Abstract
Intracranial aneurysms with poor topography are still difficult and dangerous to be catheterized during endovascular procedures. Here we report a novel coil guiding catheterization technique which could be safe and helpful. Seven aneurysms of 5 cases were embolized with the novel coil guiding catheterization technique in Zhongshan Hospital, Fudan University, from January 2017 to March 2019. All the aneurysms were with poor topography for endovascular embolization. One of the aneurysms was in the posterior communicating segment of internal carotid artery (ICA), three in the ophthalmic segment of ICA, and one in the posterior cerebral artery. Two of the cases had multiple aneurysms. All the aneurysms were catheterized by coil guiding technique and embolized. All the aneurysms were catheterized successfully and safely. The aneurysms embolization results were as follows: Raymond Class 1 occlusion was achieved in 4 aneurysms, Class 2 occlusion in 1, Class 3 occlusion in 2 immediately after the procedure. Follow-up imaging demonstrated all the aneurysms achieved total occlusion. There was no aneurysm re-rupture or patient death. The results indicated that the technique has good safety and effectiveness, with a high rate of technical success and a low rate of periprocedural complications in the reported cases. Thus, appling coil guiding aneurysm catheterization could be considered as a safe and effective method, especially for aneurysms with poor topography.
Introduction
Endovascular treatment for cerebral aneurysms has emerged as an important approach to treat patients with ruptured and unruptured intracranial aneurysms due to the development of endovascular techniques and invention of new devices [Citation1–3]. However, aneurysm embolization is not always safe. Embolization remains technically challenging for some aneurysms [Citation4, Citation5], especially for those with poor interventional topography, such as very tortuous access [Citation6], the artery harboring the aneurysm being very tortuous, unfavorable aneurysm-to-parent-vessel angle, and extreme irregular configuration of the aneurysm sac. Catheterization of these aneurysms’ sac can be very challenging and dangerous. The accumulated tension makes it very difficult to control the microcatheter tip. That would be a potential risk for intra-procedural rupture (IPR) of aneurysm [Citation7–9]. IPR is a rare but catastrophic complication with a high rate of mortality and poor outcomes.
Here we report our experience of applying an innovative embolization technique with coil guidance catheterization, which achieved good outcomes in a series of aneurysms with poor interventional topography.
Subjects and methods
Ethics statement
All procedures performed in this study were in accordance with the ethical standards of ethics committee of Zhongshan Hospital, Fudan University, with the national legislation in China [Citation10], and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (No.B2021-819/16-12-2021). Written informed consent was obtained from all the 5 patients.
Consent for publication
Written informed consent was obtained from all the 5 patients.
Patients
Among all 215 aneurysm embolization cases in Zhongshan Hospital, Fudan University, Shanghai, China between January 2017 and March 2019, seven aneurysms in 5 patients (2 males and 3 females) were treated with the novel coil guiding microcatheter catheterization technique. Two of these cases presented with aneurysms rupture and the Hunt-Hess grade was 2. The demographic characteristics are shown in .
Table 1. Demographic and clinical characteristics, Lesion characteristics and treatment results of the cases.
Aneurysm and access for interventional procedure
All the aneurysms were with hightly tortuous Type III access according to the classification of interventional access for intracranial stenosis treatment [Citation11] (shown in ). Only one of the aneurysms was narrow necked. Two of the cases had multiple aneurysms. In one case, the aneurysm involved the total P1 segment of right PCA and was too irregular to measure its neck (Illustrative case 2, demonstrated in ).
Figure 1. Embolization of irregular posterior cerebral artery aneurysms. (A) very tortuous supra-arch vessels. (B) left VA angiogram in working projection revealed highly irregular multi-lobulated left PCA aneurysm. (C) 3D reconstruction demonstrated the complex topography of the aneurysm. There were multiple aneurysms with different diameters located from the origin of the left PCA to the conjunction of P1 and P2. (D) the microcatheter tip could not be advanced further beyond the origin of the largest aneurysm neck (the black arrow) over the microwire because of the extremely high tension at the acute turning point of P1(the white arrow). We kept the microcatheter tip at the aneurysm neck and inserted the coil. Some of the loops entered the distal sac of the aneurysm. (E) with the coil being held stable, the microcatheter was advanced until its tip got into the distal sac of the aneurysm. (F) and (G) the aneurysms were completely occluded with the embolization. (H) Follow-up 3D reconstruction after seven months demonstrated ongoing occlusion of the aneurysm.
