Abstract
A number of scientific papers consider the specific and multifunctional pharmacological characteristics of Nattokinase, including those in the context of neuroprotection, neuroregeneration and neuroplasticity. We present the results from a 12-month observational study of 129 ischemic stroke and transient ischemic attack (TIA) patients treated with Nattokinase and unchanged antihypertensive, antilipidemic and neuroprotective therapy. The main objectives were: (1) to determine the presence or absence of a positive effect of Nattokinase administration on the mean values of systolic and diastolic blood pressure, on the mean values of total and low-density lipoprotein cholesterol, as well as the positive impact on cognitive function through improving cerebral blood flow; and (2) to analyze vascular complications and adverse drug reactions and interactions. After 12 months, lower mean values of systolic and diastolic blood pressure were recorded. No statistically significant lipid profile changes were found except for those in men with stroke. At month 12, the cognitive assessment showed no significant difference compared to initial levels in women, while in men, the improvement was significant. The complication rates were similar between the treatment and control groups. Our findings suggest that Nattokinase is actively involved in the complex mechanisms of vascular prevention. It has a beneficial effect on the aforementioned risk factors with possible time- and gender-related differences. We also hypothesize that both intake of Nattokinase and neuroprotective agents is associated with improvement in vascular cognitive impairment, increased function in daily activities and better outcomes.
Introduction
Stroke is a serious health condition—one of the leading causes of death, disability, various degrees of cognitive deficits, changes in personality and impaired social and professional functioning [Citation1, Citation2].
Recurrent stroke is associated with a greater number of risk factors and more frequent atherosclerotic changes in the large arteries compared to the first stroke [Citation3]. During an average of 5.3 years of follow-up for 987 participants with a first cerebral infarction in the ARIC study (the atherosclerosis risk in communities), 183 recurrences were observed in 147 patients [Citation4]. Approximately 70% of recurrent infarctions were of the same subtype, but there was a recurrence in only 28% of lacunar strokes [Citation4]. The frequency of recurrent ischemic strokes within 1 year was 7.9% for thrombotic, 6.5% for cardioembolic and 6.5% for lacunar occurrences [Citation4]. In the cohort study FUTURE (follow-up of TIA and stroke patients and unelucidated risk factor evaluation), 724 patients with a first episode of TIA, ischemic stroke and/or cerebral hemorrhage, aged 18–50, were prospectively studied for a period of 9 years. The cumulative 20-year risk of recurrent stroke was 19.4% (95% CI 14.6–24.3%) after experiencing an ischemic attack and 9.8% (95% CI 1.0–18.7%) after a cerebral hemorrhage [Citation5, Citation6].
Primary and secondary prophylaxis in cerebrovascular diseases is extremely important. Primary prophylaxis includes the identification of modifiable risk factors and their targeted elimination. They are well known: arterial hypertension, diabetes mellitus, dyslipidemia, obesity, atrial fibrillation, asymptomatic carotid stenoses, reduced physical activity, smoking, alcohol and drug abuse, hyperhomocysteinemia, obstructive sleep apnea, migraine and others. Antiplatelet and antithrombotic medications are used for secondary prophylaxis. In patients with cardiac pathology (persistent or paroxysmal atrial fibrillation), the so-called new anticoagulants (NOACs) or Acenocoumarol are indicated [Citation1, Citation2].
Nattokinase is a proteolytic fibrinolytic enzyme that is derived from the traditional Japanese food Natto. It is a natural product for oral use. The evidence for the effectiveness and safety of its use has grown in volume and scientific value over the past few years [Citation7–10].
The aims of our study were, first, to evaluate the presence or absence of a positive effect of Nattokinase on the mean values of systolic blood pressure (SBP) and diastolic blood pressure (DBP), the mean values of total and LDL cholesterol and the level of cognitive functioning in a group of patients by gender and time period. Second, we performed a comparative analysis of vascular complications and the frequency of possible adverse drug reactions and interactions.
Subjects and methods
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University First MHAT “St. Joan Krastitel”—Sofia (number 28/14.05.2019). Written informed consent was obtained from all participants and caregivers in the study.
Study design
The present study continues our previous research [Citation11, Citation12]. In the period October 2019–October 2022, 129 Caucasian patients with ischemic stroke and TIA who underwent outpatient consultations and/or treatment at the Neurology Clinic at the University First MHAT “St. Joan Krastitel”—Sofia, were enrolled. For secondary prevention, Nattokinase [Nataspin H ©—100 mg Nattokinase, 3000 Fibrinolytic units (FU) and 6 mg Hydroxytyrosol] was started in a single daily dose and, after a change in the dosage of one capsule to 2000 FU (since January 2022), patients started taking two capsules daily (4000 FU/day). The spectrum of monitoring indicators was expanded with the inclusion of clinical and laboratory parameters. A control group of 126 other patients with cerebral infarction and TIA, who underwent secondary prophylaxis with antithrombotic medications, acetylsalicylic acid (ASA, 325/100 mg) and/or Clopidogrel (75 mg) for 24 h was retrospectively considered. The diagnostic and therapeutic algorithm was in accordance with the requirements set by the National Consensus on Prevention, Diagnosis and Treatment of Cerebrovascular Diseases [Citation2]. Regular visits to the Clinic were done at 6- and 12-month intervals. Due to the COVID-19 pandemic, online meetings and consultations were also implemented. During the observation period, there was no change in the antihypertensive, antilipidemic or neuroprotective therapy that the patients received.
