Abstract
Using supplements in different sports is a common practice for many athletes. Unfortunately, the growth of this market has entailed some speculations, such as the addition of excessive doses of potentially toxic ingredients. Sometimes the doses listed on the package do not correspond to the real content. Some nutritional supplements on sale may contain undeclared ingredients. Some of those supplements are prohibited substances according to different regulations. Herein, a simple HPLC/DAD procedure that is easy to apply in conventional laboratory practice was developed for the simultaneous determination of 13 substances with steroid structure in nutritional supplements for sport: testosterone, testosterone propionate, testosterone enanthate, methyltestosterone, nandrolone, nandrolone propionate, nandrolone decanoate, methandienone, androstenedione, trenbolone, trenbolone acetate, trenbolone enanthate and boldenone undecylenate. The methodology includes gradient elution with mobile phase A: MeOH:ddH2O (55:45) and mobile phase B: 100% MeOH in a standard HPLC system containing a Halo 90 Å, C18 (150 x 4.6 mm, 2.7 µm) column, flow rate 0.6 mL/min, UV wavelength of 254 nm, temperature of 40 °C and 20 μL injection volume. The developed methodology was validated according to the corresponding official documents. The key parameters used for the selection of the optimal HPLC conditions were the ability of the mobile phase and solvents to be used with both an HPLC/MS and a GC/MS chromatographic system. The obtained total run time, the reproducibility of the retention times, the separation of all peaks and peak characteristics meet all requirements.
Introduction
Chromatography is a main technique used for qualitative and quantitative control of many biotechnological and chemical products and processes. It is part of the control of raw materials and final products in the food, pharmaceutical, diagnostic industry, etc. [Citation1, Citation2]. It also finds application in the control of food supplements and prohibited substances in doping regulation in sports.
The use of some supplements in the field of professional sports is a common practice. In many cases, these supplements are not very effective, and some of them could be harmful to health especially if administered in high doses for a long period. In some supplements, there are excessive doses of potentially toxic ingredients or amounts that do not correspond to those listed on the package. There is growing evidence that some nutritional supplements in the market have ingredients that are not listed on the label [Citation3]. Some of those supplements are prohibited substances according to the doping regulations of the International Olympic Committee and the World Anti-Doping Agency. The identified contaminants include various anabolic androgenic steroids such as testosterone and nandrolone and/or their prohormones, ephedrine and caffeine. In most cases, such contamination is a result of poor manufacturing practice, but there is also evidence of intentional contamination [Citation3, Citation4].
Synthetic derivatives of testosterone, aka. androgenic anabolic steroids (AAS), increase both lean body mass and muscle strength. As a potential approach to enhance speed and strength for athletes and to satisfy the need of muscle transformation, some dietary supplements get adulterated with illegal AAS. Thus, testosterone and its derivatives can cause immediate or long-term undesired side effects, such as androgenetic alopecia, acne, hypertension, hormonal dysfunction, especially hypogonadism and dyslipidemia as well as metabolic disturbances and cardiotoxicity [Citation4, Citation5]. Oral administration of higher doses of AAS could also cause hepatotoxicity, and injection of some steroids can cause nephrotoxicity [Citation6–8].
Contaminated dietary supplements pose risks of undesired positive doping results and serious health problems in professional sport. To detect a wide range of AAS contaminants and/or adulterants in dietary supplements, there is a need for rapid and reliable screening methods [Citation9]. The screening for different doping substances intensively utilizes gas chromatography coupled with mass spectrometry (GC/MS). Thus, a large body of spectral data has been deposited in standard reference databases. However, liquid chromatography coupled to mass spectrometry (HPLC/MS) has gained popularity for the determination of AAS in recent years [Citation10], but it cannot use the existing GC/MS libraries [Citation11]. Most of the literature sources describe the application of GC/MS with derivatization and HPLC/MS/MS for the determination of AAS in dietary supplements [Citation10, Citation12–14].
