Abstract
Bacterial cultures are commonly preserved for long periods of time via freeze-drying (lyophilization). Lyophilized bacteria typically retain viability from 5 to 35 years. We investigated the vitality and preservation of some of the characteristic morphological, serotypic and biochemical features of 14 Escherichia coli strains following lyophilized storage for over 40–50 years. We also investigated their susceptibility to conventional antibiotics used in the therapy of infections caused by Enterobacteriaceae representatives. In our study, 14 strains of E. coli related to 11 serological types – O1, O2 (two strains), O5, O7, O11, O20, O25, O26, O29, O111 (two strains) and O125 (two strains) – were used. The lyophilized microorganism ampules were produced in the period of 1971 to 1973 and were stored at 4 °C in a microbial collection for educational purposes at the Medical College – Varna, Bulgaria. Control strains were E. coli ATCC25922, an E. coli strain (used for educational purposes) and three clinical E. coli isolates from urine and wound secretions. The E. coli strains stored for 40–50 years had preserved the studied morphological and biochemical characteristics, as well as those related to their antigenic characteristics and antibiotic sensitivity. Their susceptibility to the tested antimicrobials was analogical to the control reference strain E. coli ATCC25922, indicating that despite the long storage time, all strains retained and demonstrated the typical morphological, stereotypic and biochemical characteristics of the species.
Introduction
Bacterial cultures are commonly preserved for long periods of time via freeze-drying (lyophilization). Lyophilized bacteria typically retain viability within 5 to 35 years [Citation1,Citation2]. The survival of bacterial cells depends on intrinsic factors (strain resistance) and specific conditions (e.g. growth phase, initial cell concentration, cell density, growth conditions and the (cryo-) protectants).
Many reports have shown good survival of bacteria, yeasts and fungi after lyophilization and long storage periods [Citation3–7]. A study demonstrated that lyophilized microorganisms retained their viability after 50 years of storage [Citation3]. This period is longer than commonly known in the literature.
In our study, we investigated the vitality and preservation of some of the characteristic morphological, serotypic and biochemical features of Escherichia coli strains stored in lyophilized form for over 40–50 years. We also investigated their susceptibility to conventional antibiotics used in the therapy of infections caused by members of the Enterobacteriaceae family.
E. coli is a representative of the normal digestive tract microflora in humans and animals. At the same time, there are versatile pathogenic E. coli strains that can affect multiple organs [Citation8]. In connection with the microbiological diagnosis of E. coli infections, it is important to note that the bacteria are gram-negative, they are often cultivated in differential media in which the presence of lactose-positive colonies is reported. Glucose is fermented along with other carbohydrates to form pyruvate and various other acids. Bacteria of this type are indole-positive and usually do not form hydrogen sulfide.
E. coli is divided into serogroups based only on the O-antigens and serotypes on a complex of O-, H- and possibly K-antigens. Virulence factors in E. coli genomes and phenotypic traits serve as a basis to differentiate the human pathotypes of diarrheagenic E. coli (DEC) from non-pathogenic E. coli and extraintestinal pathogenic E. coli (ExPEC) (for review see Braz et al. [Citation9]). The ExPEC are classified as uropathogenic E. coli (UPEC), sepsis-causing E. coli (SEPEC) and neonatal meningitis-associated E. coli (NMEC) [Citation10].
As reviewed by Braz et al. [Citation9], recent pathogenomics and phenotypic classification have revisited the DEC group as nine distinct pathotypes, proposed by their differential features and the essential virulence genes defining each subgroup, such as Shiga toxin-producing E. coli (STEC), enterohemorrhagic E. coli (EHEC), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), diffusely-adhering E. coli (DAEC), adherent-invasive E. coli (AIEC), and cell-detaching E. coli (CDEC) [Citation9–11].
The aim of this study was to investigate the survival of lyophilized E. coli strains stored at 4 °C for a period of 40–50 years and to study their morphological, serotypic, biochemical characteristics and susceptibility to a range of antimicrobials.
Materials and methods
Bacterial strains
In this study, 14 strains of E. coli related to 11 serological types – O1, O2 (two strains), O5, O7, O11, O20, O25, O26, O29, O111 (two strains) and O125 (two strains) were used. They were isolated in Copenhagen (years of isolation between 1971 and 1981) and the former Soviet Union (USSR, in 1976). According to the manufacturers’ product inserts, the microorganisms are suspended in a preservation medium that provides protection of the cell walls during freeze-drying and subsequent extended storage. The type of protective medium and the technique for treating the cell suspensions prior to freeze-drying were not described.
