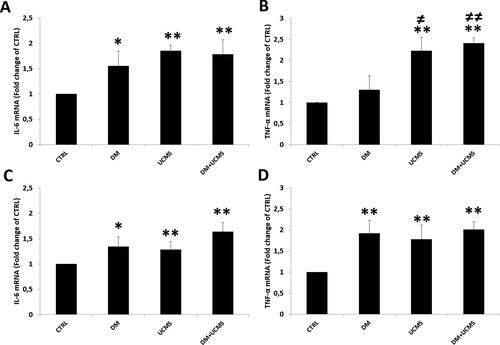
We demonstrated that IL-6 mRNA expression was enhanced in the diabetic group by 55% and 34% (p < 0.05) respectively in the liver and kidney (). Similarly, exposure to UCMS alone or in combination with diabetes significantly increased the mRNA level of IL-6 respectively by 85% and 78% in the liver and by 28% and 63% in the kidney (p < 0.01 for all) ().
The TNF-α mRNA expression profile was similar to that of IL-6 in kidney but not in liver tissue. The TNF-α mRNA level showed non-significant changes in the diabetes group, whereas in the UCMS group and in the combined UCMS and diabetes group it increased by 123% and 141% (p < 0.01), respectively, compared to the control group ().
Assessment of IL-6 level by Elisa showed that, on the one hand, diabetes and UCMS increased the two cytokines levels significantly compared to controls and on the other hand, the combination aggravated the effect induced by diabetes or UCMS alone. Thus, the IL-6 levels in liver and kidney were significantly increased in the diabetes group and UCMS group by about 30%. The group with diabetes combined with UCMS had enhanced IL-6 levels in the liver and kidney by 90% and 55% (p < 0.01), respectively, compared to the control group ().
Figure 2. Levels of proinflammatory cytokines, IL-6 and TNF-α in liver (A, B) and kidney (C, D) determined by ELISA. Data are mean ± SEM. *p < 0.05, **p < 0.01 versus CTRL; ≠ ≠ p < 0.01 versus DM; # p < 0.05, ## p < 0.01 versus UCMS. CTRL: control group, DM: Alloxan-induced diabetic rats, UCMS: unpredictable chronic mild stress group, DM + UCMS: combined group.
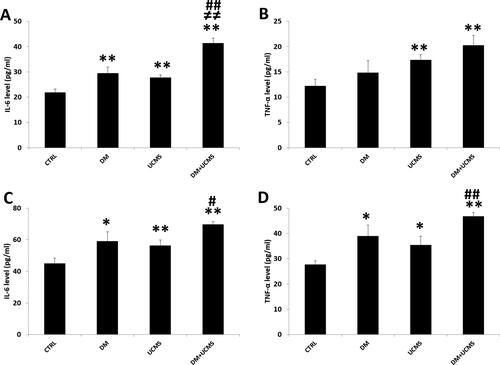
The TNF-α level in the liver remained unchanged in the diabetes group when compared to the controls and was enhanced by 42% and 66% (p < 0.01) in the UCMS group and the UCMS and diabetes group, respectively, when compared to the controls (). In the kidney, the TNF-α level was enhanced in the diabetes group and the UCMS group by 40% and 27% (p < 0.01), respectively, and diabetes combined with UCMS treatment amplified significantly the effect of diabetes or UCMS alone ().
Antioxidant enzyme activities and MDA level
The activities of the antioxidants enzymes CAT and SOD and the MDA level in the liver and kidney are illustrated in . Our results showed a similar trend of variation of SOD and CAT in the two studied organs. Diabetes and UCMS separately or in combination inhibited significantly (p < 0.01) the activities of the two anti-oxidant enzymes when compared to controls. On the other hand, the combined effect of diabetes and UCMS on CAT and SOD activities remained similar to the effects of diabetes or UCMS alone ().
Figure 3. Antioxidant enzymes activities, SOD (A), CAT (B) and MDA level (C) in liver and kidney from control and treated rats. Data are mean ± SEM. **p < 0.01 versus CTRL; ≠ ≠ p < 0.01 versus DM; # p < 0.05, ## p < 0.01 versus UCMS. CTRL: control group, DM: Alloxan-induced diabetic rats, UCMS: unpredictable chronic mild stress group, DM + UCMS: combined group.