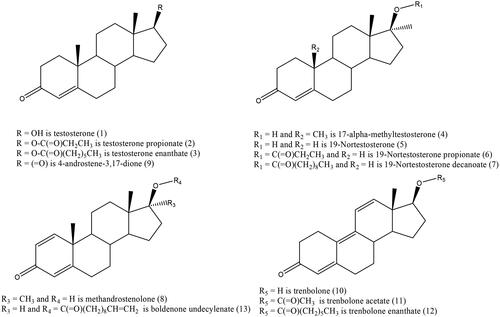
In this context, the aim of the present work was to develop and validate an HPLC-DAD procedure that is easy to use with standard laboratory equipment for the quantitative simultaneous determination of the 13 substances with a steroid structure most commonly found as contamination in nutritional supplements. The target structures include testosterone, testosterone propionate, testosterone enanthate, methyltestosterone, nandrolone, nandrolone propionate, nandrolone decanoate, methanedienone, androstendione, trenbolone, trenbolone acetate, trenbolone enanthate and boldenone undecylenate ().
Materials and methods
Chemical reagents and standards
Methanol (Lot no. K1700S) was purchased from Honeywell; Water (Lot no. 1850302)—HPLC Gradient Grade from Fisher; Testosterone (Batch no. BCBS8823V)—Sigma Aldrich; Testosterone enanthate (Batch no. 2) CRS, EDQM; Testosterone propionate (Lot no. G711859), 17-alpha-Methyltestosterone (Lot no. G149565), 19-Nortestosterone (Lot no. G833394), 19-Nortestosterone-17-propionate (Lot no. G1023048) 19-Nortestosterone-17-decanoate (Lot no. 834119), Methandrostenolon (Lot no. 1063323), 4-Androstene-3,17-dione (Lot no. G984414), Trenbolone (Lot no. 1066343), Trenbolone-acetate (Lot no. 1037748), Trenbolone enanthate (Lot no. 1092869), and Boldenone undecylenate (Lot no. 1060038) were from Dr. Ehrenstorfer. All reagents and standards are used without any additional treatment.
Standard stock solutions of all analytes were prepared by weighing amounts of standard substances according to and brought to the volume presented in the table with methanol.
Table 1. Preparation of standard stock solutions in methanol.
HPLC system
Shimadzu HPLC20AD 101-GT-INS with Halo 90 Å, C18/150 × 4.6 mm, 2.7 µm (Lot: AH182207) column was used. The developed HPLC system also included gradient elution with mobile phase A: MeOH:ddH2O (55:45) and mobile phase B: 100% MeOH presented in .
Table 2. Elution conditions.
All compounds were monitored at λ = 254 nm, column temperature was 40 °C, elution rate 0.6 mL/min, and the injection volume was fixed at 20 μL.
Determination of repeatability, precision and recovery
The samples were prepared as follows: about 2.5 g of placebo (crushed tablet mass of a food supplement) was weighed in a measuring flask, then it was spiked with a certain amount of standard of each substance, and a solvent (methanol) was added. The sample was treated by ultrasound for 15 min in the Unitra Unima OLSZTYN UM-4 ultrasonic bath (Poland) and topped up to 25 mL. Furthermore, the resulting solution was injected into the chromatographic system.
The placebo was a food supplement containing the following active ingredients: extract of the herb Tribulus terrestris, ecdysterone and diosgenin and the auxiliary substances magnesium stearate, corn starch and povidone. The capsule contents were previously removed from the capsules and homogenized in a mortar. A blank placebo solution was prepared and injected.
Results
Standard stock solutions of all analytes in methanol were prepared with quantities of compounds and volumes of methanol described in . Furthermore, the target compounds were identified in the final developed chromatographic system (, ). It consisted of a Halo 90 Å, C18 (150 × 4.6 mm, 2.7 µm) column, flow rate of 0.6 mL/min, UV wavelength of 254 nm, temperature of 40 °C, 20 μL injection volume and gradient of the mobile phase A: MeOH:ddH2O (55:45) and mobile phase B: 100% MeOH ().
Figure 2. Chromatogram of a mix of targeted compounds at λ = 254 nm, where 13.229 is trenbolone, 13.784 is nandrolone, 14.485 is androstenedione, 16.426 is methanedienone, 19.613 is testosterone, 25.020 is methyltestosterone, 26.073 is trenbolone acetate, 34.209 is nandrolone propionate, 36.748 is testosterone propionate, 46.508 is trenbolone enanthate, 49.498 is testosterone enanthate, 52.780 is nandrolone decanoate and 54.477 is boldenone undecylenate.