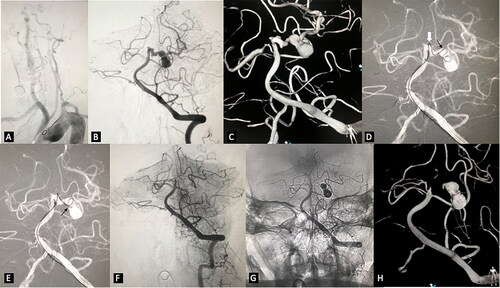
Endovascular treatment
All procedures were performed with general anesthesia. Aneurysm configuration and arterial architecture were evaluated using a Philips FD 20 system (Philips Medical System, Best, the Netherlands). After systemic heparization, a 6 F long sheath (Cook Medical Inc, Bloomington, IN, USA) and 6 F or 5 F intermediate catheter (Navien, Medtronic; Irvine. CA, USA) was placed in the distal internal carotid artery (ICA) or basilar artery to obtain a stable support. The aneurism morphology and topography were studied via a regular whole cerebral angiography including rotational angiography followed by 3D reconstruction (Philips Allura Xper FD20, 3D-RA 6.1.2). The size of aneurysm and parent artery was measured by the operator using the above-mentioned instrument and software. A microcatheter (Headway 17, MicroVention; Tustin, CA, USA) was navigated over a microwire (synchrone 2, Stryke; Salt Lake City, UT, USA) to approach the aneurysm. The microwire was difficult to maneuver due to tortuous access. There was a concern over microcatheter ‘jump’ which may cause aneurysm rupture. The microcatheter tip was placed just close to the entrance of the aneurysm, from where deployment of the first framing coil was started. After deploying several coil loops, one or two loops of the coil would enter the aneurysm sac. The coil was then held stable and the microcatheter was gently advanced into the aneurysm along the coil loop. Once the tip of the microcatheter reached a satisfying position in the aneurysm, the framing coil out of the aneurysm sac was withdrawn and the aneurysm was recoiled with regular framing and packing techniques. If stent assistance was needed, the stenting microcatheter was navigated crossing the aneurysm neck to the distal vessel segment after the aneurysm was primarily protected by several loops of the framing coil. Subsequently, standard stent assisted coiling (SAC) techniques were performed.
Anticoagulation and antiplatelet management
Patients received dual antiplatelet therapy if SAC was planned before the procedure. If stenting was decided during the procedure, intravenous tirofiban infusion was initiated before stent deployment and continued for 24 h postoperatively while being bridged to dual antiplatelet therapy.
Results
The technique described here was used in 3.3% (7/215) of aneurysms during the study period in our center. All aneurysms were catheterized and coiled with 100% technical success. No significant catheterizing difficulty or catheter jumping was encountered when the microcatheter was advanced along the coil. All patients awoke from anesthesia without neurologic deficit. The angiography post coiling demonstrated the initial embolization results as follows: Raymond Class 1 in 4 aneurysms, Class 2 in 1 and Class 3 in 2. All the 5 patients underwent follow-up DSA at intervals ranging from 6 to 9 months. According to the DSA follow-up, all the aneurysms had radiological cure ().
Illustrative case 1
A 74-year-old female had a sudden onset of headache without loss of consciousness and with right oculomotor paralysis. On arrival in the Emergency Department, she exhibited photophobia and nuchal rigidity. Emergency CT visualized a diffuse spontaneous subarachnoid hemorrhage (SAH) and no signs of hydrocephalus. Angiography revealed a posterior communicating artery aneurysm. The aneurysm was embolized with the novel coil guiding catheterization technique (). The procedure was uneventful and the patient had a good clinical outcome, with the oculomotor paralysis recovering during follow-up.