The Nattokinase group consisted of 59 women (45.74%) and 57 men (44.18%) with ischemic stroke and 8 women (6.20%) and 5 men (3.88%) with TIA. The control group included 59 women (46.83%) and 57 men (45.24%) with cerebral infarction and 6 women (4.76%) and 4 men (3.17%) with TIA. Demographic data and risk factors are presented in .
Table 1. Demographic and clinical data of patients in both groups—with Nattokinase intake and control.
An arterial blood pressure indicator evaluates systolic and diastolic value determined by three consecutive measurements in a sitting position.
Two parameters from the lipid panel were assessed, total and low-density lipoprotein (LDL) cholesterol, with upper reference limits of 6.1 and 2.6 mmol/L, respectively.
Cognitive potential was studied via the Mini Mental State Examination (MMSE) [Citation13] in the first 72 h after the diagnosis of an acute cerebrovascular event. In 11.63% of patients with ischemic stroke (7 women and 8 men) this was not feasible due to pronounced focal neurological symptoms; therefore, their results were not summarized. In patients with TIA, the Montreal Cognitive Assessment (MoCA) [Citation14] was also applied. In the 6th and 12th months, all participants completed a depression screening test.
Data analysis
The statistical methods used were: descriptive, t-test for independent and dependent samples with significance level p ≤ 0.05, one-factor analysis of variance (ANOVA) with post-hoc Tukey HSD test, correlation analysis with Pearson’s correlation coefficient (r), regression analyses with Multiple R (r), R-squared (r2), Odds Ratio (OR) and graphical representations [Citation15, Citation16]. All analyses were performed using Microsoft Excel 2019 and Statistical Package for Social Sciences version 26.0 (IBM SPSS Statistics 26.0).
Results
Arterial blood pressure and blood cholesterol status
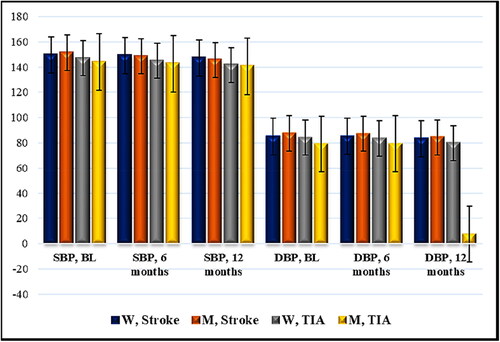
In women with ischemic stroke and Nattokinase intake, after a 6-month interval, no difference was observed in terms of mean systolic values (SBP 149.67 mmHg/SBP 149.13 mmHg, r 0.9816, r2 0.9635, 95% CI (141.80) − (157.53)/(141.54) − (156.73), p = 0.46) and mean diastolic values (DBP 85.0 mmHg/DBP 85.13 mmHg, r 0.9416, r2 0.8865, 95% CI (80.32) − (89.68)/(81.03) − (89.23), р = 0.86) of blood pressure in comparison with the initial measurements. For the same group, after a 12-month period, there was a statistically significant difference in mean systolic values (SBP 149.67 mmHg/SBP 147.07 mmHg, r 0.9926, r2 0.9853, 95% CI (141.80) − (157.53)/(139.26) − (154.87), p < 0.0001) and mean diastolic values (DBP 85.0 mmHg/DBP 83.4 mmHg, r 0.9857, r2 0.9715, 95% CI (80.32) − (89.68)/(79.17) − (87.63), р = 0.001) of blood pressure. In women with cerebral infarction, after 12 months, the mean systolic and diastolic values of arterial blood pressure were lower than the initial ones.
In men with ischemic stroke and Nattokinase, after a 6-month interval, the following results were obtained: mean systolic value (SBP 151.30 mmHg/SBP 148.53 mmHg, r 0.9636, r2 0.9285, 95% CI (145.85) − (156.82)/(142.88) − (154.19), p = 0.001) and mean diastolic value (DBP 87.52 mmHg/DBP 87.24 mmHg, r 0.9247, r2 0.8550, 95% CI (83.65) − (91.41)/(83.83) − (90.64), р = 0.67) of blood pressure. After a 12-month period, the indicators were: mean systolic value (SBP 151.30 mmHg/SBP 145.54 mmHg, r 0.9437, r2 0.8905, 95% CI (145.85) − (156.82)/(139.71) − (151.37), p < 0.0001) and mean diastolic value (DBP 87.52 mmHg/DBP 84.23 mmHg, r 0.9747, r2 0.9501, 95% CI (83.65) − (91.41)/(80.33) − (88.14), p < 0.0001). In men with cerebral infarction, there was a decrease in the mean systolic pressure after 6 months, and lower systolic and diastolic values of arterial blood pressure were observed after 12 months.