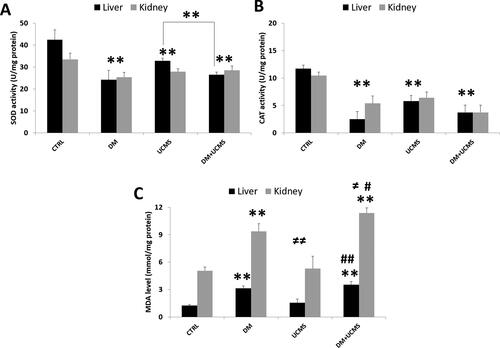
The analysis of lipid peroxidation induction showed that diabetes increased significantly the MDA level by 150% and 80% in the liver and kidney, respectively (p < 0.01). In addition, the MDA level remained unchanged under single UCMS treatment, while it increased significantly when diabetes was combined with UCMS. The MDA level was significantly enhanced in the group with diabetes combined with UCMS when compared to UCMS alone in both organs by about two folds and to the level in the kidney in the diabetes group (p < 0.05).
Discussion
Actually, it is well known, firstly, that diabetes is associated with an enhanced inflammatory state which contributes to several complications and, secondly, that social and environmental stress activates the immune response and thus inflammatory processes. However, the inflammatory state and oxidative stress have not been investigated in diabetes combined with stress conditions. Our main results showed 2 important findings regarding the effect of UCMS alone and in combination with diabetes: 1) UCMS enhances the release of proinflammatory cytokines in the liver and kidneys and induces oxidative stress imbalance; and 2) diabetes comorbidity with depression aggravates the IL-6 and TNF-α expression in the liver and kidneys and enhances lipid peroxidation as compared to single exposure to UCMS or diabetes.
This study, to our knowledge, is the first that describes the cytokine expression in liver and kidneys under chronic mild stress conditions in a rat model. For this we used the UCMS animal model to reproduce chronic stress and depression. The UCMS model in rodents is one of the most commonly used depression models which has been validated for use in chronic stress investigations to reproduce depressive symptoms [Citation27,Citation28]. In this model, repeated exposure to several stressors may potentiate the vulnerability of rats to many neuropsychiatric disorders such as depression [Citation29].
We demonstrated that UCMS was associated with overexpression of proinflammatory cytokines IL-6 and TNF-α as assessed by quantitative real time RT-PCR and ELISA. Moreover, we established that UCMS can inhibit the antioxidant enzymes SOD and CAT and enhances the MDA level in liver and kidney. Although there are no previous studies that have reported similar effects in the liver and kidneys, it has been established that UCMS increases the plasma levels of cortisol and nitrotyrosine, which indicate a disrupted oxidative stress balance in mice [Citation29].
Similarly, it has been reported that UCMS impairs aortic endothelial-dependent dilation and upregulates oxidative products and pro-inflammatory cytokines and provokes perivascular adipose tissue impairment of aortic function in Zucker Rats exposed to UCMS [Citation30]. Therefore, the level of proinflammatory cytokines in plasma and cerebrospinal fluid have been studied in patients with depression [Citation31], as such cytokines play an important role in aggravation of behavioral, neuroendocrine disorders or depressive disorders [Citation32,Citation33].
The sympathetic nervous system is activated in stress situations and in depression, and cytokine production was enhanced by noradrenergic fibers through β-adrenergic receptors [Citation34,Citation35]. Moreover, β-adrenergic receptors stimulation enhances the TNF-α, IL-1β, and IL-6 mRNA expression in the rat heart [Citation36]. On the other hand, parasympathetic stimulation and β-adrenergic receptors antagonists inhibit cytokines overexpression [Citation37,Citation38]. In the same context, our previous study showed that chemical inhibition of sympathetic nervous system reduces significantly TNF-α in the rat heart [Citation39].