During the entire storage period, the ampoules with the lyophilized strains were stored in the Microbial Bank of the Medical College-Varna, Bulgaria, at 4 °C in a refrigerator.
As controls we used E. coli ATCC25922, three clinical E. coli isolates from urine and wound secretions and one E. coli strain for educational purposes. The training strain is used for the purposes of the study activity in Medical College-Varna. It was purchased and first cultivated nearly ten years ago, being repeatedly preserved in skim milk and recultivated again.
Opening of stored strains, bacterial culturing and biochemical profile of the strains
For initial cultivation and viability assay of the lyphilized E. coli strains, we made cultures in nutrient broth, brain heart infusion broth (BHI) broth and blood agar (all media provided by HiMedia, Ridacom, Bulgaria).
The hermetically sealed ampoules were opened with a dental micromotor with a diamond separator for ceramic separation. Then, the lyophilized microbes were mixed with 1 mL of sterile saline solution. The suspensions were mixed by using swabs, and blood agar cultures were grown. Inoculations of 0.2 mL bacterial suspensions in liquid nutrient media were also made. All the Petri dishes and the test tubes were incubated aerobically at 37 °C for 24 h.
After the first colonies of the 14 E. coli strains appeared on blood agar, we transferred individual colonies onto Kligler’s iron agar (KIA) and onto Eosin methylene blue (EMB). They were incubated under the same conditions. To read the biochemical profile of the cultivated strains in KIA, a yellow color appearing on the straight part of the agar was scored as an indication of glucose degradation, and on the slant part of the agar, as an indication of lactose degradation; rising of the agar from the bottom of the test tube indicated gas production. Lactose-positive colonies on EMB were differentiated during the appearance of dark-blue-colored bacterial colonies, as well as the appearance of the characteristic metallic sheen when cultivating some lactose-fermenting strains in EMB.
Gram stain, rapid agglutination test and indol test
For identification of E. coli strains, Gram staining was performed. The serological group affiliation of some of the strains was tested with rapid agglutination reaction on a slide using polyvalent agglutinating serums (BulBio, Bulgaria) in the first and the third serological groups: I – O25, O26, O55, O111, O119 and III – O6, O20, O124, O125, O126. We performed the test by mixing five to six colonies of each strain with a drop of agglutinating serum until the suspensions were completely homogenized. We reported the results within 1-2 min. We scored the appearance of fine-grained agglutinates in the solution as positive agglutination and interpreted the result as negative if the suspension remained homogenous.
We performed the indole test with Kovac’s reagent (BulBio, Bulgaria) for biochemical identification of ability to degrade tryptophan. The test was performed with both the initial cultures of the strains in nutrient broth and BHI broth as well as in the fresh tryptophan broth. For this purpose, we dripped 3-4 drops of Kovac`s reagent into the suspensions with microorganisms and detected the appearance of a red ring on the surface of the solution as a positive indole reaction and, respectively, its absence as a negative result.
Disk-diffusion susceptibility test
We used the Kirby-Bauer disk diffusion susceptibility test to determine the susceptibility of E. coli strains to antimicrobial agents. The isolates were tested against the action of 19 antibiotics selected according to the established criteria and recommendations in EUCAST [Citation12]. For this purpose, we used factory-loaded antibiotic discs provided by Oxoid, Elta90, Bulgaria.
The test for antimicrobial sensitivity was performed by making dense seed on the surface of Mueller-Hinton agar with a bacterial suspension standardized with a densitometer (0.5MF). We left all the Petri dishes at room temperature for 15 min and then applied the antibiotic disks to the media at an appropriate distance from one another by using sterile tweezers. We incubated the Petri dishes for 24 h at 37 °C and after that reported the results by measuring the diameter of the inhibition zones in millimeters, assigning them to the S (susceptible) or R (resistant) categories, by using EUCAST [Citation12].
As control strains in the antimicrobial susceptibility test, we used five more E. coli strains: E. coli ATCC25922, one strain used for the educational activity at Medical College – Varna and three clinical isolates which were not reported as beta-lactamase producers. The drug sensitivity of these strains was tested as described above.