In women with TIA and Nattokinase intake, after 6 months, a borderline mean systolic value (SBP 146.88 mmHg/SBP 144.88 mmHg, r 0.9791, r2 0.9586, 95% CI (137.95) − (155.8)/(136.85) − (152.9), p = 0.046) and mean diastolic value (DBP 84.12 mmHg/DBP 83.5 mmHg, r 0.9841, r2 0.9685, 95% CI (78.63) − (89.62)/(77.61) − (89.39), р = 0.22) of arterial blood pressure were detected. After 12 months, there was statistically significant difference compared to the initial measurements: mean systolic value (SBP 146.88 mmHg/SBP 141.63 mmHg, r 0.9839, r2 0.9680, 95% CI (137.95) − (155.8)/(133.48) − (149.77), p = 0.0002) and mean diastolic value (DBP 84.12 mmHg/DBP 79.88 mmHg, r 0.9915, r2 0.9830, 95% CI (78.63) − (89.62)/(73.94) − (85.81), р < 0.0001).
In men with TIA and Nattokinase, after a 6-month period, there was a significant difference in the mean systolic values (SBP 144.0 mmHg/SBP 142.6 mmHg, r 0.9966, r2 0.9931, 95% CI (132.06) - (155.94)/(130.2) - (155.0), p = 0.03) and non-significant difference in the mean diastolic value (DBP 79.0 mmHg/DBP 79.2 mmHg, r 0.8481, r2 0.7192, 95% CI (69.79) - (88.21)/(70.76) – (87.64), р = 0.92). After 12 months, mean systolic (SBP 144.0 mmHg/SBP 140.6 mmHg, r 0.9918, r2 0.9837, 95% CI (132.06) - (155.94)/(127.65) – (153.55), p = 0.01) and mean diastolic values (DBP 79.0 mmHg/DBP 74.6 mmHg, r 0.8936, r2 0.7985, 95% CI (69.79) - (88.21)/(65.79) - (83.41), р = 0.043) were observed, which are statistically different compared to the initial values. The results in women and men with TIA were similar to the ones in women and men with cerebral infarction ().
Figure 1. Time distribution (6- and 12-month period) of mean SBP and DBP (mmHg) in patients with acute cerebrovascular event and Nattokinase. W, women; M, men; TIA, transient ischemic attack; BL, baseline; SBP, systolic blood pressure; DBP, diastolic blood pressure. Data are mean values (n = 129) with standard deviation (SD).
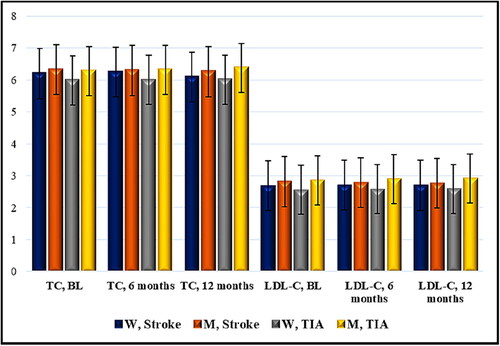
During the follow-up study period for women with ischemic stroke receiving Nattokinase, there was no difference regarding the mean value of total cholesterol 6.2 mmol/L/6.25 mmol/L, r 0.9874, r2 0.9750, 95% CI (5.74) − (6.66)/(5.83) − (6.67), p = 0.22 (6 months) and 6.2 mmol/L/6.09 mmol/L, r 0.8507, r2 0.7237, 95% CI (5.74) − (6.66)/(5.73) − (6.44), p = 0.33 (12 months). There was also no difference in the mean value of LDL cholesterol: 2.69 mmol/L/2.71 mmol/L, r 0.9580, r2 0.9177, 95% CI (2.51) − (2.86)/(2.54) − (2.89), p = 0.34 (6 months) and 2.69 mmol/L/2.70 mmol/L, r 0.9577, r2 0.9171, 95% CI (2.51) − (2.86)/(2.53) − (2.87), p = 0.63 (12 months).
