?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
With the developments in wireless technologies, living beings are increasingly exposed to electromagnetic fields (EMFs). EMFs are known to affect bone metabolism and muscle tissue. However, their effects on bones and skeletal muscles are controversial, as some studies have reported positive effects while others have reported adverse effects. In this study, the effects of radiofrequency radiation (RFR) on bone biomechanics and skeletal muscle tissues were investigated in diabetic and healthy rats. Rats were exposed to 3.5 GHz RFR for 2 h per day for 30 days. Bone biomechanics measurements were taken to evaluate the effects of RFR on bone quality, flexibility and durability. The whole-body specific absorption rate (SAR) was found to be 37 mW/kg. The results showed that RFR exposure had adverse effects on bone biomechanics, including decreased elasticity coefficient and Young’s modulus, increased maximum displacement and decreased maximum force. However, oxidative stress parameters in diabetics were also altered by 3.5 GHz RFR to a greater extent than in healthy rats. In conclusion, 3.5 GHz RFR may have potential to alter bone quality and structural integrity including muscle oxidative stress parameters in rats. It should be emphasized that the observed changes were more obvious in diabetic rats. In addition, the changes observed in healthy and diabetic rats exposed to RFR showed a statistically significant difference according to the sham groups.
Introduction
In recent years, living beings have begun to be exposed to electromagnetic fields (EMFs) along with developments in wireless technologies. However, EMFs are important external factors affecting bone metabolism and muscle tissue [Citation1, Citation2]. Various effects of EMFs on bones and skeletal muscles have been reported [Citation2, Citation3]. Researchers have indicated that EMFs accelerate healing in different tissue types, as well. For example, increased bone healing rates after fractures and the acceleration of bone formation were noted [Citation4]. Moreover, low-frequency pulsed EMFs increased osteogenesis and stimulated mRNA expression of morphogenetic proteins in osteoblast cells [Citation5, Citation6]. On the contrary, some studies have stated that EMFs cause adverse health effects on bone and skeletal muscle tissues [Citation3, Citation7]. This diversity in results may be due to the fact that the evaluated EMFs differed in intensity, frequency and other properties.
Mobile phones use radiofrequency radiation (RFR) for the transmission of images, audio files and other data. 5 G is the fifth generation of wireless communication technology, providing faster data transfer rates, lower latency, and higher capacity compared to previous generations (2 G, 3 G and 4 G). The 3.5 GHz frequency band is one of the most widely used frequency bands for 5 G, and it is well-suited for providing high-speed and high-capacity wireless communications. It offers a good balance between speed and range, making it a popular choice for 5 G networks. The 3.5 GHz frequency band has several advantages for 5 G. Firstly, it has a relatively wide bandwidth, which allows for higher data transfer rates and capacity. Secondly, it has a longer range than the higher millimeter-wave frequencies, which makes it easier to provide coverage over larger areas [Citation8–10].
Many studies have shown that RFR has adverse effects on various tissues [Citation8, Citation11, Citation12]. Bone tissue is known to be radiosensitive [Citation13]. A previous study concluded that exposure to RFR of 1800 MHz in the prenatal period increased apoptosis through oxidative stress in fetal bone and muscle tissues and caused excessive osteoblast inhibition by suppressing calcineurin [Citation13]. On the other hand, no studies to date have investigated the RFR effects on bone and muscle tissues of healthy individuals and diabetics.
Bone biomechanics provide information about the quality, flexibility and durability of bones. The main purpose of measurements in bone biomechanics is to determine the load on the bone and any resulting deformity. With bone biomechanics, and particularly with considerations of bone fragility, information can be obtained about the structural and/or material properties of bone, what kinds of changes in structural and/or material dimensions facilitate fractures in pathological conditions, and treatment procedures for particular pathologies. In load deformation curves, the region where the load is applied to the bone and the amount of deformation is linear represents the elastic region, and the bone returns to its initial state upon the removal of the load. However, the deformation that occurs in the bone is irreversible when the applied force surpasses the elastic limit point or the yield point. Damage to the bone in this area, which is called the plastic zone, is permanent due to trabecular microfractures, lamellar shifts and resulting fractures. The yield point is located between these two zones and it shows how much the bone can be deformed without losing its flexibility [Citation14]. Maximum displacement reflects the extent to which deformation occurs in tissues up to the time of fracturing and is related to the bone’s brittleness. The maximum force constitutes the value of the force occurring as the fracture occurs and it signifies the overall structural integrity of the tissues. On the other hand, the elasticity coefficient expresses the intrinsic stiffness of the tissue and shows how much the bone can deform against the applied stress without permanent damage. The elasticity coefficient is called Young’s modulus if tension or compression forces are applied to the tissue [Citation15].
In this study, to gain insight into the relation between 3.5 GHz RFR and bone and muscles, we investigated the effects of the RFR under investigation, which is one of the frequencies used in 5 G technology, on bone biomechanics and skeletal muscle tissues in diabetic and healthy rats.
Materials and methods
Ethics statement
All phases of the research involving animals presented here were undertaken in Van Yuzuncu Yil University’s Experimental Research and Application Center (Van, Turkey). The guidelines of that university’s Animal Experiments Local Ethics Committee were applied in designing and performing all steps of the study (Protocol No: 2021/05-10). The experiment was run under blind conditions.
Animals and housing contiditons
The animals used in this research included 28 healthy male Wistar Albino rats, which were 8–10 weeks of age and weighed 200–250 grams. They were provided by the same Experimental Animal Research Center at which the research was performed. These animals were divided into 4 groups with 7 rats per group and each group was kept together in a stainless steel cage, with a total of 4 such cages. They were maintained in a laboratory setting with a 12-h light/dark cycle and ad libitum pellet feed (with 21% crude protein; Purina, Istanbul, Turkey) and water under optimized conditions of temperature of 22 ± 2 °C and 60 ± 5% humidity.
Animal groupings and protocol
As stated above, the animals were divided into 4 experimental groups of 7 rats each. In Group 1 (healthy sham), 7 healthy rats were held in a Plexiglas carousel for 2 h daily for 30 days with the generator turned off. In Group 2 (healthy RFR), 7 healthy rats were exposed to 3.5 GHz RFR in the Plexiglas carousel for 2 h daily for 30 days. In Group 3 (diabetes sham), 7 rats with diabetes were held in the Plexiglas carousel for 2 h daily for 30 days with the generator turned off. In Group 4 (diabetes + RFR group), 7 rats with diabetes were exposed to 3.5 GHz RFR for 2 h daily for 30 days.
Experimental model of diabetes
To obtain animal models of type 1 diabetes mellitus in laboratory settings, streptozotocin (STZ) is commonly used as an antibiotic capable of destroying pancreatic islet β-cells [Citation16]. The induction of diabetes in the present experimental model was carried out as previously described by Bektas et al. [Citation8]. Briefly, the rats in Groups 3 and 4 were subjected to a one-night fast, followed by a single intraperitoneal injection of STZ at a dosage of 45 mg/kg, which was dissolved in 0.1 mol/L phosphate-citrate buffer. In contrast, the control rats received an injection of an equivalent volume of sterile citrate buffer solution. After 72 h, blood samples were collected to confirm the clinical presence of diabetes, and an IME-DC glucometer was used to determine the blood glucose levels. The presence of diabetes was confirmed by any values exceeding 250 mg/dL [Citation8].
Exposure to radiofrequency radiation and measurement processes
The rats in Groups 2 and 4 were exposed to 3.5 GHz RFR by means of a signal generator (model 3500 PM10, Everest, Adapazari, Turkey). The antenna of the generator was placed at the center of the Plexiglas Carousel to provide ideal exposure conditions, as it is stated in our previous study [Citation17]. A TES 593 R field probe (TES Electric Electronical Corporation, Taiwan) was used for performing all measurements of the electrical field and power density. The measured electric field and power density values were 28.1 V/m and 1.6 W/m2.
Evaluation of specific absorption rate (SAR)
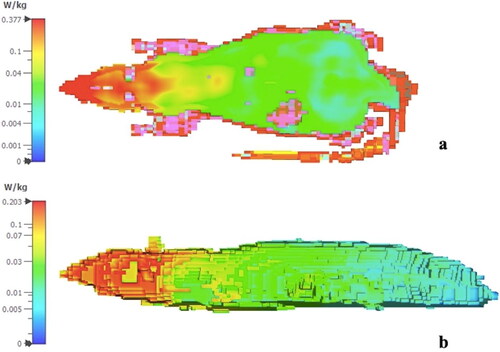
An additional setup for simulated research was designed to closely mirror the previously described experimental setup. In this process, a model that included a Faraday cage, antenna and rats was established with three-dimensional electromagnetic field solver software in the CST Studio (CST AG, Darmstadt, Germany). The SAR value was determined as described by Bektas et al. [Citation17]. A detailed rat model based on computerized tomography scans was used in the simulation, which included all tissues in the rat body. Although 2 W peak power is typical of GSM burst signals, the average power used for SAR evaluation was 250 mW. The CST model uses a technique called finite-integration technique (FIT) for electromagnetic and thermal modeling, which combines integral and differential solvers to act on space and time. The simulated electric field values were compared to the measured field values using the TES field probe to verify the accuracy of the simulation setup. The distribution of SAR was calculated using the method outlined in IEEE/IEC 62704-1.
Radiological evaluations
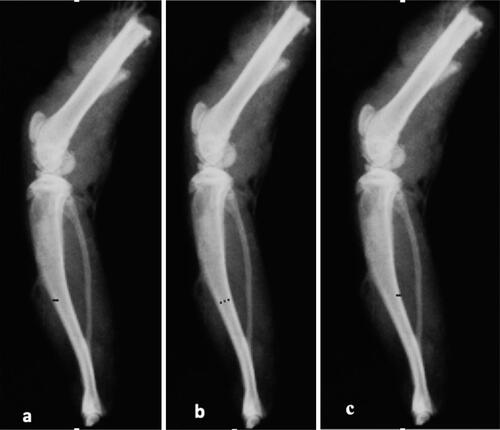
At the conclusion of the experiment, the rats were anesthetized with ketamine (Ketasol, Richter Pharma AG, Vienna, Austria) and bacillazine (Rompin, Istanbul, Turkey), and then euthanized via cervical dislocation. After the rats were euthanized, radiological imaging was performed to determine the values of the lateral cortex, the medial cortex and the medulla for the right tibia bones that had been removed from the rats (). Radiological evaluations were performed as previously described by Bektas et al. [Citation17].The thickness of the anterior cortex was measured by determining the width of the cortex from the midpoint of the tibia’s length (proximal-distal) towards its front end. The thickness of the posterior cortex was measured by determining the width of the cortex from the posterior midpoint of the tibia’s length (proximal-distal). Additionally, the width of the medulla was measured by determining its width from the midpoint of the tibia’s length (proximal-distal) and between the anterior border of the posterior cortex and the posterior border of the anterior cortex [Citation17].
Biomechanical testing
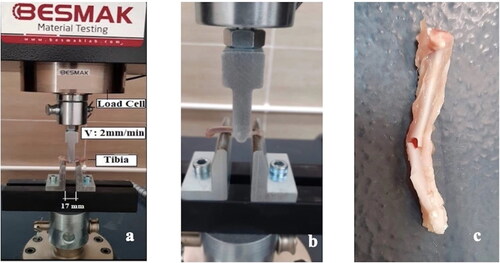
The three-point bending (3PB) test is one of the most commonly applied methods for the evaluation of loading conditions in fracture mechanics. In this research, the 3PB test was applied to samples obtained from the experimental groups using the BESMAK BMT-100E universal testing device (). Tibia diameters were taken as 4 mm. In all tests, the loading speed was 2 mm/s and the distance between the two end supports was adjusted and fixed as 14.5 mm according to tibia lengths. Before starting the tests, preloading of 20 N was applied to prevent soft tissue blanks on the bone surfaces.
Preparation of skeletal muscle tissue homogenates
At the end of the experiment, under ketamine (Ketasol, Richter Pharma AG, Vienna, Austria) and bacillazine (Rompin, Istanbul, Turkey) anesthesia, the rats were sacrificed by cervical dislocation and the skeletal muscles were removed immediately. Then, 0.5 g part of the muscle was used for the preparation of a homogenate. Skeletal muscle tissue homogenization was performed as previously described by Bektas et al. [Citation17]. Briefly, 9.5 mL of 5 mmol/L phosphate buffer (pH 7.4) containing 458 mmol/L of sucrose was used. To a plastic tube lacking a conical bottom, buffer was added along with the tissue sample. Using the TissueLyser LT (QIAGEN, The Netherlands), the samples were homogenized for 20 min at the highest speed. Following this, the homogenates were immediately centrifuged at 8000 g for 60 min, and the filtrate was collected in a separate tube [Citation17].
Determination of catalase activity
To measure the catalase activities in the muscle tissue homogenates, hydrogen peroxide was used as the substrate, and the Aebi method was employed [Citation18]. To begin the process, two replicate tubes were prepared, and 2.8 mL of 30 mmol/L H2O2 was added to the blank tube, with an additional 0.2 mL of phosphate buffer. In contrast, the sample tube contained 2.8 mL of 30 mmol/L H2O2, followed by the addition of 0.2 mL of appropriately diluted sample. After vortexing the tubes to ensure thorough mixing, the absorbance values reflecting the catalase activities were read twice at 240 nm at 30-second intervals (T80 + UV/VIS) . Catalase activty (U/L) was calculated as follows:
Where Δx = 30 s; and optical density of 2.3 = 1 µmol H2O2 in 1 cm light path [Citation17, Citation18].
Determination of reduced glutathione (GSH)
GSH levels were evaluated based on the yellow color that arose upon reacting erythrocyte sulfhydryl groups with 5,5′-dithiobis (2-nitrobenzoic acid). This spectrophotometric assay was performed at 412 nm (T80 + UV/VIS) using muscle homogenate samples with EDTA within 24 h [Citation19].
Determination of malondialdehyde (MDA) content
MDA is a product of peroxidation that occurs when fatty acids react with free radicals. MDA values were determined based on observing the color that became visible upon the application of thiobarbituric acid [Citation20]. Muscle homogenate samples (200 µL) were pipetted into test-tubes. Then, 800 µL of phosphate buffer and 25 µL of BHT solution and 500 µL of 30% TCA were added. The tubes were mixed by vortex and kept in an ice bath for 2 h after the caps were closed. The tubes were brought to room temperature. After removing the caps of the tubes, they were centrifuged at 2000 rpm for 15 min. Then, 1 mL of the obtained supernatant (filtrate) was taken and transferred to another tube; 75 µL of EDTA and 25 µL of TBA were added to the filtrate. The tubes were vortex mixed and kept in a hot water bath for 15 min (70 °C). Then, the reaction mixtures were brought to room temperature and the absorbance values were read in a spectrophotometer (T80 + UV/VIS) at 532 nm. The malondialdehyde content (µmol/L) was calculated as follows:
Where C is the concentration; F is the dilution factor and A is the absorbance
Determination of ischemia-modified albumin (IMA)
The levels of IMA in the obtained samples, were determined as previously described [Citation17]. To determine the IMA levels in the collected samples, a solution of cobalt chloride (1 g/L) was added to a mixture of serum (200 µL) and 50 µL of the solution, and was incubated for 10 min at room temperature while being stirred. After that, a solution of DTT (1.5 g/L) was added to the samples, mixed again, and left to incubate for an additional 2 min. Next, a solution of NaCl (9.0 g/L) was added, and the absorbance values were measured at 470 nm for all sample mixtures, including blanks prepared without DTT. The recorded absorbance values were used to calculate adjusted-IMA (D-IMA) levels for all rats, using albumin values and the following formula: [D-IMA = IMA × (Albumin/Median Albumin Level of the Group)]. The resulting D-IMA values were also recorded in absorbance units [Citation17, Citation21].
Statistical analysis
Descriptive statistics for the continuous variables (characteristics) were presented as Mean values and Standard deviation (±SD), while counts and percentages were given for the categorical variables. Normality assumption of the continuous variables was tested with Kolmogorov-Smirnov test. After the normality test, one-way analysis of variance (ANOVA) was performed for the normal distribution characteristics and Kruskal-Wallis test was performed for the non-normal distribution characteristics to compare the groups. Following the analyses, Tukey’s multiple comparison test (post-hoc) was used. The statistical significance level was set to 5%, and SPSS (ver: 21) statistical program was used for all statistical computations.
Results
Specific absorption rate
Specific absorption rate (SAR) calculations based on the IEEE/IEC 62704-1 method were used to determine the SAR distribution [Citation22]. Whole-body SAR was 37 mW/kg. The SAR distributions for 1 g and 10 g averaging for different cut-planes are displayed in .
Biomechanical testing results
The Young modulus was seen to be lower in Group 2 in comparison to Group 1 and this difference was statistically significant (p = 0.006). Among the diabetic rats, the Young modulus of Group 4 was higher in comparison to Group 3 (p = 0.036). Group 3 had lower Young modulus values than Group 1 (p = 0.001) ().
Table 1. Descriptive statistics and results of comparisons for biochemical characteristics.
The maximum load values and maximum stress values were significantly higher among the healthy rats exposed to RFR (Group 2) than in the diabetic rats exposed to RFR (Group 4) (p = 0.001). The maximum load and maximum stress values in Group 1 (healthy sham group) were generally higher than those in Group 3 (diabetic sham group), but this difference did not reach statistical significance (p = 0.064) ().
The duration values were observed to be significantly lower among healthy rats exposed to RFR (Group 2) in comparison to healthy rats not exposed to RFR (Group 1) (p = 0.005). Furthermore, the duration values of Group 3 were found to be statistically significantly lower in comparison to Group 1 (p = 0.015). The duration values of Group 4 were generally lower than those measured for Group 3, but this difference did not reach the level of statistical significance (p = 0.197) ().
The yield point values were statistically significantly lower among the healthy rats exposed to RFR (Group 2) than the healthy rats without RFR exposure (Group 1) (p = 0.014). Additionally, the yield point values were higher in the healthy sham group (Group 1) than the diabetic sham group (Group 3) (p = 0.001) ().
Displacement values were found to be lower among healthy rats exposed to RFR (Group 2) than the healthy rats without RFR exposure (Group 1) (p = 0.005), and they were higher among diabetic rats exposed to RFR (Group 4) than diabetic rats without RFR exposure (Group 3) (p = 0.017). In addition, the displacement values of the healthy sham group (Group 1) were considerably higher than those of the diabetic sham group (Group 3) (p = 0.001) ().
Radiological evaluation results
The lateral cortex and medial cortex values were significantly lower among the diabetic rats not exposed to RFR (Group 3) compared to the healthy rats without RFR exposure (Group 1) (p = 0.004) (). These values also tended to be lower in the groups with RFR exposure compared to the sham groups, but these differences were not statistically significant. The width of the medulla was greater in Group 2 in comparison to the other groups, and it was considerably greater in Group 1 compared to Group 3 ().
Table 2. Descriptive statistics and results of comparisons for radiological characteristics.
Biochemical analysis results
The GSH values in muscle tissue were higher in the healthy RFR group (Group 2) compared to the healthy sham group (Group 1), and they were also higher in the diabetic RFR group (Group 4) compared to the diabetic sham group (Group 3) (p = 0.001). On the other hand, the GSH values of Group 3 were quite low compared to Group 1 (p = 0.001). The CAT values in muscle tissue were higher in Group 1 compared to all other groups (p = 0.001). The IMA values in muscle tissue were significantly higher in Group 4 compared to Group 3, and they were higher in Group 2 compared to all other groups (p = 0.001). The MDA levels in muscle tissue were higher in Group 2 (healthy RFR) compared to all other groups (p = 0.001) ().
Table 3. Measurements of GSH, CAT, IMA, and MDA in skeletal muscle.
Discussion
This study was undertaken to investigate the effects of 3.5 GHz RFR on skeletal muscle tissue and biomechanical properties of the tibias in diabetic and healthy rats. Bones constitute two-component composite materials, containing minerals and collagen, and the mechanical properties of these materials are different from each other. While the mineral phase imparts strength (or load-carrying capacity) and mechanical rigidity (or stiffness) to the bone, collagen provides resistance to mechanical forces, durability and partial flexibility [Citation23, Citation24]. With the parameters that we measured in the course of our research, tissue size properties could be determined as material properties of the bones.
According to our results, exposure to 3.5 GHz RFR for 2 h daily for 30 days decreased the Young modulus values in healthy rats, thus causing a decrease in the bone flexibility of healthy rats. While the maximum load and stress values among healthy rats not exposed to RFR were statistically insignificantly higher compared to those of diabetic rats not exposed to RFR, with exposure to RFR the maximum load and stress values of bones in healthy rats were found to be considerably higher compared to diabetic rats (i.e. Group 2 vs. Group 4). This finding shows that RFR increased the damage caused to bones by diabetes.
Similarly, it was reported that the maximum load and stress values decreased in the bones of rats upon exposure to EMFs [Citation7]. Decreases in maximum load and maximum stress values indicate a decrease in bone strength [Citation7]. Our results showed that RFR at 3.5 GHz caused decreased bone strength. It has been previously established that bone tissues constitute a potential avenue for absorbed environmental energy, including the RFR from mobile phones [Citation25].
The duration values decreased considerably after healthy rats were exposed to RFR. In addition, the duration values of the diabetic sham group (Group 3) were significantly lower than those of the healthy sham group (Group 1). This finding indicates that both 3.5 GHz RFR and diabetes cause decreased durability of bone tissue.
The yield point and displacement values were also lower in the diabetic sham group in comparison to the healthy sham group. Moreover, the values measured for healthy rats were considerably decreased after the administration of RFR. Bones with higher strength are more resistant to fractures and have better deformation abilities [Citation15]. Our findings indicate that both diabetes and RFR negatively affect bone strength.
In addition, displacement, yield point, maximum load, maximum stress and modulus of elasticity are important variables associated with the mineral components of bones and they provide meaningful information about bone biomechanics [Citation7]. Lower values of these variables indicate that the bone is poorly mineralized [Citation7, Citation24]. The results obtained in the present study showed that 3.5 GHz RFR reduced yield point, maximum load, maximum stress, and modulus of elasticity values in the bones of both healthy and diabetic rats, indicating a decrease in the mineralization of the bones. Thus, these results support our hypothesis that RFR reduces bone mineral density (BMD), subsequently leading to bone loss.
Among the radiological measurement results, a significant decrease was found in the lateral and medial cortex thicknesses of diabetic rats compared to healthy rats in both the RFR and sham groups. This finding is an important indicator of the bone damage caused by diabetes. However, the medullary widths of the rats in the healthy RFR group (Group 2) were found to be significantly greater than those of the other groups. This could be attributed to RFR negatively affecting bone development. It has been previously reported that RFR reduced the calcium concentrations in the bones of rats while leading to small increases in bone diameter and length [Citation4]. In a human study, a reduction in BMD was found in the iliac wings after RFR exposure [Citation26], while in a rat study, it was concluded that RFR (900 MHz and 1.04 mW/cm2 with a specific absorption rate (SAR) value of 0.008 W/kg) did not have a significant effect on BMD [Citation27]. Other researchers demonstrated that microwave radiation of 9.5 GHz administered after the establishment of femur fractures in rats did not have a positive effect on healing but did delay the endochondral ossification [Citation28]. In another study, 2100 MHz RFR applied after the establishment of mandibular fractures in rabbits increased the energy absorption capacity, toughness and maximum strength and had a positive effect on fracture healing based on histopathological evaluations, although no significant differences from the control group were noted in radiological evaluations [Citation2].
It was determined that EMFs (50 Hz, 0.8 mT) increased the intracellular calcium level in osteoblasts [Citation29]. Intracellular calcium is also involved in many physiological functions in bone tissue as a secondary messenger [Citation30]. However, RFR exposure below the safety margins was found to lead to significant changes in the probability of the binding of light ligands such as Ca2+. RFR exposure was also found to increase intracellular Ca2+ concentrations and induce apoptosis, and it has been confirmed that increased Ca2+ input depolarizes inner mitochondrial membranes as a result of increased levels of reactive oxygen species (ROS and heightened electron transport chain activities [Citation31]. Ca2+ ion regulation is a process of critical importance for bone health [Citation7]. On the other hand, variables of bone biomechanics are related to the properties of the bone materials, such as tissue composition and arrangement, matrix calcification and the characteristics of collagen fibers and lamellae [Citation32, Citation33]. The results of the present study, which showed inverse correlations in this regard, may be due to different reasons. Porosity is another factor affecting bone biomechanics. In various diseases, the level of porosity of the bone tissue may increase. Porosity is closely related to the strength and flexibility coefficients of bone, and it is generally accepted that a small increase in porosity causes large decreases in mechanical strength and intrinsic hardness [Citation15]. A further factor that determines the biomechanical properties of bone is bone mass. Structural behaviors of trabecular bone are mainly related to bone mass, microarchitecture (trabecular geometry, spatial distribution and interconnections), and material properties.
IMA occurs as a result of the modification of albumin by ROS formed as a result of ischemia. IMA may also result from high oxidative stress in different models of ischemia affecting various organs and it is used as an oxidative stress marker [Citation34, Citation35]. Various researchers have demonstrated the presence of increased IMA in skeletal muscles due to ischemia [Citation36–39]. In our study, exposure to 3.5 GHz RFR caused a significant increase in IMA values in both healthy and diabetic rats. This is an indicator of oxidative stress in the skeletal muscles. Exposure to 3.5 GHz RFR also produced significantly higher levels of MDA in the muscle tissues of healthy rats. Similarly, low-frequency EMFs (40 Hz and 7 mT for 0.5 h/day or 1 h/day for 14 days) were reported to cause statistically significant rises in the values of oxidative stress parameters as measured in the muscles of rats [Citation3]. It was reported that low-frequency EMFs (50 Hz and 0.1 mT or 1 mT for 30 min) induced the production of ROS in myoblasts and myotubes alongside a reduction of mitochondrial membrane potential in the C2C12 myoblastic cell line [Citation40]. In the same study, EMFs also induced the uptake of calcium, an important secondary messenger, into muscle cells and caused increases in the amounts of MDA [Citation40]. In the present study, 3.5 GHz RFR triggered increases in GSH levels in both healthy and diabetic rats and decreases in CAT levels in healthy rats. This may have been due to increases in the amounts of reactive oxygen species in these animals. There are many studies in the literature concluding that RFR causes decreased activity levels of the antioxidant defense system and increased amounts of free radicals [Citation12, Citation41].
The studies described above differ from the present study in terms of the types of animals used in the experiments, the characteristics of the RFR used, the doses and durations of exposure, and the applied methodologies. The effects on living organisms of RFR emissions from wireless communication technologies and mobile phones are fundamentally dependent upon the frequency, SAR and power density of the RFR [Citation42]. Therefore, differences will inevitably be seen among the results of different studies with different experimental designs. It is also reported in our previous study [Citation17] that 900, 1800 and 2100 MHz RFR may alter some characteristics of tibia bone and some skeletal muscle oxidative stress parameters. Therefore the results of our previous study are supported by the results obtained in this study.
Our results have finally showed that 3.5 GHz RFR may alter some charasteristics of bone and skeletal muscles of diabetic to a greater extent than in healthy rats. This may be originated from the density of minerals in bone tissues such as calcium and phosphorus, which are important in bone strength. It is also important to know how radiofrequency radiations affect the parameters such as collagen etc. in bones or muscles. Therefore, more detailed studies are needed.
Conclusions
This study showed that exposure to 3.5 GHz RFR can have negative effects on bone biomechanics in rats, leading to a reduction in the flexibility coefficient, maximum force, and an increase in maximum displacement. Diabetic rats may be more susceptible to the hazardous effects of RFR, with significant alterations observed in oxidative stress parameters. These observations suggest that exposure to 3.5 GHz RFR could potentially jeopardize bone quality and structural integrity, as well as affect muscle oxidative stress parameters in rats. More research is required to investigate the potential impacts of RFR on human health, especially for individuals with pre-existing medical conditions.
Authors’ contributions
CD analyzed the data. AN, SK and MBA collected the data. HB integrated and preprocessed the data. HB and SD wrote the paper. All authors read and approved the final manuscript.
Acknowledgments
We would like to thank Prof. Dr. Halit Demir (Department of Chemistry at Van Yuzuncu Yil University) for his support in the biochemical analyses, and Prof. Dr. Korkut Yegin for his valuable contributions in determining the specific absorption rate distribution.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential competing interest was reported by the authors.
Additional information
Funding
References
- Bektas H, Dasdag S, Bektas MS. Evaluation of 900 and 1800 mhz radiofrequency radiation emitted from mobile phones on pregnant women. J Int Dent Med Res. 2021;14(4):329–338.
- Durgun M, Dasdag S, Erbatur S, et al. Effect of 2100 MHz mobile phone radiation on healing of mandibular fractures: an experimental study in rabbits. Biotechnol Biotechnol Equip. 2016;30(1):1–10.
- Ciejka E, Skibska B, Kleniewska P, et al. Influence of low frequency magnetic field on chosen parameters of oxidative stress in rat’s muscles. Polski merkuriusz lekarski. Organ Polskiego Towarzystwa Lekarskiego. 2010;29(174):361–364.
- Sieroń-Stołtny K, Teister Ł, Cieślar G, et al. The influence of electromagnetic radiation generated by a mobile phone on the skeletal system of rats. Biomed Res Int. 2015;2015:1–11.
- Schwartz Z, Simon B, Duran M, et al. Pulsed electromagnetic fields enhance BMP‐2 dependent osteoblastic differentiation of human mesenchymal stem cells. J Orthop Res. 2008;26(9):1250–1255.
- Luther G, Wagner R, Zhu E, et al. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther. 2011;11(3):229–240.
- Gürgül S, Erdal N, Yılmaz ŞN, et al. Deterioration of bone quality by long-term magnetic field with extremely low frequency in rats. Bone. 2008;42(1):74–80.
- Bektas H, Algul S, Altindag F, et al. Effects of 3.5 GHz radiofrequency radiation on ghrelin, nesfatin-1, and irisin levels in diabetic and healthy brains. J Chem Neuroanat. 2022;126:102168.
- Dasgupta S, Wang G, Simonich MT, et al. Impacts of high dose 3.5 GHz cellphone radiofrequency on zebrafish embryonic development. PLoS One. 2020;15(7):e0235869.
- Wang Y, Jiang Z, Zhang L, et al. 3.5-GHz radiofrequency electromagnetic radiation promotes the development of Drosophila melanogaster. Environ Pollut. 2022;294:118646.
- Dasdag S, Akdag MZ. The link between radiofrequencies emitted from wireless technologies and oxidative stress. J Chem Neuroanat. 2016; Sep75(Pt B):85–93.
- Yakymenko I, Tsybulin O, Sidorik E, et al. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn Biol Med. 2016;35(2):186–202.
- Erkut A, Tumkaya L, Balik MS, et al. The effect of prenatal exposure to 1800 MHz electromagnetic field on calcineurin and bone development in rats. Acta Cir Bras. 2016;31(2):74–83.
- Nordin M, Frankel VH. Basic biomechanics of the musculoskeletal system. New York: Lippincott Williams & Wilkins; 2001.
- Cowin SC. Bone mechanics handbook. Boca Raton: CRC Press; 2001.
- Furman BL. Streptozotocin‐induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70(1):5.47.1–5.47.20.
- Bektas H, Nalbant A, Akdag MB, et al. Adverse effects of 900, 1800 and 2100 MHz radiofrequency radiation emitted from mobile phones on bone and skeletal muscle. Electromagnetic Biology and Medicine. 2023;42(1):1–9.
- Aebi H. Catalase in vitro. Meth. Enzymol. 1984;105:121–126.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888.
- Gutteridge JM. Biological origin of free radicals, and mechanisms of antioxidant protection. Chem Biol Interact. 1994;91(2-3):133–140.