In our study, we observed disrupted cytokine expression under UCMS conditions associated with an imbalance of oxidative stress and biochemical renal and hepatic serum markers, indicating some organ damage. Our results are concordant with anterior investigations that demonstrated that overexpression of TNF-α in mice brain injected with methamphetamine are associated with oxidative stress state [Citation40]. Moreover, UCMS was shown to decreases CAT and SOD activities and to increase the level of thiobarbituric acid-reactive substances in mice and rat brain [Citation41,Citation42]. Brain damage was reported after UCMS exposure and cytokine overexpression as abnormalities in the external capsule and prefrontal cortex in rats [Citation43].
The experimental model of diabetes was conducted by a single injection of alloxan to rats. Previously, it was reported that alloxan causes rapid destruction of pancreatic β cells acting through GLUT2 glucose transporters [Citation44].
We showed that diabetes enhanced IL-6 and TNF-α in rat kidneys and liver associated with a fall in antioxidant enzymes and enhanced MDA production. Moreover, we demonstrated disrupted renal and hepatic serum markers. Our results corroborate previous finding that diabetes provokes an overexpression of TNF-α in kidney in animal models of diabetes and leads to tissue damage [Citation45,Citation46].
Studies have described that diabetes is strongly associated with increased concentrations of proinflammatory cytokines [Citation47,Citation48]. Cytokines are produced by numerous types of cells, such as adipose tissues, leukocytes, T cells fibroblasts, endothelial cells [Citation7]. Previously, it has been reported that inflammation and oxidative stress associated with diabetes cause renal microvascular complications [Citation49,Citation50] and that antioxidant and anti-inflammatory molecules improve kidney function in diabetic patients [Citation51]. We demonstrated that diabetes is associated with enhanced AST and ALT levels in our rat model, which can be considered as a sign of organ damage. Similar results have been reported previously [Citation52].
To our knowledge, we are the first to investigate the effect of coexistence of diabetes and UCMS on proinflammatory cytokines and oxidative stress in the liver and kidneys in a rat model. There were two reasons for the use of a combined animal model: 1) there were no previous studies reporting proinflammatory and oxidative stress states in the two target organs under similar conditions and 2) in humans, depression aggravates diabetes when they occur simultaneously [Citation53] and, inversely, the prevalence of depression increases in prediabetic patients and in undiagnosed diabetic patients, and markedly increases in previously diagnosed diabetic patients [Citation54]. We showed that diabetes comorbidity with depression aggravates the proinflammatory state (i.e. the cytokines IL-6 and TNF-α expression) in the two target organs and enhances lipid peroxidation as compared to single exposure to UCMS or diabetes. Moreover, we demonstrated that UCMS enhanced significantly the diabetes-induced rise in fasting blood glucose, ALT and CRP levels. These results corroborate previous studies in which stress conditions and anxiety enhanced the cortisol and blood glucose levels and decreased the insulin secretion by the release of sympathetic hormones [Citation55]. Studies have described a higher correlation between elevated glycosylated hemoglobin levels in American adults with type 2 diabetes and depression and anxiety [Citation56]. The aggravation of proinflammatory cytokines and oxidative stress by stress combined with diabetes observed in our study was in agreement with previous evidence that diabetes and depression symptoms are associated with an inflammatory response and that diabetes is recognized as chronic systemic inflammation [Citation5,Citation6].
Disrupted levels of transaminases, creatinine and urea resulting from association of stress and diabetes can lead to liver and kidney damage. Similarly it has been reported that elevated depressive symptoms are associated with an increased risk of coronary arteries diseases and cardiac disorders [Citation57], which can be explained by alterations in the autonomic nervous system function and elevated pro-inflammatory cytokines [Citation58]. Moreover, cell damage and apoptosis are reported to be caused by reactive oxygen species [Citation59,Citation60]. In our combined model, we demonstrated that the MDA level was significantly higher than in the diabetic group. In fact, MDA resulting from lipid peroxidation reflects the severity of the organ exposure to free radicals [Citation61] and is acknowledged to be an index of cell damage and excessive oxidative stress imbalance [Citation62]. In addition, oxidative stress is reported to stimulate proinflammatory cytokines and to aggravate chronic diseases [Citation63].