In men with cerebral infarction and Nattokinase intake, no difference was observed after a 6-month interval: mean value of total cholesterol 6.33 mmol/L/6.30 mmol/L, r 0.9904, r2 0.9809, 95% CI (6.02) − (6.64)/(5.98) − (6.62), p = 0.22; mean value of LDL cholesterol: 2.82 mmol/L/2.79 mmol/L, r 0.9804, r2 0.9612, 95% CI (2.58) − (3.06)/(2.56) − (3.02), р = 0.13. After 12 months, there was a statistically significant change: mean value of total cholesterol 6.33 mmol/L/6.26 mmol/L, r 0.9846, r2 0.9694, 95% CI (6.02) − (6.64)/(5.95) − (6.57), p = 0.02; mean value of LDL cholesterol: 2.82 mmol/L/2.77 mmol/L, r 0.9838, r2 0.9679, 95% CI (2.58) − (3.06)/(2.53) − (3.0), р = 0.01.
In women with TIA and Nattokinase, no significant difference was observed: mean value of total cholesterol 5.99 mmol/L/6.0 mmol/L, r 0.9965, r2 0.9930, 95% CI (5.31) − (6.67)/(5.37) − (6.63), p = 0.69 (6 months), and mean value of total cholesterol 5.99 mmol/L/6.01 mmol/L, r 0.9464, r2 0.8956, 95% CI (5.31) − (6.67)/(5.37) − (6.65), p = 0.79 (12 months). The mean values of LDL cholesterol were: 2.56 mmol/L/2.58 mmol/L, r 0.9751, r2 0.9508, 95% CI (2.35) − (2.77)/(2.40) − (2.75), р = 0.60 (6 months) and 2.56 mmol/L/2.59 mmol/L, r 0.9501, r2 0.9027, 95% CI (2.35) − (2.77)/(2.42) − (2.75), р = 0.45 (12 months).
Similar results were seen among men with TIA: mean value of total cholesterol 6.28 mmol/L/6.32 mmol/L, r 0.9909, r2 0.9820, 95% CI (5.5) − (7.06)/(5.51) − (7.13), p = 0.37 (6-month period) and mean value of total cholesterol 6.28 mmol/L/6.38 mmol/L, r 0.9874, r2 0.9749, 95% CI (5.5) − (7.06)/(5.6) − (7.16), p = 0.089 (12-month period); mean value of LDL cholesterol: 2.86 mmol/L/2.90 mmol/L, r 0.9916, r2 0.9834, 95% CI (2.29) − (3.43)/(2.25) − (3.55), p = 0.37 (6-month period) and mean value of LDL cholesterol 2.86 mmol/L/2.92 mmol/L, r 0.9962, r2 0.9924, 95% CI (2.29) − (3.43)/(2.30) − (3.54), p = 0.07 (12-month period). The data are presented in .
Figure 2. Time distribution (6- and 12-month period) of mean values of total and LDL cholesterol (Mmol/L) in patients with acute cerebrovascular event and Nattokinase. W, women; M, men; TIA, transient ischemic attack; BL, baseline; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol. Data are mean values (n = 129) with standard deviation (SD).
Cognitive capacity
The mean values of the selected marker for cognitive capacity assessment, MMSE, in women with ischemic stroke and Nattokinase intake (n = 52) demonstrated a change at the 6th month (lower parameters compared to baseline levels): mean value of MMSE 25.31 pt./24.92 pt., r 0.9343, r2 0.8730, 95% CI (24.69) − (25.93)/(24.29) − (25.56), p = 0.001. At month 12, there was improvement compared to the previous indicators, which was statistically insignificant as compared to the initial score: MMSE 25.31 pt./25.17 pt., r 0.9263, r2 0.8580, 95% CI (24.69) − (25.93)/(24.52) − (25.83), p = 0.28.
In men with cerebral infarction and Nattokinase (n = 49), after a 6-month interval, there was significant difference with a preserved tendency for lower values: mean value of MMSE 25.76 pt./25.30 pt., r 0.9376, r2 0.8792, 95% CI (25.26) − (26.25)/(24.77) − (25.84), p < 0.0001. At month 12, statistically significant improvement was observed, including compared to the baseline values: mean value of MMSE 25.76 pt./25.57 pt., r 0.9605, r2 0.9225, 95% CI (25.26) − (26.25)/(25.07) − (26.07), p = 0.01.
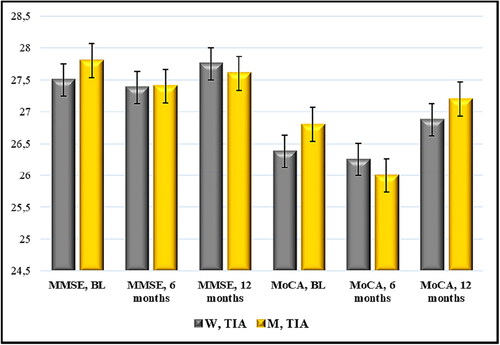
Non-significant difference was found in women with TIA and Nattokinase intake: mean value of MMSE 27.50 pt./27.38 pt., r 0.8001, r2 0.6402, 95% CI (26.73) − (28.27)/(26.49) − (28.26), p = 0.60 (6 months) and mean value of MMSE 27.50 pt./27.75 pt., r 0.6547, r2 0.4286, 95% CI (26.73) − (28.27)/(27.16) − (28.34), p = 0.35 (12 months). In the MoCA test, the compared scores were statistically insignificant: mean value of MoCA 26.38 pt./26.25 pt., r 0.7767, r2 0.6033, 95% CI (25.29) − (27.46)/(25.28) − (27.22), p = 0.68 (6 months) and MoCA 26.38 pt./26.88 pt., r 0.8162, r2 0.6662, 95% CI (25.29) − (27.46)/(26.05) − (27.70), p = 0.10 (12 months).