Results
We used E. coli strains stored for over 40–50 years in lyophilized form in ampoules in our facilities ().
Figure 1. Ampoules with 14 lypophilized strains of Escherichia coli (А), with production dates between 1971 and 1981. Opening of the ampoules (B) by using a dental micromotor with a diamond separator.
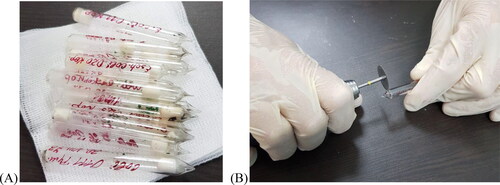
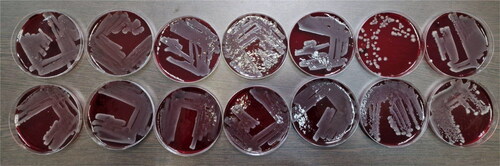
We opened the ampoules by using a dental micromotor with a diamond separator to separate the ceramics (); after that we made two cultures in liquid nutrient medium each – nutrient broth and BHI broth, and one each in blood agar to ‘awaken’ the strains. We cultivated all test tubes and Petri dishes for 24 h. After incubation, turbidity was observed macroscopically in all liquid media and densely grown bacterial colonies without hemolytic activity in all dishes with solid medium ().
Figure 2. First seeding of fourteen Escherichia coli strains after 40–50 years of storage.
Note: Top row from left to right (number in the study 1–7) – O7, O111, O25, O20, O11, O125 and O111; bottom row from left to right (number in the study 8–14) – O29, O26, O5, O1, O125, O2 and O2.
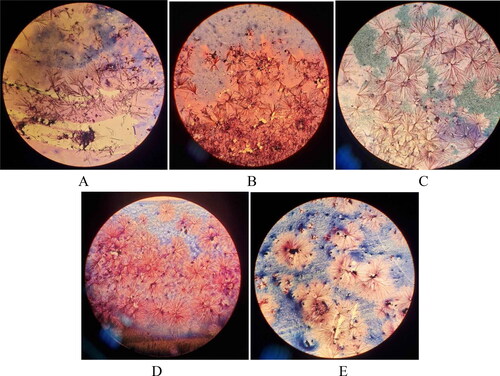
Gram staining was performed on material taken from microbial colonies from each blood agar. None of the preparations showed typical gram-negative staining on the microscopic field. This was probably because of the still specific crystalline structure of the lyophilized substances (А–E).
Figure 3. Microscopic pictures of Escherichia coli (А-E). after initial cultures of the strains from the lyophilized form stored for four to five decades. Slides were prepared by Gram staining and were observed under immersion system, 100x (Carl Zeiss Microscopy GmbH, Primo star).
Note: E. coli strains O7 (A), O11 (B), O25 (C), O111 (D) and O125 (E).
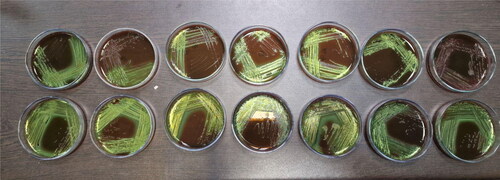
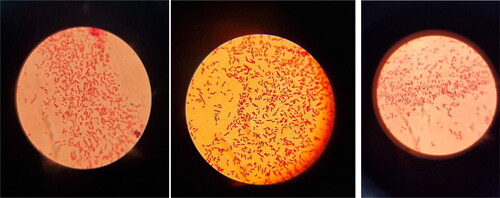
Separate microbial colonies were transferred from blood agar on EMB, cultivated under standard conditions, after which the characteristic E.coli lactose-positive colonies were clearly observed in dark blue color with a green metallic sheen on the agar ().
Figure 4. Bacterial colonies of Escherichia coli on Eosin methylene blue.
Note: Top row from left to right (number in the study 1–7) – O7, O111, O25, O20, O11, O125 and O111; bottom row from left to right (number in the study 8–14) – O29, O26, O5, O1, O125, O2 and O2.
After we repeated the microscopic preparations using the Gram staining procedure, pure cultures of gram-negative rod-shaped bacteria were clearly visible in the field ().