- Bar–Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia—a preliminary report. J Emerg Med. 2000;19(4):311–315.
- Bektas H. The effects of mobile phones on diabetes and appetite. J Int Dent Med Res. 2022;15:441–447.
- An YH, Draughn RA, editors. Mechanical testing of bone and the bone-implant interface. Boca Raton: CRC Press; 1999.
- Burr D. The contribution of the organic matrix to bone’s material properties. Bone. 2002;31(1):8–11.
- Aslan A, Kırdemır V, Kocak A, et al. Influence of 1800 MHz GSM-like electromagnetic radiation exposure on fracture healing. Arch Med Res. 2014;45(2):125–131.
- Atay T, Aksoy BA, Aydogan NH, et al. Effect of electromagnetic field induced by radio frequency waves at 900 to 1800 MHz on bone mineral density of iliac bone wings. J Craniofac Surg. 2009;20(5):1556–1560.
- Aslan A, Aydoğan NH, Atay T, et al. The effects of electromagnetic field exposure at short and long term of 900 mhz frequency emitted from mobile phones on rat bone tissue. Dicle Medical Journal/Dicle Tip Dergisi. 2011;38(4):452–457.
- Sen B, Dasdag S, Celik S, et al. The effect of low densitiy 9450 MHz microwave irradiation on fracture healing. Proc. of. International Symposium of Millimeter Waves of Non-Thermal Intensity in Medicine, Moscow, Russia. 1991. 1–2.
- Zhang X, Liu X, Pan L, et al. Magnetic fields at extremely low-frequency (50 hz, 0.8 mT) can induce the uptake of intracellular calcium levels in osteoblasts. Biochem Biophys Res Commun. 2010;396(3):662–666.
- Zhou J, Ming L-G, Ge B-F, et al. Effects of 50 hz sinusoidal electromagnetic fields of different intensities on proliferation, differentiation and mineralization potentials of rat osteoblasts. Bone. 2011;49(4):753–761.
- Carrasco C, Rodriguez AB, Pariente JA. Melatonin as a stabilizer of mitochondrial function: role in diseases and aging. Turk J Biol. 2015;39(6):822–831.
- Ferretti J, Cointry G, Capozza R, et al. Analysis of biomechanical effects on bone and on the muscle-bone interactions in small animal models. J Musculoskelet Neuronal Interact. 2001;1(3):263–274.
- Comelekoglu U, Yalin S, Bagis S, et al. Low-exposure cadmium is more toxic on osteoporotic rat femoral bone: mechanical, biochemical, and histopathological evaluation. Ecotoxicol Environ Saf. 2007;66(2):267–271.
- Sbarouni E, Georgiadou P, Voudris V. Ischemia modified albumin changes–review and clinical implications. Clin Chem Lab Med. 2011;49(2):177–184.
- Özsürekci C, Şengül Ayçiçek G, Çalışkan H, et al. Thiol–disulfide homeostasis and ischemia‐modified albumin as a marker of oxidative stress in patients with sarcopenia. Geriatr Gerontol Int. 2021;21(7):584–589.
- Apple FS, Quist HE, Otto AP, et al. Release characteristics of cardiac biomarkers and ischemia-modified albumin as measured by the albumin cobalt-binding test after a marathon race. Clin Chem. 2002;48(7):1097–1100.
- Sinha MK, Gaze DC, Tippins JR, et al. Ischemia modified albumin is a sensitive marker of myocardial ischemia after percutaneous coronary intervention. Circulation. 2003;107(19):2403–2405.
- Zapico-Muñiz E, Santaló-Bel M, Mercé-Muntañola J, et al. Ischemia-modified albumin during skeletal muscle ischemia. Clin Chem. 2004;50(6):1063–1065.
- Piwowar A, Knapik-Kordecka M, Warwas M. Ischemia-modified albumin level in type 2 diabetes mellitus–preliminary report. Dis Markers. 2008;24(6):311–317.
- Morabito C, Rovetta F, Bizzarri M, et al. Modulation of redox status and calcium handling by extremely low frequency electromagnetic fields in C2C12 muscle cells: a real-time, single-cell approach. Free Radic Biol Med. 2010;48(4):579–589.
- Akdag MZ, Dasdag S, Canturk F, et al. Does prolonged radiofrequency radiation emitted from Wi-Fi devices induce DNA damage in various tissues of rats? J Chem Neuroanat. 2016;75(Pt B):116–122.
- Ahlbom A, Bridges J, De Seze R, et al. Possible effects of electromagnetic fields (EMF) on human health–opinion of the scientific committee on emerging and newly identified health risks (SCENIHR). Toxicology. 2008;246(2-3):248–250.