Figure 2. Right posterior communicating aneurysm was embolized with the novel coil guiding catheterization technique. (A) working projection revealed an irregular shaped aneurysm located in the posterior communicating artery segment. (B) 3D reconstruction demonstrated very tortuous vessel access. (C) the microcatheter tip was advanced near the aneurysm neck (small black arrow). The coil was inserted. Coil loops were protruded to the distal ICA and A1, some were in the aneurysm sac. (D) the microcatheter was advanced while the coil was slightly retrieved until the microcatheter tip was totally in the aneurysm sac. (E) the coil outside the aneurysm sac was retrieved before the true framing process started. (F) final control after the coiling demonstrated no contrast filling of the aneurysm sac.
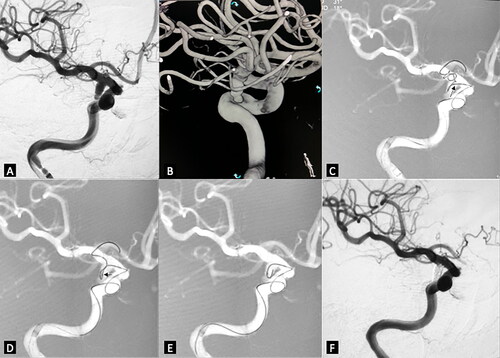
Illustrative case 2
A 63-year-old man was referred to our center after sustaining a severe headache for one day. The emergency CT scan revealed subarachnoid hemorrhage. The patient underwent angiography and revealed an irregular multi-lobulated aneurysm of the P1 segment of the left posterior cerebral artery. The interventional embolization was performed and the novel coil guiding catheterization technique was used (). The procedure was successful and the patient showed no neurologic deficits when awaking. The examination to follow up on the patient after 7 months showed stable condition, without de novo aneurysm formation.
Discussion
Although interventional embolization for intracranial aneurysm has been the most popular treatment modality and has been recommended by different guidelines [Citation4, Citation6], there are still challenges and potential risks in this treatment. Life-threatening complications including IPR may potentially occur [Citation7, Citation9, Citation12]. Several risk factors are significantly associated with IPR, such as small-sized aneurysm (<3.5 mm), presence of bleb and poor interventional topography [Citation8]. One of the steps with highest risk of aneurysm rupture is the procedure when a microcatheter enters the aneurysm. This procedure may cause aneurysm rupture, which results in severe hemorrhage and adverse prognosis [Citation12–14]. Kawabata et al. [Citation12] reported that the rate of good clinical outcome following IPR induced by a coil, microwire, and microcatheter was 90%, 100%, and 57%, respectively.
There are several techniques to avoid IPR during catheterization, such as establishing stable access support, sound shaping of the microcatheter tip, effort to advance the microcatheter just across the aneurysm neck and then withdraw it to help the tip enter the aneurysm without tension [Citation15, Citation16]. However, if the vessel access is too tortuous and the operation lacks a high-quality intermediate catheter, the microwire and microcatheter would be very difficult to manipulate. The microwire and microcatheter have the tendency to jump forward because of accumulated tension and torsion. Some of the access problems may be addressed by using distal access or intermediate catheters, such as Navien (Medtronic; Irvine, CA, USA), Sofia (MicroVention Terumo, Tustin, California, USA), etc. However, these devices are not always available in a low-resource environment, and the intermediate catheter does not solve all problems. Also, in the circumstance of very poor topography, the tension of the microwire and microcatheter would induce vascular displacement. This would increase the risk of perforators torn.
Although the novel coil guiding aneurysm catheterization technique was used only in 3.3% of aneurysms’ embolization in this case series, it showed safety and effectiveness in treating aneurysms with poor interventional topography. In such situations, it is more difficult to control the microcatheter and microwire. Here our primary experience demonstrated that the coil guiding technique achieved a high technical success rate (100%) with no complications (perioperative stroke incidence, 0%).