The evaluation of cognitive potential in men with TIA via MMSE showed similar values: mean value of MMSE 27.80 pt./27.40 pt., r 0.8750, r2 0.7656, 95% CI (27.24) − (28.36)/(26.29) − (28.51), p = 0.18 (6-month period) and mean value of MMSE 27.80 pt./27.60 pt., r 0.6124, r2 0.3750, 95% CI (27.24) − (28.36)/(26.92) − (28.28), p = 0.37 (12-month period). When MoCA was applied at the 6th month, the result was lower and statistically reliable: mean value of MoCA 26.80 pt./26.0 pt., r 0.8964, r2 0.8036, 95% CI (25.76) − (27.84)/(24.76) − (27.24), p = 0.02. After 12 months, there was improvement compared to month 6, but with no significant difference compared to the initial sums: mean value of MoCA 26.80 pt./27.20 pt., r 0.7857, r2 0.6173, 95% CI (25.76) − (27.84)/(26.16) − (28.24), p = 0.18.
One-factor ANOVA with post-hoc Tukey HSD test confirmed the results. In women with ischemic stroke: p = 0.002, post-hoc Tukey HSD [0.05] = 0.25, HSD [0.01] = 0.31, Month (M)1/M6 p < 0.01, M6/M12 p < 0.05, M1/M12 NS. In men with cerebral infarction: p < 0.0001, post-hoc Tukey HSD [0.05] = 0.19, HSD [0.01] = 0.24, M1/M6 p < 0.01, M6/M12 p < 0.01. In women with TIA there was no statistically significant difference when MMSE (p = 0.26) and MoCA (p = 0.09) were applied. In men with TIA there was no change in relation to MMSE (p = 0.41), but there was statistical reliability in MoCA р = 0.002, post-hoc Tukey HSD [0.05] = 0.62, HSD [0.01] = 0.86, М1/М6 р < 0.05, M6/M12 p < 0.01, M1/M12 NS ( and ).
Figure 3. Time distribution (6- and 12-month period) of mean scores of MMSE (points) in patients with acute cerebrovascular event and Nattokinase. W, women; M, men; TIA, transient ischemic attack; BL, baseline; MMSE, Mini Mental State Examination. Data are mean values (n = 114) with standard deviation (SD).
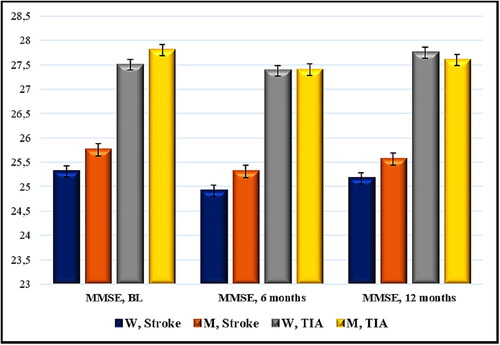
Figure 4. Time distribution (6- and 12-month period) of mean scores of MMSE and MoCA (points) in cases with TIA and Nattokinase. W, women; M, men; TIA, transient ischemic attack; BL, baseline; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment. Data are mean values (n = 13) with standard deviation (SD).
Vascular complications
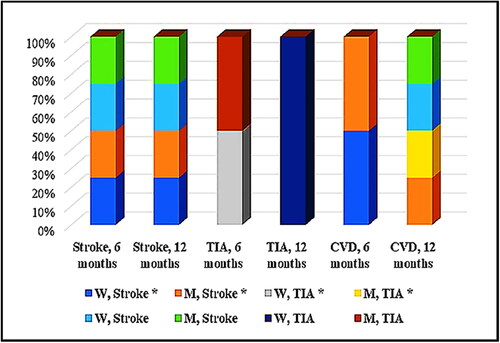
During the observation period, vascular complications were of particular interest. They were divided into cerebrovascular (stroke and TIA) and cardiovascular for the purpose of this study. In the Nattokinase group, within 6 months, there were two recurrent ischemic strokes, one patient with recurrent TIAs and two other subjects with cardiovascular problems. For 12 months, there were two more strokes and two cases of cardiovascular complications. In the ASA/Clopidogrel control group, throughout the 6-month follow-up period, strokes and TIA were registered in three patients. By the end of the period, there had been one case of TIA, two cases of cerebral infarction and two cases of cardiovascular complications (). The difference between the total number of vascular complications in both groups—with Nattokinase and the control group with standard secondary prophylaxis – was non-significant (р = 0.55, calc. t = −0.63, crit. t = 2.31). When applying categorical logistic analysis, there was a relative risk or risk ratio (RR) = 1.1 and an odds ratio (OR) = 1.11, at 95% CI 0.413–2.964, z 0.201 and significance level p = 0.84.