Conclusions
In this study, we provided evidence suggesting that the coexistence of stress and diabetes can lead to impaired proinflammatory cytokine and oxidative stress state in liver and kidney as well as to perturbation of biochemical serum markers. We used a combined model of diabetes and stress in an attempt to fill some of the gaps in the knowledge about the proinflammatory and oxidative stress states in liver and kidney under such conditions. Our findings will be useful for a better understanding of depression-induced organ damage and progression of chronic health disorders in diabetic patients. Further investigations are necessary for a better clarification of the physiological mechanism responsible for the observed proinflammatory and oxidative stress disturbance.
Authors’ contributions
HD conceived the research problem, developed the design, conducted experiments and wrote and corrected the paper. SBH developed the design of methodology and collected data. WH conducted proinflammatory experiments. LZ supervised the study and validated the paper.
Data availability statement
The data that support the findings of this study are available at from the corresponding author, upon reasonable request.
Disclosure statement
The authors report no conflict of interest
Additional information
Funding
References
- Harding JL, Pavkov ME, Magliano DJ, et al. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16.
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
- Pugliese G, Penno G, Natali A, et al. Diabetic kidney disease: new clinical and therapeutic issues. Joint position statement of the italian diabetes society and the italian society of nephrology on the natural history of diabetic kidney disease and treatment of hyperglycemia in patients with. Nutr Metab Cardiovasc Dis. 2019;29(11):1127–1150.
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107.
- Korczak DJ, Pereira S, Koulajian K, et al. Type 1 diabetes mellitus and major depressive disorder: evidence for a biological link. Diabetologia. 2011;54(10):2483–2493.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119.
- Balahura LR, Selaru A, Dinescu S, et al. Inflammation and inflammasomes: pros and cons in tumorigenesis. J Immunol Res. 2020;2020:1–15.
- Holdsworth SR, Gan P-Y. Cytokines: names and numbers you should care about. Clin J Am Soc Nephrol. 2015;10(12):2243–2254.
- Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, et al. Inflammatory cytokines in diabetic nephropathy. J Diabetes Res. 2015;2015:1–9.
- Iranshahi N, Assar S, Amiri SM, et al. Decreased gene expression of epstein–barr Virus-Induced gene 3 (EBI-3) may contribute to the pathogenesis of rheumatoid arthritis. Immunol Invest. 2019;48(4):367–377.
- Pérez-Morales RE, Del Pino MD, Valdivielso JM, et al. Inflammation in diabetic kidney disease. Nephron. 2019;143(1):12–16.
- Yang J, Park Y, Zhang H, et al. Feed-forward signaling of TNF-α and NF-κB via IKK-β pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;296(6):H1850–H1858.
- Lee J, Lee S, Zhang H, et al. Interaction of IL-6 and TNF-α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS One. 2017;12(11):e0187189.
- Park Y, Capobianco S, Gao X, et al. Role of EDHF in type 2 diabetes-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;295(5):H1982–H1988.
- Park Y, Yang J, Zhang H, et al. Effect of PAR2 in regulating TNF-α and NAD (P) H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol. 2011;106(1):111–123. Springer
- Bădescu SV, Tătaru C, Kobylinska L, et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120–125. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27453739.
- Foyet HS, Tchinda Deffo S, Koagne Yewo P, et al. Ficus sycomorus extract reversed behavioral impairment and brain oxidative stress induced by unpredictable chronic mild stress in rats. BMC Complement Altern Med. 2017;17(1):502.
- Ali S, Stone MA, Peters JL, et al. The prevalence of co‐morbid depression in adults with type 2 diabetes: a systematic review and meta‐analysis. Diabet Med. 2006;23(11):1165–1173.
- Ting EY-C, Yang AC, Tsai S-J. Role of interleukin-6 in depressive disorder. IJMS. 2020;21(6):2194. MDPI
- Brooks S, Branyan KW, DeVallance E, et al. Psychological stress-induced cerebrovascular dysfunction: the role of metabolic syndrome and exercise. Exp Physiol. 2018;103(5):761–776.