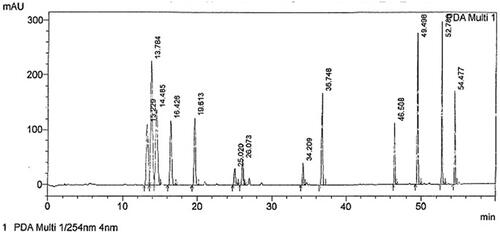
Table 3. Coefficient of determination R2 and RSD.
Data on the selectivity and efficiency of the chromatographic system are presented in .
To determine the repeatability, a standard solution with a concentration of 0.1 mg/mL was injected 10 times and the RSD was determined for each of the compounds ().
To determine the linearity for all targeted compounds, solutions at six concentrations (10%, 20%, 50%, 80%, 100% and 120%) of targeted concentration 0.1 mg/mL were prepared for all analytes. Every solution was injected in triplicate in the chromatographic system and the coefficient of determination R2 was calculated for all compounds ().
The obtained intervals of linearity for the targeted compounds are summarized in .
Table 4. Linearity range for the target compounds.
Discussion
AAS is a large and rapidly growing group of synthetic androgens used both clinically and illicitly. Protein supplements are the most useful additives for improving the performance of athletes.
The new synthetic AAS named ‘designer steroids’ are created to avoid anti-doping tests in professional sports, but also for consumption by non-professional bodybuilders. These new compounds could cause health risks due to the lack of toxicity and safety testing. Distributors of such new AAS avoid control and regulation by offering them on the market as nutritional or dietary supplements [Citation15]. Thus, the efforts to achieve maximal anabolic effects and minimal androgenic ones have focussed on a few structural modifications of testosterone [Citation16]. The current classification of AAS distinguishes three main classes depending on the substitution of the parent molecule. Class I have C-17 esterification. Class II has a demethylated group at C-19 and may also have C-17 esters. Class III has alkylation at C-17 [Citation16].
The development of new more efficient and sensitive methods for the analysis of designer AAS will help the control authorities to stay abreast of the modern trends in this business [Citation15, Citation17]. A recent technique that has been proposed for this purpose is High Field Asymmetric Waveform Ion Mobility Spectrometry (FAIMS) [Citation15]. It works at atmospheric pressure separating gas-phase ions due to their behaviour in strong and weak electric fields [Citation18]. FAIMS is easy to combine with electrospray ionization. Thus, it is implemented as an additional separation mode in LC/MS, most often in proteomic analysis. As Swearingen and Moritz [Citation18] described in their review of the FAIMS technique, the separation is orthogonal to both LC and MS. It can improve the detection in complex samples via online fractionation of ions. In addition, this technique filters out chemical noise and thus improves the dynamic range and concomitantly the detection limits of targeted ions. Moreover, FAIMS can remove interfering ions and can select target compounds’ charge states, which is optimal for identification by tandem MS. The main advantage of the technique is also that when it is coupled with MS, FAIMS improves the separation of analytes from other interfering compounds with little or no increase in analysis time [Citation15, Citation18].
Some investigations have reported that protein supplements may contain undeclared AAS [Citation19–21]. Unfortunately, their large distribution could be dangerous due to the possible side effects. To minimize the possibility of adverse health effects for athletes, adequate screening methods to detect a wide range of steroids are essential [Citation22, Citation23]. The developed GC/MS techniques work well, but their main disadvantage is that the samples need pre-treatment. The pre-treatment is often related to hydrolysis and derivatization reactions. On the other hand, the HPLC/MS technique is successfully applied to determine AAS in various matrices and also to study the mechanism of their metabolism [Citation17, Citation24–26]. For example, in 2020, Khandhra et al. reported the application of HPLC/DAD and HPLC/MS for the determination of similar prohibited substances: synephrine, caffeine, clenbuterol, nandrolon, testosterone and methylhexaneamine in nutritional supplements [Citation10]. A QuEChERS method for screening and simultaneous quantitative analysis of 28 AAS in protein supplements using HPLC-MS/MS that also meet the analytical requirement is described by Lee et al. [Citation23]. The validated method was applied to 198 protein supplements from online and offline markets via direct purchase from abroad between 2019 and 2020. The data from the scientific literature from the last 10 years reveal HPLC/MS technique as the most powerful technique for the detection of prohibited substances in real matrices. However, this technique is quite expensive and not easily accessible in many analytical laboratories with standard equipment in developing countries. Moreover, good separation and adequate molecule ionization are essential in HPLC/MS systems and depend on the careful selection of the mobile phase and the gradient conditions. The sample preparation procedure is also critical for the success of further separation and LOD and LOQ determination. On the other hand, the HPLC/UV method is cheaper and more widely applicable in laboratory practice than HPLC/MS. In our work, we aimed to simplify the analysis using a single wavelength for the detection of the target compounds and to create an HPLC-DAD protocol that is easy to use in a conventional analytical laboratory.