Figure 5. Distribution (by gender and time period) of vascular complications in both groups: with Nattokinase intake (*) and control. Data are mean values (Nattokinase group, n = 129 versus control group, n = 126) with standard deviation (SD).
Adverse drug reactions were rare and were represented mainly by skin and gastrointestinal manifestations. The frequency in both groups was low: 4.65% compared to 5.56% (control) and without statistical reliability (р = 0.68, calc. t = −0.42, crit. t = 2.18).
Discussion
The specific and multifunctional pharmacological characteristics of Nattokinase have been presented in a number of scientific publications. Randomized, double-blind, placebo-controlled studies reviewed the antihypertensive properties of Nattokinase and differentiated sex-specific mechanisms in terms of the effect on SBP and DBP and a better therapeutic response in male patients [Citation17, Citation18]. Our results are similar and contribute to our understanding of the beneficial properties of Nattokinase. After a 6-month interval and continuous antihypertensive therapy in men with ischemic stroke and TIA, lower mean values of SBP (−2.77/−1.4 mmHg) were observed. After a 12-month interval, the mean values of SBP and DBP were lowered by (−5.76/−3.4 mmHg) and (−3.29/−4.4 mmHg) respectively. After 6 months, in women with TIA, the mean value of SBP was borderline (−2.0 mmHg, p = 0.046), but overall, the changes in arterial blood pressure in women with acute cerebrovascular events were non-significant. After 12 months, in women with ischemic stroke and TIA, the mean values of SBP (−2.6/−5.25 mmHg) and DBP (−1.6/−4.24 mmHg) were lower than the initial ones.
The application of Nattokinase has a lipid lowering effect by reducing the serum levels of LDL and triglycerides, which also reflects in decreasing the values of total cholesterol [Citation19–24]. In the follow-up study group, no statistically significant change in the investigated total and LDL cholesterol was found, except for the subcategory of men with stroke, who took Nattokinase and standard statin therapy for 12 months (total cholesterol −0.07, p = 0.02; LDL cholesterol −0.05, р = 0.01). A clinical study with 1062 participants reported significant improvement in lipid profile, which was dose-dependent [Citation25]. The recommended daily dose of Nattokinase is 10 800 FU and the lower dose of 3600 FU for 24 h is considered ineffective [Citation25]. Our patients received 3000 FU daily and, after the change, 4000 FU a day.
The neuroprotective properties of Nattokinase are related to protective action against the external (receptor) and internal (mitochondrial) mechanisms of apoptosis and to the process of amyloid fibril degradation (under strictly defined conditions) [Citation26–29]. The frequency of cognitive impairment after stroke is ≥50% and may progress to vascular dementia [Citation2, Citation30, Citation31]. In the observed women and men with ischemic stroke, after 6 months, lower mean values of the MMSE compared to baseline scores were reported. At month 12, the scores in women were non-significantly different as compared to the initial ones (better compared to month 6), while in men the improvement was statistically significant (−0.19, p = 0.01). Over the entire follow-up study period, there was no development with respect to the mean MMSE and MoCA values in women with TIA. In men with TIA, the compared MMSE scores should no significant change, MoCA at month 6 showed lower values, which after 12 months were not different from the baseline scores. Through intragroup ANOVA analysis, the results between the separate subcategories were confirmed in support of the absence of statistically significant cognitive decline one year after an acute cerebrovascular event on the background of continuous neuroprotective therapy and Nattokinase.
Nattokinase is characterized by its fibrinolytic and anti-inflammatory activity and limits the development of oxidative stress and the inflammatory cascade [Citation32–35]. It has an antithrombotic effect and all listed properties are the basis of successful vascular prevention [Citation8, Citation36–38]. During the monitoring period, the rate of vascular (cerebral and cardiac) complications in the Nattokinase group was similar to the one in the control group (6.98%/6.35%).
Our study has some limitations. For example, patients with cardioembolic and cryptogenic accidents were not included; the number of participants was small, about 5% of them did not comply with the visit requirements, etc.