- Yazir Y, Demirtaş Şahin T, Furat Rençber S, et al. Restorative effect of resveratrol on expression of endothelial and neuronal nitric oxide synthase in cavernous tissues of chronic unpredictable mild stress-exposed rats: an impact of inflammation. Int J Impot Res. 2018;30(6):318–326.
- Dab H, Hachani R, Hodroj W, et al. Differential control of MMP and t-PA/PAI-1 expressions by sympathetic and renin-angiotensin systems in rat left ventricle. Auton Neurosci. 2009;150(1-2):27–32.
- Dab H, Hachani R, Dhaouadi N, et al. Physiological regulation of MMPs and tPA/PAI in the arterial wall of rats by noradrenergic tone and angiotensin II. J Renin Angiotensin Aldosterone Syst. 2012;13(1):36–45.
- Lindau-Shepard BA, Shaffer JB. Expression of human catalase in acatalasemic murine SV-B2 cells confers protection from oxidative damage. Free Radic Biol Med. 1993;15(6):581–588.
- Polle A, Krings B, Rennenberg H. Superoxide dismutase activity in needles of norwegian spruce trees (Picea abies L.). Plant Physiol. 1989;90(4):1310–1315.
- Buege JA, Aust SD. 1978. [30] microsomal lipid peroxidation. pp. 302–310.
- Golbidi S, Frisbee JC, Laher I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am J Physiol Heart Circ Physiol. 2015;308(12):H1476–H1498.
- Xavier J, Farias CP, Soares MSP, et al. Ayahuasca prevents oxidative stress in a rat model of depression elicited by unpredictable chronic mild stress. Arch Clin Psychiatry (São Paulo). 2021;48(2):90–98.
- Frisbee JC, Brooks SD, Stanley SC, et al. An unpredictable chronic mild stress protocol for instigating depressive symptoms, behavioral changes and negative health outcomes in rodents. JoVE. 2015;(106)
- DeVallance ER, Branyan KW, Olfert IM, et al. Chronic stress induced perivascular adipose tissue impairment of aortic function and the therapeutic effect of exercise. Exp Physiol. 2021;106(6):1343–1358.
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. Elsevier
- Dantzer R, Wollman E, Vitkovic L, et al. Cytokines and depression: fortuitous or causative association? Mol Psychiatry. 1999;4(4):328–332.
- Serafini G, Pompili M, Seretti ME, et al. The role of inflammatory cytokines in suicidal behavior: a systematic review. Eur Neuropsychopharmacol. 2013;23(12):1672–1686. Elsevier
- Guirao X, Kumar A, Katz J, et al. Catecholamines increase monocyte TNF receptors and inhibit TNF through β2-adrenoreceptor activation. Am J Physiol. 1997;273(6):E1203–E1208.
- Prabhu SD, Chandrasekar B, Murray DR, et al. β-Adrenergic blockade in developing heart failure. Circulation. 2000;101(17):2103–2109.
- Murray DR, Prabhu SD, Chandrasekar B. Chronic β-Adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation. 2000;101(20):2338–2341.
- Breit S, Kupferberg A, Rogler G, et al. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44.
- Kan H, Xie Z, Finkel MS. Norepinephrine-stimulated MAP kinase activity enhances cytokine-induced NO production by rat cardiac myocytes. Am J Physiol. 1999;276(1):H47–H52.
- Dab H, Hachani R, Sakly M, et al. Physiological regulation of pro-inflammatory cytokines expression in rat cardiovascular tissues by sympathetic nervous system and angiotensin II. gpb. 2013;32(04):569–575.
- Flora G, Lee YW, Nath A, et al. Methamphetamine-induced TNF-α gene expression and activation of AP-1 in discrete regions of mouse brain. Neuromol Med. 2002;2(1):71–85. Springer
- Kumar B, Kuhad A, Chopra K. Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: behavioral and biochemical evidences. Psychopharmacology (Berl). 2011;214(4):819–828. Springer
- Schaalan MF, Nassar NN. Effects of octreotide in chronically mild stressed rats: possible role of immune and oxidative stress pathways. Neurochem Res. 2011;36(10):1717–1723. Springer
- Yang P, Gao Z, Zhang H, et al. Changes in proinflammatory cytokines and white matter in chronically stressed rats. Neuropsychiatr Dis Treat. 2015;11:597–607.