Figure 5. Microscopic images of Escherichia coli after re-cultivation (24 h at 37 °C) of the strains from the lyophilized form stored for four to five decades. Slides were prepared by Gram staining and were observed under immersion system, 100x (Carl Zeiss microscopy GmbH, Primo star).
Note: E. coli strains from left to right O11, O25 and O111.
Serotype determination
We performed rapid agglutination tests on a slide with the corresponding diagnostic agglutinating sera to determine the serotype affiliation of the E. coli strains to the first or third serological group. All tested strains gave a positive agglutination result with the serum homologous to their designated serotype ().
Table 1. Results of test agglutination with Escherichia coli strains stored for four to five decades in lyophilized form.
We also carried out agglutination reactions as controls for each strain with other randomly selected mismatched diagnostic sera. All agglutination reactions were negative, with homogenous suspensions.
Biochemical identification
We performed the indole test with the 14 tested strains to determine the typical indole-positive E. coli showing ability for tryptophan degradation. All 14 strains of E. coli gave a positive reaction for indole, after Kovac’s reagent dripping, both in the broths from the first inoculation of the strains and in fresh tryptophan broth. We made also inoculations in KIA to detect the characteristic ability of E. coli strains to degrade lactose, often with gas and acid production. All strains demonstrated the typical biochemical profile of E. coli, after one-day cultivation. All were lactose-positive and glucose-positive with a red-to-yellow change in the straight and slant part of the agar. In some of the strains, gas production was observed with rising of the agar medium from the bottom of the test tube. All of the tested strains were negative for hydrogen sulfide production ().
Table 2. Biochemical profile of Escherichia coli serotypes on Kligler’s iron agar (KIA).
Antimicrobial susceptibility testing of E. coli strains
All of the 14 tested strains were investigated for susceptibility to 19 antimicrobial agents using the Kirby-Bauer disk diffusion test. The results of the conducted research are presented in .
Table 3. Antimicrobial sensitivity of Escherichia coli strains tested by the disk-diffusion test.
All of the tested strains demonstrated susceptibility to all used antibiotics. The strains had completely identical antibiotic susceptibilities to that demonstrated by E. coli ATCC25922. For the other four control strains (one educational and three clinical isolates), we reported resistance to some of these drugs. The educational strain demonstrated resistance to 10 of the 19 antimicrobial agents and susceptibility to ampicillin/sulbactam, piperacillin/tazobactam, ceftazidime, ceftriaxone, imipenem, amikacin, ciprofloxacin, levofloxacin. In the clinical isolates, we also reported a lack of susceptibility to a large number of the tested drugs. All were susceptible to piperacillin/tazobactam, ceftazidime, ceftriaxone, imipenem, amikacin, ciprofloxacin and levofloxacin. All tested strains showed susceptibility to these six antibiotic preparations.
Overall the E. coli strains stored for 40–50 years had preserved the morphological and biochemical characteristics, as well as those related to their antigenic characteristics and antibiotic sensitivity. The results showed that they are all gram-negative rods. Their susceptibility to the tested antimicrobials was analogical to the control reference strain E. coli ATCC25922. The tested strains gave positive agglutination with their homogenous agglutinating sera, both glucose- and lactose-positive in KIA culture, in most of the cases with gas production. They also showed ability to degrade lactose when cultured in EMB agar, with dark blue colonies and metallic sheen appearance.
These results give us a reason to consider that the lyophilized E. coli strains stored for over 40–50 years had preserved both vitality and largely their genotypic and phenotypic characteristics. These results give us the basis for more in-depth biochemical and molecular genetic studies of Escherichia coli strains to obtain more data.
Discussion
Overview of E. coli groups
Diarrheagenic E. coli (DEC) distinguished as the causative agents of E. coli diarrheal diseases are the following four groups:
Enteropathogenic E. coli (EPEC) cause severe enteritis and enterocolotis with diarrhea and are characterized with high mortality among children up to 3 years of age [Citation8]. They belong to the following serogroups: O6, O20, O26, O44, O55, O86, O111, O114, O119, O125, O126, O127, O128, O142, O158, O159 etc. Sporadic cases of diarrhea among children have been observed in O1, O2, O4 etc. EPEC are highly invasive to the enterocytes of the intestinal mucosa, often producing enterotoxins similar to those of S. dysenteriae 1 – with neurotoxic, cytotoxic enterotoxic effects.