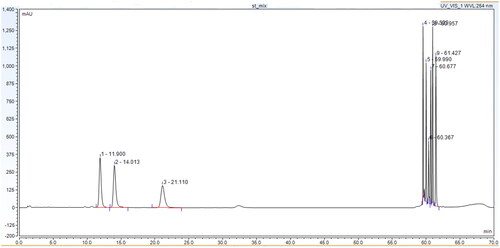
Herein, the experimental approach that we used for the development of a new procedure for the simultaneous determination of 13 substances with an AAS structure was developed by analogy to our previous report for the development of an HPLC/DAD technique for the simultaneous determination of six prohibited substances in model matrices [Citation27]. Thus, in this study, initially a conventional C18 Agient Zorbax Eclipse column with parameters 150 × 4.6 mm x 5 µm and an isocratic method with methanol: water (55: 45) mobile phase was tested. From the obtained results (), the separation of AAS base forms was poor, the peak shape for all substances and the retention time were not acceptable.
Figure 3. Chromatogram of a standard solution—a mixture of all 13 target compounds obtained by optimizing the chromatographic conditions.
The process of optimization of the conditions of the chromatographic system started by varying the stationary phase. The conventional C18 Agient Zorbax Eclipse column was replaced by a Halo 90 Å, C18 (150 × 4.6 mm, 2.7 µm) column, which has the same inner diameter, but with a smaller particle size, resulting in better efficiency. At the same time, the isocratic mode of elution was changed with a gradient one, with the aim of reducing the total analysis time and achieving better separation. Thus, good peak separation and excellent peak properties for all compounds were obtained. In addition, different methanol-to-water ratios in the mobile phase and gradients were tested for better peak characteristics and shorter run times. During the experiments with gradient conditions, the quantity of methanol varied between 45% and 70% in the mobile phase. Finally, it was found that, as the amount of methanol increased up to 45%, the time was shorter and reached 15 min but the separation became worse.
Therefore, the gradient method was optimized to obtain a good separation, but the total chromatography time remained quite long −60 min. A rapid increase in the concentration of methanol resulted in a good separation between the peaks of the base forms, while the esters remained poorly separated. Thus, as a result of a series of experiments, the best gradient was achieved, as described in . The optimum flow rate was 0.6 mL/min, because smaller particle size results in higher pressure operation, and our goal was to develop a method for a standard chromatographic system rather than a very high pressure (HPLC) system. A UV wavelength of 254 nm was chosen because all target analytes have characteristic absorption at this wavelength and can be detected with good sensitivity. The optimal temperature was 40 °C at 20 μL injection volume. As previously highlighted [Citation24], in this case too, the specific features used to select the optimal HPLC conditions are the ability of the mobile phase and solvents to be used with the HPLC/MS and GC/MS chromatographic system, the total run time, the reproducibility of the retention times, the separation of all peaks and peak shape, resolution and symmetry.
After the development of an appropriate HPLC/DAD procedure for simultaneous determination of all 13 target compounds, it was validated according to the requirements of the European Medicines Agency set out in the document ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology [Citation28]. The identification of the analysed compounds was done by comparing the retention times (tR), that is, by comparing the peaks from the resulting chromatogram with the peaks from the chromatogram of the standard solutions of any single target compound. The tR values determined for all AAS are presented in . Perfect separation for quantitative analysis was achieved for the analytical time of 60 min. An acceptance criterion for quantification methods is that the coefficient of determination R2 is greater than 0.99. The obtained standard curves for each of the 13 targeted substances met the requirement of a coefficient of determination for active substances R2 > 0.99 (). The obtained analytical results show that the method is linear in the ranges summarized in .