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226.
- Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. JASN. 2008;19(3):433–442.
- Ortiz A, Gonzalezcuadrado S, Bustos C, et al. Tumor-necrosis-factor as a mediator of glomerular damage. J Nephrol. 1995;8(1):27–34.
- Chen T-L, Xu E-L, Xu H, et al. The influence of diabetes enhanced inflammation on cell apoptosis and periodontitis. ABB. 2012;03(06):712–719.
- King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79(8 Suppl):1527–1534.
- Bassi R, Vergani A, D’Addio F, et al. Positive effects of a novel non-peptidyl low molecular weight radical scavenger in renal ischemia/reperfusion: a preliminary report. Springerplus 3. 2014;3(1):1–4. Springer:
- Yu C-C, Fornoni A, Weins A, et al. Abatacept in B7-1–positive proteinuric kidney disease. N Engl J Med. 2013;369(25):2416–2423.
- Alshali KZ, Karawagh AM. A review of glycemic efficacy of liraglutide once daily in achieving glycated hemoglobin targets compared with exenatide twice daily, or sitagliptin once daily in the treatment of type 2 diabetes. SMJ. 2016;37(8):834–842.
- Abdel-Wahhab KG, Daoud EM, El Gendy A, et al. Efficiencies of low-level laser therapy (LLLT) and gabapentin in the management of peripheral neuropathy: diabetic neuropathy. Appl Biochem Biotechnol. 2018;186(1):161–173.
- Wang D, Wang H, Gao H, et al. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci. 2020;10(1):28.
- Chen S, Zhang Q, Dai G, et al. Association of depression with pre-diabetes, undiagnosed diabetes, and previously diagnosed diabetes: a meta-analysis. Endocrine. 2016;53(1):35–46.
- Wong H, Singh J, Go RM, et al. The effects of mental stress on non-insulin-dependent diabetes: determining the relationship between catecholamine and adrenergic signals from stress, anxiety, and depression on the physiological changes in the pancreatic hormone secretion. Cureus. 2019;11(8):e5474.
- Chlebowy DO, Batscha C, Kubiak N, et al. Relationships of depression, anxiety, and stress with adherence to Self-Management behaviors and diabetes measures in african American adults with type 2 diabetes. J Racial Ethn Health Disparities. 2019;6(1):71–76.
- Lichtman JH, Bigger JT, Blumenthal JA, et al. Depression and coronary heart disease. Circulation. 2008;118(17):1768–1775.
- Sreckovic MJ, Jagic N, Miloradovic V, et al. Association of coronary ischemia estimated by fractional flow reserve and psychological characteristics of patients. pwki. 2017;2:117–121.
- Jonas CR, Ziegler TR, Gu LH, et al. Extracellular thiol/disulfide redox state affects proliferation rate in a human Colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33(11):1499–1506.
- oz HS, Chen TS, Nagasawa H. Comparative efficacies of 2 cysteine prodrugs and a glutathione delivery agent in a colitis model. Transl Res. 2007;150(2):122–129. Elsevier
- Yang F, Liao J, Yu W, et al. Copper induces oxidative stress with triggered NF-κB pathway leading to inflammatory responses in immune organs of chicken. Ecotoxicol Environ Saf. 2020;200:110715. Elsevier
- Wang F, Liu J, Hu X, et al. The influence on oxidative stress markers, inflammatory factors and intestinal injury-related molecules in wahui pigeon induced by lipopolysaccharide. PLoS One. 2021;16(5):e0251462. USA
- Sturza A, Popoiu CM, Ionică M, et al. Monoamine oxidase-related vascular oxidative stress in diseases associated with inflammatory burden. Oxid Med Cell Longev. 2019;2019:1–8. Hindawi.