Enterotoxigenic E. coli (ETEC) cause diarrheal diseases because they produce thermo-stable (ST) and/or thermo-labile (TL) enterotoxin. The most common groups are the following: O6, O8, O15, O20, O25, O27, O63, O78, O128, O148, O153, O159, O167 etc.
Enteroinvasive E. coli (EIEC) ressemble Shigella in several aspects. They are often immobile, lactose-negative and antigenetically similar to different serogroups of Shigella. O-serogroups in which EIEC, include O28ас, O112ас, O124, O136, O143, O144, O147, O152, O164.
Enterohemorrhagic E. coli (EHEC) cause epidemics and sporadic cases of infections of bloody diarrhea and colitis, mainly caused by E. coli O157:H7. They are invasive and produce cytotoxin, probably identical to S. dysenteriae 1 and important in the pathogenesis of the disease [Citation13].
Uropathogenic E. coli (UPEC), most common etiological agents of uroinfections, include certain strains of E. coli found in serogroups O1, O2, O4, O6, O7, O8, O9, O11, O22, O25, O62, O75 etc. These microbes often possess Р- and Х-pili, due to which they attach to uroepithelial cells.
Neonatal meningitis-associated E. coli (NMEC) are isolated in 40-80% cases of neonatal bacterial meningitis. Most of them have capsular antigen K1. They belong to different O-groups: O1, O6, O7, O16, O18, O83 [Citation14].
Sepsis-causing E. coli (SEPEC) are a group of E.coli strains belonging to serogroups O1, O2, O4, O6, O7, O8, O9, O11, O18, O22, O25, O75, etc. that have been proven to cause septic conditions.
The strains used in our study belong to UPEC group (O2, O7, O11 and O25); NMEC group (O7); EPEC group (O20, O26, O111 and O125) and ETEC group (O20 and O25). Оur results demonstrate that lyophilization of microorganisms is a reliable microbial preservation method. Its effectiveness is longer than previously known in some E. coli serotypes, and probably also in other bacterial species of clinical significance.
Preservation of characteristics following long-term storage in lyophilized form
Lyophilization is a preferred method for strain preservation at room temperature. Owing to zero water activity (aw), lyophilized preparations show no growth and at the same time preserve their viability and purity over a long period of storage [Citation15,Citation16]. To prevent damage to bacterial cells during the freeze-drying procedure, cryoprotectants are added [Citation17]. There are many cryoprotectants such as skimmed milk or different carbohydrates (trehalose, glycerol, sucrose, among others) [Citation18].
Recent studies have tracked the effectiveness of lyophilization over shorter periods of time than our study - up to one year [Citation18–21]; two years [Citation22]; four years [Citation6, Citation23] and ten years [Citation7]. Thus, our results are in agreement with the report by [Citation3], who also demonstrated that after 50-year of storage, the ampoules contained considerable amounts of viable cells (in many cultures, 106–109 cells) that were quite sufficient for culture maintenance. In their study, all lyophilized microorganisms (pro- and eukaryotes) retained their viability after 50-year storage, a longer period than those previously known in literature.
Most research has primarily aimed at investigating the effectiveness of lyophilization of microbes with probiotic potential, lactic acid bacteria, acetic acid bacteria and yeasts [Citation19,Citation20] and less often of microbes of clinical significance [Citation7]. Most reports focus on the degree of survival of microbes, without conducting subsequent research on whether their characteristic features are preserved - serological affiliation, resistance to antimicrobial agents, and others.
All accumulated evidence demonstrates that freeze-drying is a reliable technique in terms of stability and survival of microorganisms for a long time; however, the process effectiveness depends on the choice of cryoprotectant.
Conclusions
The tests carried out with E. coli strains belonging to eleven different O-serological types stored for four to five decades in lyophilized form showed that, despite the long storage time, all strains retained and demonstrated the typical morphological, serotypic and biochemical characteristics of the species. We consider that these studies provide valuable information related to the time performance of lyophilized bacteria forms, an approach extremely useful and applied for preservation of microorganisms for various scientific and commercial purposes, including bacteria with probiotic potential, as well as those with wide application in the biotechnological processes.
Author contribution
N. E. conceived of the study, carried out the experiments, supervised the findings of this work and wrote the manuscript. E.G., G.T., G.N., K.L. and T.K. carried out the experiments and contributed to the English version of the manuscript. S.S. carried out the experiments and contributed to the drug sample preparation in the experimental part. All authors provided critical feedback and helped shape the research, analysis and the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data that support the findings reported in this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Adams J. The principles of freezedrying. Methods Mol Biol. 2007;368:15–38.
