Abstract
The aim of this study was to evaluate the effect of ellagic acid on 5-fluorouracil-induced oral mucositis in rats. Twenty-four 6-month-old male Wistar albino rats were used. The rats were divided into three groups: Experimental Group I, Experimental Group II and Control Group. A superficial scratch was made on the mucosa of the right cheek pouch on the 3rd day and the 5th day. Oral mucositis protocol was applied to the Control Group. Ellagic acid was administered by gavage to the Experimental Group I from the 1st day to the 5th day along with the 5-FU procedure, and to the Experimental Group II from the 5th day to the 10th day. On the 11th day, all rats were sacrificed. The effect of ellagic acid on the healing of oral mucositis was evaluated histopathologically and immunohistochemically. TNF-α expression was observed at various intensities in the infected incision sites of the Experimental Group I sample, especially in the interstitial area in the lamina propria and mostly in the cytoplasm of the inflammatory cells within the vessel. Similar to the Experimental Group I, TNF-α expression of varying intensity occurred in the inflammatory cells in the Experimental Group II, partly in the epithelium and mostly in the inflammatory cells in the lamina propria. Additional research is needed to elucidate the pathogenic inflammatory mechanisms involved in the mucositis site and the prophylactic and therapeutic roles of antioxidants in the healing of oral mucositis by ellagic acid.
Introduction
Oral mucositis (OM) represents a prevalent adverse effect of chemotherapy, affecting a significant proportion of patients. Specifically, it has been noted to afflict around 75% of individuals receiving high-dose chemotherapy before undergoing hematopoietic cell transplantation, as well as 20–60% of patients undergoing treatment for solid tumors [Citation1, Citation2]. OM manifests as erythema and ulceration of non-keratinized mucosa, resulting in a range of impairments in oral functions, including chewing, swallowing and speech. Hence, effective management of OM is crucial for promoting oral health, enhancing quality of life and improving prognosis [Citation3, Citation4]. The daily life functions of patients are directly impacted by OM. Such complications comprise of impediments during feeding and communication, leading to symptoms such as dehydration, weight loss, pain, infection, altered taste, anorexia and cachexia. Moreover, OM poses a greater risk of septicemia in patients with neutropenia. Despite being self-limiting, the condition can impede the delivery of optimal cancer treatment due to its association with clinically significant pain, malnutrition, prolonged hospitalization, bloodstream infections and reduction in antineoplastic dose. Various attempts to prevent or treat OM have been largely futile, mainly due to inadequate knowledge about its underlying pathophysiology [Citation5].
Following the onset of ulceration, the lesions become colonized by local flora, which can exacerbate the injury and impede the healing process. Overall, it is a phase marked by persistent damage, inflammation and at times, ulceration in the oral and/or alimentary canal, with healing occurring anywhere from one to several weeks following radiation and/or chemotherapy. If recuperation is protracted, adhering to the intended chemotherapy or radiation treatment regimen may prove challenging. Conversely, hastened recovery not only enhances the quality of life, but also expedites cancer treatment while necessitating fewer secondary interventions. Establishing a "therapeutic alliance" is imperative to obtain feedback and provide options, in order to enhance each chemotherapy cycle and prevent the onset of a catabolic state [Citation6].
Ellagic acid (EA) is a polyphenolic compound naturally present in various plants, including fruits and nuts commonly used in everyday cooking, possessing robust antioxidant properties [Citation7]. EA belongs to the flavonoid family and is typically synthesized by plants in the form of ellagitannins, a type of tannin. EA comprises two lactone groups and four hydroxyl groups, whereby the hydroxyl group is recognized for augmenting antioxidant activity in lipid peroxidation and safeguarding cells against oxidative harm [Citation8]. In recent times, EA has garnered considerable attention owing to its diverse biological properties, which encompass antioxidant, chemopreventive, antiapoptotic, antimutagenic, antifibrotic and anti-inflammatory activities [Citation9–11].
The aim of this study was to compare the immunohistochemical and histological effects of EA in an experimental model of OM.
Materials and methods
Ethics statement
This study was granted approval by the Bingöl University Animal Experiments Local Ethics Committee (Meeting Date: 11.03.2022 Meeting No: 2022/01 Decision No: 01/02). All efforts were made to minimize animal suffering, and procedures were selected in accordance with international standards for the humane treatment of experimental animals.
Experimental animals
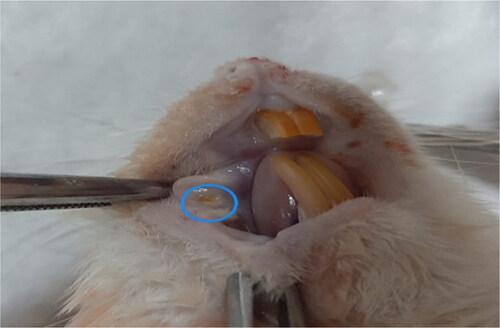
The study utilized 24 male Wistar albino rats, each of 6 months in age. Rats were obtained from Bingöl University Experimental Research Center. The rats were maintained under standard conditions of a 12-h light/dark cycle, a constant temperature of 23 ± 1 °C, and a relative humidity of 55 ± 10% in Bingöl University Centre for Experimental Research. The animals were allowed ad libitum access to both water and standard laboratory diet. The rats were randomly segregated into three groups, namely Experimental Group I, Experimental Group II and Control Group, each comprising 8 rats with body weight in the range of 350–400 g. All rats were subjected to an intraperitoneal injection of 100 mg/kg 5-fluorouracil (5-FU) on the 1st day, followed by an injection of 65 mg/kg 5-FU on the 3rd day. Before 5-FU administration, the rats were kept in a suitable position and the area was cleaned with an antiseptic solution; 5-FU was injected very slowly. On the 3rd and 5th days, a superficial scratch was made on the mucosa of the right cheek pouch using the tip of a 21-gauge needle [Citation12]. The described method has been previously utilized to induce ulcerative mucositis that is akin to human oral mucositis, as illustrated in . The Control Group was only subjected to the procedure to induce OM. In order to evaluate the preoperative and postoperative effects of EA, 50 mg/kg powder EA (Sigma Aldrich, USA) was mixed with distilled water and administered to rats by gavage (with the help of a probe) every day from day 1 to day 5 in Experimental Group I and every day from day 5 to day 10 in Experimental Group II. On day 11, all rats were anesthetized by intraperitoneal injection of ketamine (60 mg/kg) and xylazine (6 mg/kg). The right cheek mucosa was taken for examination. Then all rats were sacrificed by decapitation using a guillotine.
Control Group (n = 8): OM was induced, EA was not administered, and two rats died before being sacrificed. For this reason, their data were not included in the statistical analysis. The Control Group was completed with six rats.
Experimental Group I (n = 8): EA was given for the first five days after OM creation, and two rats died before being sacrificed. For this reason, their data was not included in the statistical analysis. The Experimental Group I was completed with six rats.
Experimental Group II (n = 8): Five days after OM creation, EA was administered for five days. Two rats died before being sacrificed. For this reason, their data were not included in the statistical analysis. The Experimental Group II was completed with six rats.
The cause of death of the rats was the lack of healthy feeding due to the induction of OM with a heavy chemotherapeutic agent such as 5-FU. As a result, six rats that lost a lot of weight died.
Histopathologic analysis
For the histopathological examination, samples from the rats that were subjected to necropsy were fixed in a 10% buffered formalin solution. The tissues were then washed in running tap water and subjected to routine tissue tracing. Following this procedure, 4 µm thick sections were cut from each sample and embedded in paraffin blocks using a rotary microtome (Leica RM2135, Wetzlar, Germany). Tissues on normal slides were kept in an oven for 1 h, followed by deparaffinization and rehydration in xylol-alcohol series. Finally, entellan was dripped on the tissues stained with hematoxin and eosin and covered with coverslips. The preparations were examined under a light microscope and pathologic findings were scored and statistically analyzed.
Immunohistochemical (IHC) staining
Tissue sections taken on adhesive slides were kept in an oven for 1 h and then deparaffinized-rehydrated by passing through a xylol-alcohol series. The sections were kept in 3% H2O2 for 20 min to stop endogenous activation in the tissues. After washing with PBS, the sections were subjected to three boiling/cooling cycles in a citrate buffer solution to expose the antigens in the tissue. After using a PAP pen to demarcate the tissue, the sections were washed with PBS and incubated with Ultra V Block for 20 min to prevent non-specific binding. Subsequently, 1/100 diluted TNF-α (sc-52B83, Santa Cruz, USA) and 8-OHdG (no. sc66036, Santa Cruz, USA) primary antibodies were applied to the tissues and the tissues were incubated at +4 °C for 16 h. The tissues were washed again with PBS and incubated with biotinized secondary antibody (No. TP-125- BN, Thermofisher, USA) for 20 min. After another PBS wash, the tissues were incubated with streptavidin-peroxidase (No. TA-125-HR, Thermofisher, US) for 20 min. After a final wash with PBS, 3,3′-diaminobenzidine (DAB) chromogen was added to the tissues to observe antigen-antibody binding, and Mayer hematoxylin was used for background staining. The preparations were examined under a light microscope (Leica DM2500, Wetzlar, Germany), and statistical analysis was performed by scoring according to the intensity of immunoreaction.
Statistical analysis
The collected data was subjected to analysis using IBM SPSS V23 software. The chi-square test was employed for categorical data. The findings were demonstrated in terms of frequency and percentage. Differences were considered statistically significant at a threshold of p < 0.050.
Results
Histopathologic examination
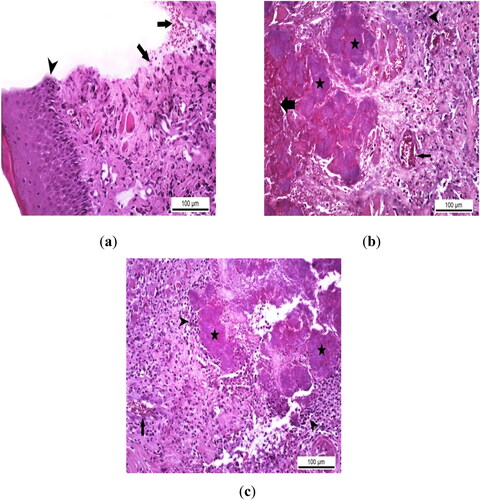
Histopathologic examination in the Control Group showed that epithelial regeneration was at a routine healing level and partial reparation was observed in the propria layer. In the wound line of the Control Group in which mild healing was noted, serofibrinous exudation was detected containing a small number of inflammatory cells and erythrocytes (). Other than these, no pathologic findings or secondary infections that would delay healing were encountered. In the Experimental Group I, infectious agents caused secondary complications at the incision line. Necrotic and hemorrhagic ulcerations progressing to the basement membrane due to the state of infection at the incision site were observed. In addition, in some cases belonging to this group, bacterial colonies, which are thought to cause secondary bacterial infection, were found to be dense especially in necrotic areas (). No regenerative and reparative changes that would indicate a complete healing or repair were noted at the site of inflammation where the secondary infection was located. Similar to the Experimental Group I, no development that would indicate healing or repair was observed in the Experimental Group II, and it was determined that the wound line was infected with pathogenic agents in the samples of this group. Hemorrhagic and necrotic ulcers with severe tissue destruction were observed at the infected incision site. In addition, inflammatory cell infiltrations, mostly mononuclear, were detected where bacterial colonies were localized ().
Figure 2. Histopathological analysis of the therapeutic effect of EA in rats with experimental oral mucositis. (a) Routine healing with serofibrinous exudation containing erythrocytes (arrows) and epithelial regeneration (arrowhead) at the incision line in the control group where only experimental oral mucositis was induced, Control Group, oral mucosa, Rat, HE, Scale bar = 100 µm. (b) Severe hemorrhagic and necrotic foci (thick arrow), bacterial colonies (stars), vascular hyperemia in the lamina propria (thin arrow) and inflammatory cell infiltration in the interstitial connective tissue at the infected wound site (arrowhead) in Group I rats with experimental oral mucositis after EA administration for the first 5 days. Group I, oral mucosa, Rat, Scale bar = 100 µm. (c) Bacterial colonies (asterisks) with mononuclear cell infiltration around the necrotic area (arrowheads) and hyperemia in the basal lamina propria (arrow) in Group II rats given EA for 5 days after experimental oral mucositis was induced. Group II, oral mucosa, Rat, HE, Scale bar = 100 µm.
Immunohistochemical (IHC) staining
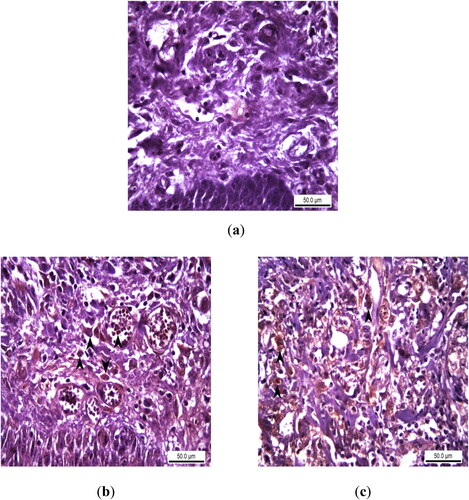
In immunohistochemical (IHC) staining, no TNF-α expression indicating acute inflammation was found in the epithelial layers and basement membranes of the samples of Control Group. In the infected incision sites of the samples of the Experimental Group I, TNF- α expression was observed at various intensities, especially in the interstitial area in the lamina propria and in the cytoplasm of the inflammatory cells within the vessel (). Similar to the Experimental Group I, TNF-α expression of varying severity was observed in the Experimental Group II, partly in the epithelium and mostly in the inflammatory cells in the lamina propria ().
Figure 3. Immunohistochemical analysis of TNF alpha expression as an acute inflammation biomarker in rats with experimental oral mucositis and investigating the therapeutic effect of EA. (a) In the control group where experimental oral mucositis was induced, TNF alpha expression was not observed in the epithelial layer and lamina propria. Control Group, oral mucosa, Rat, IHC, Scale bar = 50 µm; (b) TNF alpha expression (marked by arrowheads) in the epithelial cells, lamina propria and intravascular inflammatory cells of rats in Group I, where experimental oral mucositis was induced after the first 5 days of EA administration. Group I, oral mucosa, Rat, IHC, Scale bar = 50 µm; (c) TNF alpha expression (marked by arrowheads) in the cytoplasm of inflammatory cells in the lamina propria of rats in Group II, who received EA for 5 days following the induction of experimental oral mucositis. Group II, oral mucosa, Rat, IHC, Scale bar = 50 µm.
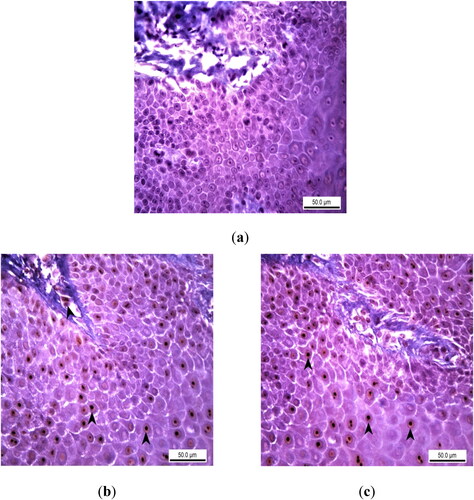
In immunohistochemical staining, no expression of 8-oxo-2-deoxyguanosine (8-OHdG), which indicates the presence of DNA damage and oxidative stress, was found in the epithelial layer and basement membranes of the samples of the Control Group. 8-OHdG expression of varying intensity was detected in the epithelial layers of the samples of the Experimental Group I, partially in the inflammatory cells in the lamina propria (). Similar to the Experimental Group I, 8-OHdG expression was observed in the nuclei of epithelial cells, especially in the lamina epithelium of the oral mucosa in the Experimental Group II ().
Figure 4. Immunohistochemical analysis of 8-OHdG expression as a DNA oxidation biomarker in rats with experimental oral mucositis investigating the therapeutic effect of EA. (a) In the control group where experimental oral mucositis was induced, 8-OHdG expression was not observed in the epithelial layer and lamina propria. Control Group, oral mucosa, Rat, IHC, Scale bar = 50 µm. (b) 8-OHdG expression (marked by arrowheads) in the nuclei of epithelial cells and inflammatory cells in the lamina propria of rats in Group I, where experimental oral mucositis was induced after the first 5 days of EA administration. Group I, oral mucosa, Rat, IHC, Scale bar = 50 µm. (c) 8-OHdG expression (marked by arrowheads) in the nuclei of epithelial cells of rats in Group II, who received EA for 5 days following the induction of experimental oral mucositis. Group II, oral mucosa, Rat, IHC, Scale bar = 50 µm.
In the histopathological examination of the groups, no statistical difference was found between the rates of regeneration (p = 0.725), repair (p = 0.370), inflammation (p = 0.062) and secondary infection (p = 0.077) results (). There was a statistical difference between the results of TNF-α staining according to the immunoreaction status in the groups (p = 0.043) (). There was a statistical difference between the results of 8-OHdG staining (p = 0.019). This difference is due to the difference in the rates of the “No expression” result according to the groups. In 8-OHdG staining, 83.3% of the Control Group showed “No expression”; whereas, 33.3% of Experimental Groups I and II showed “Mild immunoreaction” ().
Table 1. Statistical analysis of histopathologic scores in the experimental groups.
Table 2. Scoring of immunohistochemical findings of acute inflammation biomarker TNF alpha expression and DNA damage biomarker 8-OHdG expression in the control and experimental groups.
Discussion
In this study, after inducing mucositis in the cheek mucosa of rats via 5-FU induction, the effect of EA on soft tissue healing was evaluated. TNF-α and 8-OHdG were used to evaluate both infection status and DNA oxidation in cells.
Laser therapy, cryotherapy, professional oral hygiene, antimicrobial agents, royal jelly, Lactobabillus brevis lozenges, Zinc supplementation, and Benzydamine have shown to be the best treatment and/or prevention methods for OM [Citation12, Citation13]. As a result of studies on these treatments, it has been reported that Palifermin (Keratinocyte Growth Factor) [Citation14], Chlorhexidine Gluconate [Citation15], Smecta (Antidiarrhea) [Citation12], Actovegin (Antihypoxane) [Citation16], Kangfuxin (Ethanol Extract) [Citation17], royal jelly [Citation18], zinc supplement, Benzydamine, cryotherapy, L. brevis lozenges [Citation12], laser therapy [Citation14] and professional oral hygiene [Citation19] can be used in the treatment and prevention of OM [Citation12–20]. OM treatment options have been explored with extracts obtained from natural products. EA was the focus of our study because it has components with potential to improve OM.
Uthaiwat et al. [Citation21] evaluated the therapeutic efficacy of topical Melatonin Niosome Gel (MNG) in mice with OM induced by 5-FU. They reported that MNG reduced infection and lipid oxidative stress in 5-FU-induced OM. Fonseca et al. [Citation22] showed that L-cysteine decreased inflammation levels in OM induced by 5-FU in hamsters. Positive results have been seen by adding L-cysteine to the diet of patients treated with chemotherapeutic agents such as 5-FU [Citation22]. They concluded that this would be hope for cancer patients. Çelebi et al. [Citation4] evaluated the therapeutic properties of colostrum in rats with OM induced by 5-FU, but no statistically significant result was found as a result of the study. Immunohistologically, secondary infection was detected. Similarly, in our study conducted with EA, since the formation of OM with 5-FU affected the general health status of rats, it was more clearly seen that wound healing was accompanied by secondary infection.
The main reason for using EA (2,3,7,8-tetrahydroxy-chromeno [5,4,3-cde]-chromene5,10-dione) in this study is its antioxidant properties. This is clearly seen when we examine its chemical structure. The EA structure emerged with the succession of four hydroxyl groups and two lactone functional groups. The presence of more than two hydroxyl groups in components such as EA places it in the group of phenolic to polyphenolic compounds. EA has strong antioxidant properties because it has two double hydroxyl groups [Citation23]. Barch et al. [Citation24] reported that the anti-carcinogenic effect of EA was also due to these two double hydroxyl groups. At the same time, due to the role of EA as an anticancer drug, Zhang et al. [Citation25] showed that it is a potent anticancer agent for future research. Oxidative stress, which is caused by various causes, is a condition in the human natural system that causes various chronic diseases. For this reason, oxidative stress can be addressed with antioxidants such as EA, which are found in fruits and vegetables in nature and are easily obtained [Citation26].
There is important evidence that infection in the body of patients receiving radiation and/or chemotherapy treatment plays an effective role in the development and progression of OM (For review, see [Citation27]). There is a significant increase in inflammatory cell infiltrate in OM. Furthermore, mRNA levels of TNF-α and IL-1β in oral mucosal tissue are correlated with the severity of mucosal damage [Citation28]. A non-steroidal anti-inflammatory drug (NSAID) was used, which has been shown to inhibit the production of inflammatory cytokines such as TNF-α and IL-1β. Therefore, guidelines include a recommendation for the use of mouthwash containing NSAIDs to prevent OM in patients with head and neck cancer receiving moderate doses of radiotherapy [Citation27–Citation29]. In our EA study, it was observed that TNF-α expression occurred at various intensities in the Experimental Groups, partially in the epithelium and mostly in the inflammatory cells in the lamina propria.
The release of 8-OHdG, which is a result of oxidative stress and can cause changes in the DNA structure, is proof of these harmful effects. Damage to DNA can lead to mutations that cause cancer and other diseases. EA has been shown to significantly reduce the amount of 8-OHdG produced after oxidative DNA damage, which is significantly correlated with DNA binding capacity [Citation30]. This proves that EA provides a protection mechanism against free radicals, that is, against oxidative stress. For example, when EA (58.33 mg/kg body weight) was administered to a mouse model of adjuvant-induced arthritis, proinflammatory cytokines IL-1 β, TNF-α and serum levels of IL-17 were reported to be significantly reduced [Citation31, Citation32]. In our study, there was no 8-oxo-2-deoxyguanosine (8-OHdG) expression, which indicates the presence of DNA damage and oxidative stress in the epithelial layer and basement membranes of the samples of the Control Group. 8-OHdG expression of varying intensity was detected in the epithelial layers of the samples of Experimental Group I, partially in the inflammatory cells in the lamina propria. Similar to the Experimental Group I, 8-OHdG expression was observed in the Experimental Group II in the nuclei of epithelial cells, especially in the lamina epithelium of the oral mucosa. It is not possible to conclude that EA reduces oxidative stress in this sense.
Some studies have been conducted on the anticancer effect of EA on many human cancer cells. EA plays an anti-proliferative role in skin, esophageal and colon cancer cells cancer by disrupting the cell cycle and by its apoptosis ability. EA is an antioxidant used in recent studies for symptom relief of chronic diseases such as ulcerative colitis, Crohn’s disease, Alzheimer’s disease and diabetes (Reviewed in [Citation33]).
This study investigated the effect of EA on the healing of OM induced by 5-FU in rats. It is noteworthy that the effect on wound healing was not statistically significant and secondary infection occurred in the epithelial layers. Since 5-FU is an effective chemotherapeutic agent, it is thought that systemic exposure of rats to 5-FU may have contributed to the secondary infection.
Conclusions
EA had positive effects on the treatment of OM. However, due to the chemotherapeutic agent used, the immunity and nutrition of the rats were affected and therefore unexpected inflammations occurred. In order to obtain clearer results on OM, further investigations are necessary that will expand upon and support our study.
Authors’ Contributions
Conceptualization, Visualization, Validation, E.E.Ç, A.Ç and A.K.; Data curation, Formal Analysis, E.E.Ç, A.Ç and M.B.D; Funding acquisition, Project administration, E.E.Ç; Methodology, H.Y. and M.B.D.; Writing – original draft, Writing – review & editing, Investigation, Supervision, Resources, Software, E.E.Ç and A.Ç. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors would like to thank Bingol University Scientific Research Projects Coordination Unit for their financial support.
Disclosure statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Vera-Llonch M, Oster G, Ford CM, et al. Oral mucocytis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer. 2007;15(5):1–9.
- Keefe DM, Schubert MM, Elting LS, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109(5):820–831.
- Silva GB, Sacono NT, Othon-Leite AF, et al. Effect of low-level laser therapy on inflammatory mediator release during chemotherapy-induced oral mucositis: a randomized preliminary study. Lasers Med Sci. 2015;30(1):117–126.
- Çelebi A, Dörtbudak MB, Keskinrüzgar A, et al. The therapeutic effect of bovine colostrum on 5-Fluorouracil-Induced oral mucositis in rats. J Stomatol Oral Maxillofac Surg. 2022;123(6):e682–e686. [GoogleScholar]
- Hong BY, Sobue T, Choquette L, et al. Chemotherapy-induced oral mucocytosis is associated with detrimental bacterial dysbiosis. Microbiome. 2019;7(1):1–18.
- Anderson P, Kaye L. The therapeutic alliance: adapting to the unthinkable with better information. Health Commun. 2009;24(8):775–778.
- García-Niño WR, Zazueta C. Ellagic acid: pharmaco- logical activities and molecular mechanisms involved in liver protection. Pharmacol Res. 2015;97:84–103.
- Pari L, Sivasankari R. Effectofellagicacidoncyclosporine A-induced oxidative damage in the liver of rats. Fundam Clin Pharmacol. 2008;22(4):395–401.
- Al-Obaidi MMJ, Al-Bayaty FH, Al Batran R, et al. Impact of ellagic acid in bone formation after tooth extraction: an experimental study on diabetic rats. Sci World J. 2014;2014:1–14. doi: 10.1155/2014/908098.
- Turk G, Çeribaşı AO, Sakin F, et al. Antiperoxidative and anti-apoptotic effects of lycopene and ellagic acid on cyclophosphamide-induced testicular lipid per- oxidation and apoptosis. Reprod Fertil Dev. 2010;22(4):587–596. doi: 10.1071/RD09078.
- Priyadarsini KI, Khopde SM, Kumar SS, et al. Free radical studies of ellagic acid, a natural phenolic antioxidant. J Agric Food Chem. 2002;50(7):2200–2206.
- Daugėlaitė G, Užkuraitytė K, Jagelavičienė E, et al. Prevention and treatment of chemotherapy and radiotherapy induced oral mucositis. Medicina. 2019;55(2):25.
- Bayer S, Kazancioglu HO, Acar AH, et al. Comparison of laser and ozone treatments on oral mucositis in an experimental model. Lasers Med Sci. 2017;32(3):673–677.
- Vadhan‐Raj S, Goldberg JD, Perales MA, et al. Clinical applications of palifermin: amelioration of oral mucositis and other potential indications. J Cell Mol Med. 2013;17(11):1371–1384. [Google Scholar] doi: 10.1111/jcmm.12169.
- Diaz-Sanchez RM, Pachón-Ibáñez J, Marín-Conde F, et al. Double-blind, randomized pilot study of bioadhesive chlorhexidine gel in the prevention and treatment of mucositis induced by chemoradiotherapy of head and neck cancer. Med Oral Patol Oral Cir Bucal. 2015;20(3):e378–e385. [Google Scholar] doi: 10.4317/medoral.20338.
- Wu SX, Cui TT, Zhao C, et al. A. prospective, randomized, multi-center trial to investigate actovegin in prevention and treatment of acute oral mucositis caused by chemoradiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2010;97(1):113–118. [Google Scholar] doi: 10.1016/j.radonc.2010.08.003.
- Baharvand M, Jafari S, Mortazavi H. Herbs in oral mucositis. J Clin Diagn Res. 2017;11(3):ZE05–ZE11. doi: 10.7860/JCDR/2017/21703.9467.
- Erdem O, Güngörmüş Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist Nurs Pract. 2014;28(4):242–246. [Google Scholar] doi: 10.1097/HNP.0000000000000033.
- Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52(1):61–77, viii. [Google Scholar] doi: 10.1016/j.cden.2007.10.002.
- Wang L, Gu Z, Zhai R, et al. Efficacy of oral cryotherapy on oral mucositis prevention in patients with hematological malignancies undergoing hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials. PLoS ONE. 2015;10(5):e0128763.doi: 10.1371/journal.pone.0128763.
- Uthaiwat P, Daduang J, Priprem A, et al. Topical melatonin niosome gel for the treatment of 5-FU-induced oral mucositis in mice. CDD. 2021;18(2):199–211.
- Fonseca KM, Macda RodriguesCosta D, Da Silva VF, et al. Anti-inflammatory effect of L-cysteine (a semi-essential amino acid) on 5-FU-induced oral mucositis in hamsters. Amino Acids. 2021;53(9):1415–1430.
- Zafrilla P, Ferreres F, Tomás-Barberán FA. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (rubus idaeus) jams. J Agric Food Chem. 2001;49(8):3651–3655.
- Barch DH, Rundhaugen LM, Stoner GD, et al. Structure-function relationships of the dietary anticarcinogen ellagic acid. Carcinogenesis. 1996;17(2):265–269.
- Zhang HM, Zhao L, Li H, et al. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol Med. 2014;11(2):92–100.
- Zeb A. Ellagic acid in suppressing in vivo and in vitro oxidative stresses. Mol Cell Biochem. 2018;448(1–2):27–41.
- Nicolatou-Galitis O, Sarri T, Bowen J, et al. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21(11):3179–3189.
- Sonis ST, Peterson RL, Edwards LJ, et al. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36(4):373–381. [Google Scholar] doi: 10.1016/s1368-8375(00)00012-9.
- Bowen JM, Elad S, Hutchins RD, et al. Methodology for the MASCC/ISOO mucositis clinical practice guidelines update. Support Care Cancer. 2013;21(1):303–308.
- Srinivasan P, Vadhanam MV, Arif JM, et al. A rapid screening assay for antioxidant potential of natural and synthetic agents in vitro. Int J Oncol. 2002;20(5):983–986.
- Allam G, Mahdi EA, Alzahrani AM, et al. Ellagic acid alleviates adjuvant-induced arthritis by modulation of pro- and anti-inflammatory cytokines. Central-Eur J Immunol. 2016;41:339–349.
- Sharifi-Rad J, Quispe C, Castillo CMS, et al. Ellagic acid: a review on its natural sources, chemical stability, and therapeutic potential. Oxid Med Cell Longevity. 2022;2022:1–24. doi: 10.1155/2022/3848084.
- Derosa G, Maffioli P, Sahebkar A. Ellagic acid and its role in chronic diseases. Anti-Inflamm Nutraceuticals Chronic Dis. 2016;928:473–479.