The acceptance criterion for the repeatability of the system is RSD <1%. For all 13 determined substances, the RSD % values obtained met the requirements of acceptability and their value was less than 1.0% (). According to the ICH Guideline Validation of Analytical Procedures text and methodology Q2 (R1) [Citation28], repeatability is assessed using a minimum of 6 determinations at 100% of the test concentration, or a total of nine samples are prepared by adding the corresponding substance to the placebo in three parallel samples for each of the selected levels 70%, 100% and 130%. The RSD % value between the concentrations found from the nine samples should be ≤3.0% and the analytical recovery should be between 95% and 105%. The data summarized in show that the developed HPLC-DAD procedure meets both requirements.
Table 5. Method repeatability, precision and recovery of HPLC-DAD procedure for simultaneous quantification of 13 target AAS.
The obtained data show that the values for RSD % fulfil the requirements of acceptability and their value is less than 3.0% for all 13 determined substances. In addition, the obtained results reveal that for all 13 investigated substances with an AAS structure, the analytical yield, recovery and RSD value are within the limits of the eligibility criteria. The described procedure is suitable for use with any HPLC-DAD equipment in standard laboratory settings.
Conclusions
This study reports the development of an HPLC/DAD method for the simultaneous determination of 13 substances with the steroid structure in dietary supplements for sport and its validation according to ICH Harmonized Tripartite Guideline Validation of Analytical Procedures: text and methodology Q2(R1). The 13 target subtances are testosterone, testosterone propionate, testosterone enanthate, methyltestosterone, nandrolone, nandrolone propionate, nandrolone decanoate, methandienone, androstenedione, trenbolone, trenbolone acetate, trenbolone enanthate and boldenone undecylenate. The specific features used to select the optimal HPLC conditions are the ability of the mobile phase and solvents to be used with the HPLC/MS and GC/MS chromatographic system. The obtained total run time, the reproducibility of the retention times, the separation of all peaks and peak characteristics like peak resolution, shape and symmetry were found to be acceptable. The described procedure could be used in standard laboratory settings with any HPLC-DAD equipment.
Authors’ contributions
S. Atanasova, S. Jaber and V. Nemska: development and validation of the method, analysis of target compounds; Z. Zaharieva, Ts. Foteva and D. Tanev: development and validation of the method, calculation and interpretation of the results, D. Danalev and N. Georgieva: conceptualization and manuscript preparation. All authors read and approved the final version of the manuscript.
Ethical approval
This article does not contain any studies with animals or humans.
Supplemental Material
Download PDF (147.4 KB)Acknowledgements
The authors would like to thank Testing Center Global Test Ltd. (Sofia, Bulgaria) for HPLC equipment.
Data availability statement
All data that support the findings reported in this study are available from the authors Z.Z. and D.D. upon reasonable request.
Disclosure statement
All authors declare that they have no conflict of interest. They are entitled to the authorship and have approved the final version of the manuscript.
Additional information
Funding
References
- Frenz J. Chromatographic separations in biotechnology. In: C. Horváth and L.S. Ettre (Eds.) Chromatography in biotechnology, ACS symposium series. Washington, DC: American Chemical Society. 1993. p. 1–8.
- Cramer SM, Jayaraman G. Preparative chromatography in biotechnology. Curr Opin Biotechnol. 1993;4(2):217–225.
- Maughan RJ. Contamination of dietary supplements and positive drug tests in sport. J Sports Sci. 2005;23(9):883–889.
- Hoff D. Doping-och antidopingforskning–En inventering av samhälls-och beteendevetenskaplig forskning och publikationer 2004–2007. 2008. https://lucris.lub.lu.se/ws/portalfiles/portal/5961243/3165184, available 17.01.2023.
- Evans NA. Current concepts in anabolic-androgenic steroids. Am J Sports Med. 2004;32(2):534–542.
- Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34(8):513–554.
- Karila TAM, Karjalainen JE, Mäntysaari MJ, et al. Anabolic androgenic steroids produce dose-dependent increase in left ventricular mass in power athletes, and this effect is potentiated by concomitant use of growth hormone. Int J Sports Med. 2003;24(5):337–343.