Despite these limitations, the results of this research are in agreement with previous reports that Nattokinase has antihypertensive effects. Its lipid-lowering properties were not confirmed except for the group of men with stroke and long-term (12 months) treatment with standard-dose statins. Our data also introduced a unique hypothesis of the potential protective role of Nattokinase against cognitive changes attributable to cerebrovascular disease. According to the presented individual and global research data, Nattokinase is well tolerated and characterized by an efficacy and clinical safety profile [Citation39, Citation40]. It is an interesting and hopeful alternative in the context of the current topics of neuroprotection, neuroregeneration and neuroplasticity [Citation41, Citation42].
Conclusions
The concept of Nattokinase use provides a series of possible directions for the future development of advanced prophylactic and therapeutic strategies. It is a new opportunity in cerebrovascular disease management. Further studies with larger sample sizes are necessary in order to explain the important role of Nattokinase in short-term and long-term disease prevention and in health promotion.
Authors’ contributions
DM and DD—conceptualization; DM, DD, JM-G and IG—writing, original draft preparation and revision; JMG, IG, DM and DD—statistical analysis and interpretation; DM—supervision. All authors have read and approved the final version of the manuscript.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University First MHAT “St. Joan Krastitel”—Sofia (Number 28/14.05.2019). Written informed consent was obtained from all participants and caregivers in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data used for the analysis are available from the corresponding author (DM) upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Additional information
Funding
References
- Maslarov D. Cerebrovascular diseases: recent evaluations and ideas for improving the public health [dissertation]. Sofia: Medical University of Sofia; 2017.
- Milanov I, Stamenova P. National consensus statement for prophylaxis, making the diagnosis and treatment of cerebrovascular diseases. Bulg Neurol. 2020;21(S4):1–10.
- Kolmos M, Christoffersen L, Kruuse C. Recurrent ischemic stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2021;30(8):105935. doi: 10.1016/j.jstrokecerebrovasdis.2021.105935.
- Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690–1696. doi: 10.1016/j.jacc.2010.11.041.
- Rutten-Jacobs LC, Maaijwee NA, Arntz RM, et al. Risk factors and prognosis of young stroke. The FUTURE study: a prospective cohort study. Study rationale and protocol. BMC Neurol. 2011;11:109. doi: 10.1186/1471-2377-11-109.
- Rutten-Jacobs LC, Arntz RM, Maaijwee NA, et al. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. 2013;309(11):1136–1144. doi: 10.1001/jama.2013.842.
- Sumi H, Hamada H, Tsushima H, et al. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43(10):1110–1111. doi: 10.1007/BF01956052.
- Chen H, McGowan EM, Ren N, et al. Nattokinase: a promising alternative in prevention and treatment of cardiovascular diseases. Biomark Insights. 2018;13:1177271918785130. doi: 10.1177/1177271918785130.
- Ahmed HH, Nevein NF, Karima A, et al. Miracle enzymes serrapeptase and nattokinase mitigate neuroinflammation and apoptosis associated with Alzheimer’s disease in experimental model. WJPPS. 2013;3:876–891.
- Urano T, Ihara H, Umemura K, et al. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J Biol Chem. 2001;276(27):24690–24696. doi: 10.1074/jbc.M101751200.
- Maslarov D, Drenska D. Nattokinase in patients with acute ischemic stroke and TIA—preliminary data. XVII National Congress of Neurology with international participation. Bulg Neurol. 2018;19(2):44A–121.
- Maslarov D, Drenska D. Use of nattokinase in patients with ischemic stroke and transient ischemic attacks. Neurol Neurosci. 2020;1(2):1–5.
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x.
- Krousel-Wood MA, Chambers RB, Muntner P. Clinicians’ guide to statistics for medical practice and research: part I. Ochsner J. 2006;6:68–83.
- Krousel-Wood MA, Chambers RB, Muntner P. Clinicians’ guide to statistics for medical practice and research: part II. Ochsner J. 2007;7(1):3–7.
- Kim JY, Gum SN, Paik JK, et al. Effects of nattokinase on blood pressure: a randomized, controlled trial. Hypertens Res. 2008;31(8):1583–1588. doi: 10.1291/hypres.31.1583.
- Jensen GS, Lenninger M, Ero MP, et al. Consumption of nattokinase is associated with reduced blood pressure and von Willebrand factor, a cardiovascular risk marker: results from a randomized, double-blind, placebo-controlled, multicenter North American clinical trial. Integr Blood Press Control. 2016;9:95–104. doi: 10.2147/IBPC.S99553.
- Ren NN, Chen HJ, Li Y, et al. A clinical study on the effect of nattokinase on carotid artery atherosclerosis and hyperlipidaemia. Zhonghua yi xue za zhi. 2017;97(26):2038–2042 (in Chinese).
- Xie S, Yu Z, Liu X. Preparation of nattokinase and study on its hypolipidemic effect. Chin J Biochem Pharm. 2015;35:17–20.