Coils were specifically designed for aneurysm embolization [Citation17, Citation18]. A coil has four degrees of spatial structure and is designed to be very soft to avoid tearing the aneurysm sac. The coils also have a stretch-resistant design and could maintain their shape. Although it could not be torqued, the shaft of the coil also has the property of supporting and guidance just as a microwire. To fine-tune the microcatheter tip position with the assistance of a coil loop is a regular technique during aneurysm embolization at present. In addition, the coil shaft is softer than a microwire. When the operator advances the microcatheter with coil guiding and supporting, small vessels will be less likely to be retracted. Retraction would increase the risk of tear of such vessels, as well as their perforators (as in illustrative case 2). For these reasons, this coil guiding technique has its advantage in such instance as the aneurysms with poor interventional topography.
Based on our experience, we found the following key points should be kept in mind when performing this coil guiding technique. First, when the patient has very tortuous vessel access, a stable supporting system will be critical. The operator should choose a long sheath and appropriate intermediate catheter to provide enough proximal support. The microcatheter tip should be accurately shaped according to the morphology of the aneurysm neck and the course of its proximal parent artery. The support and guiding force only relying on the coil is too weak. Secondly, when having the coil to guide the microcatheter, the operator needs to gently balance the force of pulling the coil and pushing the microcatheter. After one or more loops enter the aneurysm, the microcatheter should be advanced. The coil should stay still throughout the process, until the microcatheter tip advances into the aneurysm’s sac gradually. Then the first one or two loops should be retrieved into the microcatheter to get ready for the true framing in the aneurysm. Thirdly, if there is too much tension while advancing the microcatheter, the operator should check the position of the guiding catheter and adjust it to increase the support. Maintaining the continuous flushing of the system also would be helpful. As qualified neurointerventionists know, the operator should not force to advance the microcatheter or pull the coil, as that would make the coil stretched or detached prematurely [Citation19, Citation20]. Regarding the choice of the first guiding coil, we recommend a three-dimensional bare coil. It could change its direction during advancement, which will reduce the risk of potential destruction of surface modification [Citation21, Citation22].
However, some limitations should be noted. The number of participants in our study is small resulting in a lower level of evidence for the application of the technique. This study is a retrospective case series, lacking clinical trials’ data. We will further improve relevant shortcomings in subsequent research.
Conclusions
Here we reported our successful experience in performing intracranial aneurysms catheterization with coil guiding technique. The obtained results were promising and indicate that the technique has good safety and effectiveness, with a high rate of technical success and a low rate of periprocedural complications in the reported cases. The applied coil guiding aneurysm catheterization could be considered safe and effective, especially for those aneurysms with poor interventional topography and in a low-resource environment.
Availability of data and material
Anonymized data that support the findings reported in this study are available from the corresponding author on reasonable request.
Author’s contribution
ZY guarantees the integrity of the entire study, conceptualized and designed the study and reviewed the manuscript; ZJ contributed to manuscript editing; HY contributed to the clinical studies; ZJ and ZY contributed to the manuscript preparation; HY contributed to experimental studies and data acquisition; P Z and JH contributed to literature research; PZ and Z Y contributed to the data analysis. All authors have read and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Luther E, McCarthy DJ, Brunet MC, et al. Treatment and diagnosis of cerebral aneurysms in the post-International subarachnoid aneurysm trial (ISAT) era: trends and outcomes. J Neurointerv Surg. 2020;12(7):1–6.
- Thompson BG, Brown RD, Jr., Amin-Hanjani S, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention; American Heart Association, et al. American Stroke Association. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(8):2368–2400.
- Liu J, Zhang Y, Wang Y, et al. Stenting after coiling using a single microcatheter for treatment of ruptured intracranial fusiform aneurysms with parent arteries less than 1.5 mm in diameter. World Neurosurg. 2017;99:809.e7–809.e10. Epub 2017 Jan 10.
- Etminan N, Dorfler A, Steinmetz H. Unruptured intracranial aneurysms- pathogenesis and individualized management. Dtsch Arztebl Int. 2020;117(14):235–242.