- Tindall BJ. Vacuum drying and cryopreservation of prokaryotes, meth. Mol Biol. 2007;368:73–98.
- Kupletskaya MB, Netrusov AI. Viability of lyophilized microorganisms after 50-year storage. Microbiology. 2011;80(6):850–853.
- Steel KJ, Ross HE. Survival of freeze dried bacterial cultures. J Appl Bacteriol. 1963;26:370–375.
- Antheunisse J. Viability of lyophilized microorganisms after storage. Antonie Van Leeuwenhoek. 1973;39(2):243–248.
- Elkadi OА, Elanany М, Ramadan МА. Long-term survival of three fungal species in lyophilized human blood plasma. ASM J Microbiol Spectrum. 2021;9(1):e0022221.
- Miyamoto-Shinohara Y, Imaizumi T, Sukenobe J, et al. Survival rate of microbes after freeze-drying and long-term storage. Cryobiology. 2000;41(3):251–255. PMID: 11161557.
- Aijuka M, Buys EM. Persistence of foodborne diarrheagenic Escherichia coli in the agricultural and food production environment: implications for food safety and public health. Food Microbiol. 2019;82:363–370.
- Braz VS, Melchior K, Moreira GC. Escherichia coli as a multifaceted pathogenic and versatile bacterium. Front Cell Infect Microbiol. 2020;10:548492.
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140.
- Pawłowska B, Sobieszczańska BM. Intestinal epithelial barrier: the target for pathogenic Escherichia coli. Adv Clin Exp Med. 2017;26(9):1437–1445.
- EUCAST. 2022. https://www.eucast.org/eucast_news/news_singleview/?tx_ttnews%5 Btt_news%5D=464&cHash=ea8540c0fbdaa71b3bbcb3bf765239de.
- Liu Y, Thaker H, Wang C, et al. Diagnosis and treatment for Shiga toxin-producing Escherichia coli associated hemolytic uremic syndrome. Toxins. 2023;15(1):10.
- Liu Y, Zhu M, Fu X, et al. Escherichia coli causing neonatal meningitis during 2001–2020: a study in Eastern China. Int J Gen Med. 2021;14:3007–3016. PMID: 34234530; PMCID: PMC8254664.
- García MD, Uruburu F. La conservación de cepas microbianas. Actualidad SEM. 2020;30:12–16.
- Arencibia D, Rosario L, Gámez R. Métodos generales de conservación de microorganismos. Finlay, La Habana. 2008.1–14. ISBN 978-959-7076-20-9.
- Li B, Tian F, Liu X, et al. Effects of cryoprotectants on viability of Lactobacillus reuteri CICC6226. Appl Microbiol Biotechnol. 2011;92:609–616.
- Yuste A, Arosemena EL, Calvo MÀ. Study of the probiotic potential and evaluation of the survival rate of lactiplantibacillus plantarum lyophilized as a function of cryoprotectant. Sci Rep. 2021;11:19078.
- Stefanello RF, Nabeshima EH, Iamanaka BT, et al. Survival and stability of Lactobacillus fermentum and wickerhamomyces anomalus strains upon lyophilisation with different cryoprotectant agents. Food Res Int. 2019;115:90–94. ISSN 0963-9969.
- Moretti AF, Gamba RR, do Céu Costa M, et al. Protective effect of lyophilization on fermentative, microbiological and sensory properties of kefir. Int J Biochem Pharmacol. 2019;1(1):1–7. ISSN2689-7695.
- Paliy AP, Gujvinska SO, Alrawashdeh MS, et al. Selection of technological regime and cryoprotector for lyophilization of lactobacteria (Lactobacillus spp.). Ukrainian J Ecol. 2020;10(4):184–190.
- Turuvekere Sadguruprasad L, Basavaraj M. Statistical modelling for optimized lyophilization of Lactobacillus acidophilus strains for improved viability and stability using response surface methodology. AMB Expr. 2018;8:129.
- Antheunisse J, de Bruin-Tol JW, van der Pol-van Soest ME. Survival of microorganisms after drying and storage. Antonie Van Leeuwenhoek. 1981;47(6):539–545.