- Li D, Hou L, Hu M, et al. Recent advances in nattokinase-enriched fermented soybean foods: a review. Foods. 2022;11(13):1867. doi: 10.3390/foods11131867.
- Wu D, Lin C, Lee M. Lipid-Lowering effect of nattokinase in patients with primary hypercholesterolemia. Acta Cardiol Sin. 2009;25:26–30.
- Suzuki Y, Kondo K, Ichise H, et al. Dietary supplementation with fermented soybeans suppresses intimal thickening. Nutrition. 2003;19(3):261–264. doi: 10.1016/s0899-9007(02)00853-5.
- Simova I, Simov D, Klisurski M, et al. Nattokinase-fibrinolytic, antihypertensive and lipid-lowering potential: an application in clinical practice. MD XV. 2018;1:87–89 (in Bulgarian).
- Chen H, Chen J, Zhang F, et al. Effective management of atherosclerosis progress and hyperlipidemia with nattokinase: a clinical study with 1,062 participants. Front Cardiovasc Med. 2022;9:964977. doi: 10.3389/fcvm.2022.964977.
- Ji H, Yu L, Liu K, et al. Mechanisms of nattokinase in protection of cerebral ischemia. Eur J Pharmacol. 2014;745:144–151. doi: 10.1016/j.ejphar.2014.10.024.
- Wang JM, Chen HY, Cheng SM, et al. Nattokinase reduces brain infarction, fibrinogen and activated partial thromboplastin time against cerebral ischemia-reperfusion injury. J Food Drug Anal. 2012;3:686–691.
- Hsu R-L, Lee K-T, Wang J-H, et al. Amyloid-degrading ability of nattokinase from Bacillus subtilis natto. J Agric Food Chem. 2009;57(2):503–508. doi: 10.1021/jf803072r.
- Ahn YJ, Kim MH, Kim J, et al. Neuroprotective effect of nattokinase mediated by inhibition of platelet aggregation and thrombosis in photo thrombotic stroke. Stroke. 2015;46(suppl_1):A-PW262. doi: 10.1161/str.46.suppl_1.wp262.
- Lo JW, Crawford JD, Desmond DW, et al. Long-term cognitive decline after stroke: an individual participant data meta-analysis. Stroke. 2022;53(4):1318–1327. doi: 10.1161/STROKEAHA.121.035796.
- Zhu Y, Zhao S, Fan Z, et al. Evaluation of the mini-mental state examination and the Montreal cognitive assessment for predicting post-stroke cognitive impairment during the acute phase in Chinese minor stroke patients. Front Aging Neurosci. 2020;12:236. doi: 10.3389/fnagi.2020.00236.
- Wu H, Wang Y, Zhang Y, et al. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020;32:101500. doi: 10.1016/j.redox.2020.101500.
- Pais E, Alexy T, Holsworth REJr, et al. Effects of nattokinase, a pro-fibrinolytic enzyme, on red blood cell aggregation and whole blood viscosity. Clin Hemorheol Microcirc. 2006;35(1–2):139–142.
- Hsia CH, Shen MC, Lin JS, et al. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr Res. 2009;29(3):190–196. doi: 10.1016/j.nutres.2009.01.009.
- Kurosawa Y, Nirengi S, Homma T, et al. A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Sci Rep. 2015;5(1):11601. doi: 10.1038/srep11601.
- Weng Y, Yao J, Sparks S, et al. Nattokinase: an oral antithrombotic agent for the prevention of cardiovascular disease. Int J Mol Sci. 2017;18:e523. doi: 10.3390/ijms18030523.
- Petrovsky P. Stroke prevention in patients with atrial fibrillation using the natural product nattokinase as an alternative of conventional anticoagulation. Bulgarian Cardiology XXIII. 2017;4:51–55 (in Bulgarian).
- Maslarov D, Gabrovski N. [Farmakologichno I nefarmacologichno lechenie na ishemichen mozachen insult.] Pharmacological and non-pharmacological treatment of ischemic stroke. [Nauka Farmakologia] Sci Pharmacol. 2013;2(7):43–49 (in Bulgarian).
- Gallelli G, Di Mizio G, Palleria C, et al. Data recorded in real life support the safety of nattokinase in patients with vascular diseases. Nutrients. 2021;13:2031. doi: 10.3390/nu13062031.
- Liu Y, Han Y, Cao L, et al. Analysis of main components and prospects of natto. AER. 2021;9(1):1–9. doi: 10.4236/aer.2021.91001.
- Muresanu DF. Neuroprotection and neuroplasticity: a holistic approach and future perspectives. J Neurol Sci. 2007;257(1-2):38–43. doi: 10.1016/j.jns.2007.01.041.
- Muresanu DF, Buzoianu A, Florian SI, et al. Towards a roadmap in brain protection and recovery. J Cell Mol Med. 2012;16(12):2861–2871. doi: 10.1111/j.1582-4934.2012.01605.x.