- Park YK, Yi HJ, Choi KS, et al. Intraprocedural rupture during endovascular treatment of intracranial aneurysm: clinical results and literature review. World Neurosurg. 2018;114:e605–e615.
- Fukuda H, Handa A, Koyanagi M, et al. Endovascular therapy for ruptured cerebral aneurysms in the elderly: poor accessibility of the guiding catheter and use of local anesthesia as the predictors of Procedure-Related rupture. Neurosurgery. 2015;77(4):544–552; discussion 552.
- Shang W, Chang X, Wang X, et al. Risk factors for intraprocedural rupture during emergency endovascular treatment of ruptured anterior communicating artery aneurysms. Interv Neuroradiol. 2022;28(4):426–432.
- Singh DK, Pathak V, Yadav K. Risk factor assessment and outcomes of intra procedural rupture of intracranial aneurysm during endovascular treatment: a race against time. Turk Neurosurg. 2022;32(1):52–57.
- Wang JM, Chen QX. Risk factors for intraprocedural rerupture during embolization of ruptured intracranial aneurysms. J Korean Med Sci. 2020;35(48):e430.
- http://www.gov.cn/gongbao/content/2017/content_5227817.htm.
- Jiang WJ, Wang YJ, Du B, et al. Stenting of symptomatic M1 stenosis of Middle cerebral artery: an initial experience of 40 patients. Stroke. 2004;35(6):1375–1380. Epub 2004 May 6. PMID: 15131312.
- Kawabata S, Imamura H, Adachi H, et al. Risk factors for and outcomes of intraprocedural rupture during endovascular treatment of unruptured intracranial aneurysms. J Neurointerv Surg. 2018;10(4):362–366.
- McDougall CG, Halbach VV, Dowd CF, et al. Causes and management of aneurysmal hemorrhage occurring during embolization with guglielmi detachable coils. J Neurosurg. 1998;89(1):87–92.
- Santillan A, Gobin YP, Greenberg ED, et al. Intraprocedural aneurysmal rupture during coil embolization of brain aneurysms: role of balloon-assisted coiling. AJNR Am J Neuroradiol. 2012;33(10):2017–2021.
- Carvi y Nievas M, Haas E, Höllerhage HG. Severe intracranial bleedings during endovascular procedures: outcome of surgically treated patients. Neurol Res. 2007;29(1):81–90.
- Kim KH, Cha KC, Kim JS, et al. Endovascular coiling of Middle cerebral artery aneurysms as an alternative to surgical clipping. J Clin Neurosci. 2013;20(4):520–522. Epub 2013 Jan 31.
- Matsubara N, Miyachi S, Nagano Y, et al. Evaluation of the characteristics of various types of coils for the embolization of intracranial aneurysms with an optical pressure sensor system. Neuroradiology. 2011;53(3):169–175. Epub 2010 Jun 3.
- Serafin Z, Di Leo G, Pałys A, et al. Follow-up of cerebral aneurysm embolization with hydrogel embolic system: systematic review and meta-analysis. Eur J Radiol. 2015;84(10):1954–1963. Epub 2015 Jun 29.
- https://www.bostonscientific.com/en-US/products/embolization/vortx-vascular-occlusion-coils/vortx-prescriptive-information.html.
- Matsumoto Y, Kanoke A, Omodaka S, et al. How to avoid intraoperative rupture and what to do when it ruptures. No Shinkei Geka. 2021;49(1):128–134.
- Reinges MH, Krings T, Drexler AY, et al. Bare, bio-active and hydrogel-coated coils for endovascular treatment of experimentally induced aneurysms. Long-term histological and scanning electron microscopy results. Interv Neuroradiol. 2010;16(2):139–150. Epub 2010 Jul 19.
- Abrahams JM, Diamond SL, Hurst RW, et al. Topic review: surface modifications enhancing biological activity of guglielmi detachable coils in treating intracranial aneurysms. Surg Neurol. 2000;54(1)(discussion 40-1):34–40.