- Farzam K. Anabolic-androgenic steroids and cardiometabolic derangements. Cureus. 2021;13(1):e12492.
- Peters RJB, Rijk JCW, Bovee TFH, et al. Identification of anabolic steroids and derivatives using bioassay-guided fractionation, UHPLC/TOFMS analysis and accurate mass database searching. Anal Chim Acta. 2010;664(1):77–88.
- Al-Khadhra RS. The determination of common anabolic steroid and stimulants in nutritional supplements by HPLC-DAD and HPLC-MS. J Chromatogr Sci. 2020;58(4):355–361.
- Becue I, Van Poucke C, Van Peteghem C. An HPLC-MS screening method with library identification for the detection of steroids in dietary supplements. J Mass Spectrom. 2011;46(3):327–335.
- Cho SH, Park HJ, Lee JH, et al. Determination of anabolic-androgenic steroid adulterants in counterfeit drugs by UHPLC-MS/MS. J Pharm Biomed Anal. 2015;111:138–146.
- Odoardi S, Castrignanò E, Martello S, et al. Determination of anabolic agents in dietary supplements by liquid chromatography-high-resolution mass spectrometry. Food Addit Contam A Chem Anal Control Expo Risk Assess. 2015;32(5):635–647.
- Wang H, Wang P, Zhao X, et al. Determination of anabolic androgenic steroids in dietary supplements and external drugs by magnetic solid‐phase extraction combined with high‐performance liquid chromatography–tandem mass spectrometry. J Sep Sci. 2021;44(9):1939–1949.
- Wei MS, Kemperman RHJ, Palumbo MA, et al. Separation of structurally similar anabolic steroids as cation adducts in FAIMS-MS. J Am Soc Mass Spectrom. 2020;31(2):355–365.
- Patanè FG, Liberto A, Maria Maglitto AN, et al. Nandrolone decanoate: use, abuse and side effects. Medicina (Kaunas, Lithuania). 2020;56(11):606.
- Gosetti F, Mazzucco E, Gennaro MC, et al. Ultra high performance liquid chromatography tandem mass spectrometry determination and profiling of prohibited steroids in human biological matrices. A review. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:22–36.
- Swearingen KE, Moritz RL. High field asymmetric waveform ion mobility spectrometry (FAIMS) for mass Spectrometry-Based proteomics. Expert Rev Proteomics. 2012;9(5):505–517.
- De Cock KJ, Delbeke FT, Van Eenoo P, et al. Detection and determination of anabolic steroids in nutritional supplements. J Pharm Biomed Anal. 2001;25(5–6):843–852.
- Baume N, Mahler N, Kamber M, et al. Research of stimulants and anabolic steroids in dietary supplements. Scand J Med Sci Sports. 2006;16(1):41–48.
- Van Poucke C, Detavernier C, Van Cauwenberghe R, et al. Determination of anabolic steroids in dietary supplements by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2007;586(1–2):35–42.
- Rahnema CD, Crosnoe LE, Kim ED. Designer steroids–over-the-counter supplements and their androgenic component: review of an increasing problem. Andrology. 2015;3(2):150–155.
- Lee JH, Han JH, Min AY, et al. Screening for twenty-eight target anabolic-androgenic steroids in protein supplements using QuEChERS extraction followed by liquid chromatography-tandem mass spectrometry. Food Addit Contam A Chem Anal Control Expo Risk Assess. 2020;37(9):1425–1436.
- Higashi T, Ogawa S. Chemical derivatization for enhancing sensitivity during HPLC/ESI-MS/MS quantification of steroids in biological samples: a review. J Steroid Biochem Mol Biol. 2016;162:57–69.
- Marcos J, Pozo OJ. Current HPLC-MS methods and procedures applied to the identification of new steroid metabolites. J Steroid Biochem Mol Biol. 2016;162:41–56.
- Liu H, Lin T, Cheng X, et al. Simultaneous determination of anabolic steroids and β-agonists in milk by QuEChERS and iltra high performance liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1043:176–186.
- Zaharieva Z, Tanev D, Danalev D. Development and validation of HPLC/DAD method for simultaneously determination of six prohibited substances in model matrices. Acta Chromatogr. 2020;32(4):276–280.
- ICH Guideline Validation of Analytical Procedures text and methodology Q2 (R1). https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf