Abstract
Soluble receptors are important for the balance between ligands and their membrane receptors. Changes in the expression of soluble receptors are associated with human diseases. The aim of this study was to determine the serum levels of sRAGE, sRANKL and OPG in subgroups of rheumatoid arthritis (RA) patients according to the level of CRP and to assess the relationship of these parameters with markers of iron and disease status. The study involved 114 RA patients. The levels of sRAGE, sRANKL and OPG were higher in the subgroup with increased CRP level compared to the subgroup with normal CRP. sRAGE showed a significant positive correlation with sTfR (r = 0.435, p < 0.0001), prohepcidin (r = 0.232, p = 0.04), sRANKL (r = 0.636, p < 0.0001), RF (r = 0.363, p < 0.002), and antiCCP antibodies (r = 0.429, p = 0.003) in the subgroup with normal CRP. Serum sRANKL was positively associated with sRAGE (r = 0.636, p < 0.0001), sTfR (r = 0.513, p < 0.0001), CRP (r = 0.223, p = 0.048), DAS28 (r = 0.269, p = 0.016), RF (r = 0.390, p = 0.001) and antiCCP antibodies (r = 0.445, p = 0.002) also in the subgroup with normal CRP. Serum OPG was positively correlated with ferritin in the subgroup with normal CRP. The association of sRAGE, sRANKL and OPG with markers of iron and disease status in RA suggests a relationship between inflammatory state, osteoclast activation and impaired iron and immune status. Therefore, sRAGE, sRANKL and OPG can be useful markers for the assessment of early pathological changes in RA and can also assist in monitoring of the therapy.
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic and progressive autoimmune disease, in which chronic inflammation of the synovial tissue of the joints damages articular cartilages, bones and other joint structures. The global prevalence of RA is approximately 0.3% and 2% of the general population, and it is estimated that 0.5% of the Bulgarian adult population is affected by RA. The disease affects 2–3 times more women than men and is a serious, worldwide public health concern [Citation1–3]. Different factors may cause the development of RA, including genetic factors such as the presence of MHC (major histocompatibility complex) antigens, particularly HLA-DR4 antigens, environmental factors such as viral and bacterial infections, smoking and immune disorders [Citation1, Citation4].
The diagnosis of RA relies on a range of blood tests, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level, rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Recently, researchers have shown an increased interest in novel biomarkers for the differential diagnosis of RA in the early stages of the disease that will provide more information and will help in the development of effective therapeutic strategies.
Many membrane-bound cellular receptors have soluble forms that are released into the circulation. Soluble receptors affect the balance between ligands and their membrane receptors, and the changes in their expression are associated with human diseases. Therefore, the serum levels of different soluble receptors can be a useful marker for early pathological changes in different diseases such as RA and can assist in monitoring of the therapy [Citation5].
The receptor for advanced glycation end products (RAGE) is a cell surface multiligand receptor, a member of the immunoglobulin superfamily involved in the activation of the inflammatory process. RAGE is expressed by various cells, such as inflammatory cells, neutrophils, lymphocytes and synovial cells. RAGE has a secretory splice isoform, soluble RAGE (sRAGE) that circulates in plasma where it can bind RAGE ligands and inhibit RAGE activation and signaling [Citation6–11]. The alterations in sRAGE levels in rheumatoid arthritis are reported in previous studies [Citation6, Citation8, Citation12, Citation13]. However, it is not clear what factors influence sRAGE levels in RA, but little is known about the relationship between sRAGE and disease characteristics.
The receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL), also called osteoclast differentiation factor, is a key factor that controls osteoclast differentiation and activity. RANKL is a member of the TNF superfamily of cytokines. The binding of RANKL to its receptor RANK triggers signaling pathways that activate several transcriptional factors required for osteoclast differentiation, maturation and activation leading to increased bone resorption. RANKL/RANK signaling is controlled by osteoprotegerin (OPG). OPG is a soluble decoy receptor of RANKL, blocking RANKL/RANK interaction, inhibiting osteoclast formation and enhancing osteoclast apoptosis [Citation2, Citation13–17].
Patients with RA often develop iron deficiency and different types of anemia as some of the most common comorbidities. The causes of anemia in RA are complex and multifactorial [Citation1, Citation18–20]. In addition, in the presence of chronic inflammation, the major form of systemic hypoferremia is anemia of chronic disease (ACD) in which there is a retention of iron within the body stores. Proinflammatory cytokines (mainly IL-6) stimulate the synthesis of hepcidin in hepatocytes which disables the action of the iron exporter ferroportin, resulting in a decrease in serum iron levels [Citation1, Citation21–24]. Some of the markers used for the clinical assessment of iron status include hemoglobin, ferritin, total iron binding capacity (TIBC), serum iron, soluble transferrin receptor (sTfR) and hepcidin [Citation25, Citation26]. Previous studies report that systemic hypoferremia can affect bone metabolism [Citation21, Citation27] but little is known about the association between the functional iron deficiency and markers reflecting bone and cartilage destruction in rheumatoid arthritis.
Previously, our group reported that sRAGE, sRANKL and OPG serum levels were associated with inflammation, iron metabolism and diagnostic markers in RA patients [Citation22]. The present study aimed to determine how the serum levels of sRAGE, sRANKL and OPG are altered in subgroups of these RA patients distributed according to the level of CRP and to assess the relationship of these parameters with markers of iron and disease status.
Subjects and methods
Ethics statement
The research was approved by the Ethic Committee at the Medical University of Plovdiv (protocol No. 4/18.07.2013) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Subjects
The study population consisted of 114 patients with RA (16 male and 98 female, mean age 58 ± 10 years) admitted to the Rheumatology departments of the University hospitals in Medical University of Plovdiv in the period from July 2013 to January 2015 [Citation22, Citation23]. All patients were diagnosed as having RA according to EULAR (European Alliance of Associations for Rheumatology) 2010 criteria. Inclusion criteria were: women and men aged between 18 and 80 years who fulfilled the EULAR criteria for RA. The exclusion criteria were: patients with psoriatic arthritis, systemic lupus erythematosus, patients with RA having renal or hepatic impairment, hemolytic anemia, blood transfusions within the past three months, cancer patients currently receiving chemotherapy and patients currently taking iron supplements. As previously described [Citation22, Citation23], all patients were receiving NSAIDs and additional therapy as follows: DMARDs (n = 83), DMARDs and corticosteroids (n = 10), biological agents, DMARDs and corticosteroids (n = 4), corticosteroids (n = 2), biological agents and DMARDs (n = 11), biological agents and corticosteroids (n = 3). The same RA patients were included in our previous studies [Citation22, Citation23], but in the present study, these patients were distributed into two subgroups according to the level of CRP. CRP value 8 µg/mL was used as a cut-off point. The first group was composed of 79 patients with normal CRP and the second group was made up of 35 patients with increased CRP.
Biochemical analysis
Serum concentrations of sRAGE, pg/mL; sRANKL, pmol/L; osteoprotegerin, pmol/L; ferritin, ng/mL; sTfR, µg/mL; CRP, µg/mL; IL-6, pg/mL were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (BioVendor—Laboratorni medicina, Brno, Czech Republic, Cat No RD191116200R, RD193004200R, RD194003200, DKO039, RD194011100, 740001, RD194015200R). The concentrations of serum prohepcidin (ng/mL) were measured by ELISA using a commercial kit (DRG Instruments, GmbH, Marburg, Germany, Cat No EIA4644), according to the manufacturer’s instructions. RF (U/mL) was determined with ELISA kits (Nova Tec Immundiagnostica, GmbH, Germany, Cat No RFM3010), and antiCCP antibodies (U/mL) with ELISA kits (Eurodiagnostica, Sweden, Cat No RA-96 PLUS). Non-fasting serum samples were incubated in a 96-well plate precoated with a monoclonal or polyclonal antibody directed towards the antigen. After 2 h of incubation and washing with phosphate buffer, biotin labelled antibody was added and incubated for 1 h with the captured protein. After another washing, streptavidin-HRP conjugate was added. After 30 min incubation and the last washing step, the remaining conjugate was allowed to react with the substrate solution (TMB). The reaction was stopped by the addition of acidic solution and the absorbance of the resulting yellow product was measured [Citation6, Citation8, Citation16]. A standard curve was constructed by plotting absorbance values versus concentrations of standards, and concentrations of unknown samples were determined using this standard curve. The measurements were performed on ELISA reader HumaReader HS, HUMAN (Wiesbaden, Germany).
Data analysis
Statistical analysis was performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). Normal distribution was assessed using the Kolmogorov-Smirnov test and continuous variables were expressed as mean ± SD or as median and 25th-percentile—75th-percentile. Student’s t-test was used to compare two groups with Gaussian distribution and Mann-Whitney U test was used to compare groups with non-Gaussian distribution. Correlations between data were evaluated by calculating Pearson’s or Spearman’s correlation coefficient depending on the distribution of the continuous variables. Differences were considered statistically significant at the p < 0.05 level.
Results
The present study included a total of 114 patients with RA distributed in subgroups according to CRP. The main demographic and clinical characteristics of the study groups are summarized in .
Table 1. Demographic and clinical characteristics of the study groups.
The two study groups included more women than men. The study group with normal CRP consisted of 79 patients with RA (12 men and 67 women), mean age 57.3 ± 10.0 years and the subgroup of patients with increased CRP consisted of 35 patients (4 men and 31 women), mean age 58.9 ± 10.5 years.
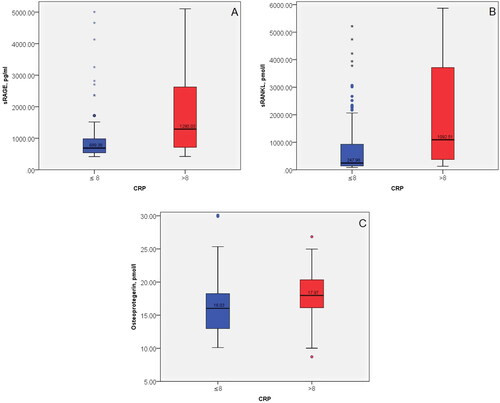
The serum sRAGE and sRANKL were significantly higher in inflammation (CRP > 8) in comparison to the group with normal CRP (CRP ≤ 8), p = 0.006 and p < 0.0001, respectively. The level of OPG showed a trend to increase in inflammation ().
Figure 1. Comparison of sRAGE (A), sRANKL (B) and OPG (C) in subgroups of RA patients according to the level of CRP (CRP ≤ 8 and CRP > 8).
Significant positive correlations of sRAGE with sTfR and sRANKL were observed, independently of CRP level, since they were found in both groups. Positive correlations of sRAGE with prohepcidin, RF and antiCCP antibodies were observed only at CRP ≤ 8 but not in the group with increased CRP ().
Table 2. Correlations of sRAGE in subgroups of RA patients according to the level of CRP (CRP ≤ 8 and CRP > 8).
sRANKL was positively associated with sRAGE, sTfR and RF independently of the CRP level since the correlations were observed in both study groups. Significant positive correlations of sRANKL with CRP, DAS28 and antiCCP antibodies were observed only in the group with CRP ≤ 8 ().
Table 3. Correlations of sRANKL in subgroups of RA patients according to the level of CRP (CRP ≤ 8 and CRP >8).
The results of OPG associations with the study parameters are shown in . OPG was positively correlated with ferritin only in the group with CRP ≤ 8 and not in the group with inflammation ().
Table 4. Correlations of OPG in subgroups of RA patients according to the level of CRP (CRP ≤ 8 and CRP > 8).
Discussion
In our previous study, we have reported a comparison of sRAGE, sRANKL and OPG levels in a general group of patients with RA and healthy controls and the relationship between these parameters and markers of iron homeostasis, inflammation and autoimmune disorders [Citation22]. To investigate the effect of inflammation on the associations of these parameters with biomarkers of iron status and disease indicators, in the present study we focused on the serum levels of sRAGE, sRANKL and OPG in subgroups of rheumatoid arthritis patients according to the level of CRP. sRAGE was significantly higher in the group with CRP > 8 compared to the group with normal CRP. These findings probably indicate a relationship of sRAGE with the increased inflammatory activity. The elevated sRAGE concentration may be attributed to the increased inflammatory status and probably reflects high RAGE expression in tissues during inflammation [Citation28]. Nakhjavani et al. [Citation6] report higher levels of sRAGE in RA patients with high and moderate DAS-28 scores versus patients in remission, and Nadali et al. [Citation8] have found that sRAGE in female patients with RA was higher compared to patients with CVD. sRAGE levels were associated with inflammation including disease activity and IL-6 [Citation8].
The correlation between sRAGE and sTfR is observed in the subgroups according to CRP, which supports the statement that this relationship has important biological significance under normal and pathological conditions. The association of sRAGE with iron homeostasis is also supported by the positive correlation between sRAGE and prohepcidin observed in the group with normal CRP.
Similarly to sRAGE, sRANKL was also increased in inflammation (CRP >8). These findings indicate that sRANKL is a marker that is sensitive to inflammation. Previous research has indicated that CRP triggers inflammatory responses and has an impact on sRANKL levels and osteoclastogenesis [Citation29].
The changes in sRANKL serum levels represent the impaired iron homeostasis in RA since sRANKL is positively associated with sTfR in the subgroups according to CRP level. These results suggest parallel changes in bone structure due to the increased osteoclast activation and manifestation of hypoferremia caused by the chronic inflammatory process.
sRAGE and sRANKL are associated with the disease status in RA assessed by the determination of their correlation with specific immunologic markers. Both receptors were strongly and positively correlated with RF and antiCCP antibodies in the subgroup of RA patients with normal CRP. Such correlations were observed also in the whole group of RA patients [Citation22]. sRANKL was positively associated with disease activity measured by DAS28 only in the group with normal CRP. These results demonstrate that sRAGE and sRANKL could be considered as parameters that reflect the pathological changes in RA induced by chronic inflammation.
The distribution of RA patients according to CRP level showed a positive correlation between OPG and ferritin observed only at normal CRP and it was not found in inflammation (CRP > 8), when ferritin serves as an acute phase protein. A previous study has indicated the effects of iron on osteoclastogenesis and osteoclast activity [Citation30]. Iron transferrin induces mitochondrial production of reactive oxygen species (ROS) in both osteoclast and osteoblast precursors and activation of CREB. This triggers signaling pathways that result in osteoblast and osteoclast differentiation.
Our results are consistent with those of other studies which report that RA patients have significantly higher circulating OPG levels compared to controls in association with disease activity, and specific variants of the OPG gene have been associated with RA susceptibility [Citation31–34].
The major limitation of our study is that the two subgroups of RA patients included more women than men and the female patients were pre- and postmenopausal women. Another limitation of this study is that both RANKL and OPG levels were assayed in serum and reflect their production by several tissues. Therefore, the circulating sRANKL and OPG levels may not precisely reflect the bone erosions in RA patients.
Conclusions
The levels of sRAGE, sRANKL and OPG were higher in the subgroup with increased CRP level compared to the subgroups with normal CRP and the difference in sRAGE and sRANKL levels was statistically significant between the study groups. The association of sRAGE, sRANKL and OPG with markers of iron (sTfR, prohepcidin and ferritin) and disease status (RF, antiCCP antibodies) in RA suggests a relationship between the inflammatory state, osteoclast activation and the impaired iron and immune status. Therefore, sRAGE, sRANKL and OPG can be useful markers for assessment of early pathological changes and treatment efficacy in RA.
Authors’ contributions
Conceptualization, G.D. and K.S.; methodology and investigation, G.D., K.S. and T.S.; sample collection, G.D. and K.S.; sample preparation G.D., K.S. and T.S.; data analysis and interpretation, G.D., K.S. and T.S.; writing-original draft preparation, G.D. and K.S.; writing-review and editing, K.S. and T.S. All authors have read and agreed to the final version of the manuscript.
Acknowledgments
The authors would like to thank Prof. Ana Maneva, PhD, DSc (Department of Medical Biochemistry, Faculty of Pharmacy, Medical University of Plovdiv) for her assistance and support in the preparation of the manuscript.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, GD. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Tański W, Chabowski M, Jankowska-Polańska B, et al. Anaemia and iron deficiency in patients with rheumatoid arthritis and other chronic diseases. Postępy Hig Med Dośw. 2021;75(1):1–7. doi: 10.5604/01.3001.0014.7838.
- Jura-Półtorak A, Szeremeta A, Olczyk K, et al. Bone metabolism and RANKL/OPG ratio in rheumatoid arthritis women treated with TNF-α inhibitors. J Clin Med. 2021;10(13):2905. doi: 10.3390/jcm10132905.
- Marinov L, Nikolova I, Mitov K, et al. Quality of life of patients with rheumatoid arthritis in Bulgaria. Pharmacia. 2016;63(2):22–29.
- Tofigh R, Hosseinpourfeizi M, Baradaran B, et al. Rheumatoid arthritis and non-coding RNAs; how to trigger inflammation. Life Sci. 2023;315:121367. doi: 10.1016/j.lfs.2023.121367.
- Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64(2):135–146. doi: 10.1002/jlb.64.2.135.
- Jafari Nakhjavani MR, Jafarpour M, Ghorbanihaghjo A, et al. Relationship between serum-soluble receptor for advanced glycation end products (sRAGE) and disease activity in rheumatoid arthritis patients. Mod Rheumatol. 2019;29(6):943–948. doi: 10.1080/14397595.2018.1551107.
- Wu SE, Chiu YL, Kao TW, et al. Elevated level of the soluble receptor for advanced glycation end-products involved in sarcopenia: an observational study. BMC Geriatr. 2021;21(1):531. doi: 10.1186/s12877-021-02487-1.
- Nadali M, Lyngfelt L, Erlandsson MC, et al. Low soluble receptor for advanced glycation end products precedes and predicts cardiometabolic events in women With rheumatoid arthritis. Front Med (Lausanne). 2020;7:594622. doi: 10.3389/fmed.2020.594622.
- Knani I, Bouzidi H, Zrour S, et al. Methylglyoxal: a relevant marker of disease activity in patients with rheumatoid arthritis. Dis Markers. 2018;2018:8735926–8735926. doi: 10.1155/2018/8735926.
- Rzepka R, Dołęgowska B, Marczuk N, et al. Novel inflammatory markers of labor following premature preterm rupture of membranes. Clin Exp Obstet Gynecol. 2019;46(3):359–367. doi: 10.12891/ceog4607.2019.
- Rzepka R, Dołęgowska B, Sałata D, et al. Soluble receptors for advanced glycation end products and receptor activator of NF-ΚB ligand serum levels as markers of premature labor. BMC Pregnancy Childbirth. 2015;15(1):134. doi: 10.1186/s12884-015-0559-3.
- Chen YS, Yan W, Geczy CL, et al. Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoantibody, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis Res Ther. 2009;11(2):R39. doi: 10.1186/ar2645.
- Park MJ, Lee SH, Moon SJ, et al. Overexpression of soluble RAGE in mesenchymal stem cells enhances their immunoregulatory potential for cellular therapy in autoimmune arthritis. Sci Rep. 2016;6(1):35933. doi: 10.1038/srep35933.
- Yao Z, Getting SJ, Locke IC. Regulation of TNF-Induced osteoclast differentiation. Cells. 2021;11(1):132. doi: 10.3390/cells11010132.
- Remuzgo-Martínez S, Genre F, López-Mejiás R, et al. Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis. Sci Rep. 2016;6(1):29713. doi: 10.1038/srep29713.
- Fadda S, Hamdy A, Abulkhair E, et al. Serum levels of osteoprotegerin and RANKL in patients with rheumatoid arthritis and their relation to bone mineral density and disease activity. Egypt Rheumatol. 2015;37(1):1–6. doi: 10.1016/j.ejr.2014.06.001.
- Al-Rubaie HA, Al-Bayaa I, Al-Amiri Y. The value of soluble transferrin receptor and soluble transferrin receptor-ferritin ındex in discriminating ıron deficiency anaemia from anaemia of chronic disease in patients With rheumatoid arthritis. TORJ. 2019;13(1):9–14. doi: 10.2174/1874312901913010009.
- Jarlborg M, Gabay C. Systemic effects of IL-6 blockade in rheumatoid arthritis beyond the joints. Cytokine. 2022;149:155742. doi: 10.1016/j.cyto.2021.155742.
- Xu S, Wang Y, Lu J, et al. Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arthritis-induced osteoporosis. Rheumatol Int. 2012;32(11):3397–3403. doi: 10.1007/s00296-011-2175-5.
- Sato H, Takai C, Kazama JJ, et al. Serum hepcidin level, iron metabolism and osteoporosis in patients with rheumatoid arthritis. Sci Rep. 2020;10(1):9882. doi: 10.1038/s41598-020-66945-3.
- Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102(6):1585–1594. doi: 10.3945/ajcn.114.103366.
- Stefanova K, Delcheva G, Stankova T, et al. sRANKL, OPG and sRAGE as markers of bone metabolism in rheumatoid arthritis: relation to indicators of impaired iron homeostasis and inflammation. CR Acad Bulg Sci. 2021;74(8):1238–1246. doi: 10.7546/CRABS.2021.08.16.
- Stefanova K, Delcheva G, Maneva A, et al. Pathobiochemical mechanisms relating iron homeostasis with parameters of inflammatory activity and autoimmune disorders in rheumatoid arthritis. Folia Med (Plovdiv). 2018;60(1):124–132. doi: 10.1515/folmed-2017-0068.
- Wahle M. Anemia in patients with rheumatoid arthritis. Z Rheumatol. 2012;71(10):864–868. doi: 10.1007/s00393-011-0925-0.
- Smith JT, Schneider AD, Katchko KM, et al. Environmental factors ımpacting Bone-Relevant chemokines. Front Endocrinol (Lausanne). 2017;8(8):22. doi: 10.3389/fendo.2017.00022.
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809.
- Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790(7):682–693. doi: 10.1016/j.bbagen.2008.08.006.
- Pullerits R, D'Elia HF, Tarkowski A, et al. The decrease of soluble RAGE levels in rheumatoid arthritis patients following hormone replacement therapy is associated with increased bone mineral density and diminished bone/cartilage turnover: a randomized controlled trial. Rheumatology (Oxford). 2009;48(7):785–790. doi: 10.1093/rheumatology/kep079.
- Kim KW, Kim BM, Moon HW, et al. Role of C-reactive protein in osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther. 2015;17(1):41. doi: 10.1186/s13075-015-0563-z.
- Roodman GD. Osteoclasts pump iron. Cell Metab. 2009;9(5):405–406. doi: 10.1016/j.cmet.2009.04.005.
- Wang P, Li S, Liu LN, et al. Circulating osteoprotegerin levels are elevated in rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2017;36(10):2193–2200. doi: 10.1007/s10067-017-3747-x.
- Saidenberg-Kermanac’h N, Cohen-Solal M, Bessis N, et al. Role for osteoprotegerin in rheumatoid infammation. Joint Bone Spine. 2004;71(1):9–13. doi: 10.1016/S1297-319X(03)00131-3.
- Van Steenbergen HW, Van Der Helm-van Mil AHM. Osteoprotegerin as biomarker for persistence of rheumatoid arthritis. Rheumatology (Oxford). 2016;55(5):949–950. doi: 10.1093/rheumatology/kev415.
- Arida A, Nezos A, Papadaki I, et al. Osteoprotegerin and MTHFR gene variations in rheumatoid arthritis: association with disease susceptibility and markers of subclinical atherosclerosis. Sci Rep. 2022;12(1):9534. doi: 10.1038/s41598-022-13265-3.
