Abstract
Plant diseases and insect pests cause tremendous losses in agriculture, forestry and fruit tree production worldwide. The continuous application of chemical pesticides in the control of pests and diseases is increasingly becoming undesirable due to the associated health risks, environmental pollution and pesticide resistance. Biological control strategies are generally safer and more sustainable alternatives in plant disease and insect pest management. Bacillus species have been widely studied and commercialized as biocontrol agents, due to their ability to produce a wide range of versatile antimicrobial lipopeptides, polyketides, insecticidal toxins and the elicitation for induced systemic resistance (ISR). This review focuses on the biocontrol prospects of antimicrobial peptides from Bacillus sp. and the elicitation of ISR against plant diseases and insect pests of economic importance in agriculture, forestry and fruit tree production. The review summarizes the biocontrol reports of antimicrobial and insecticidal peptides from Bacillus sp. including lipopeptides, polyketides, bacterial volatile compounds and the elicitation of ISR against various phytopathogens and insect pests and the mechanisms involved in their antimicrobial/insecticidal activity and plant defence responses. This study will help researchers identify the gaps for future research in the application of Bacillus sp. in the eco-friendly management of plant diseases and insect pests.
Introduction
Plant diseases and insect pest damage cause tremendous losses in forestry and fruit production industry worldwide, by lowering the yield and quality of products, which has a negative impact on the livelihood of farmers by reducing the economic returns and hinders the afforestation efforts by lowering the survival of seedlings [Citation1,Citation2]. The use of chemical pesticides (fungicides, bactericides and insecticides) has been the main strategy for controlling pests and diseases since the mid-1990s, with most commercial farmers adopting a routine of pesticide application [Citation3–5]. However, the continuous use of chemical pesticides not only poses health and environmental risks, but the sub-lethal exposure of these chemicals has also led to an increasing challenge of pesticide resistance in various insect pests and phytopathogens of economic importance [Citation6–8]. The increasing awareness of such previously underestimated health and environmental risks caused by the continuous application of pesticides has stimulated strong consumer-based demand for eco-friendly and safer alternatives to chemical pesticides [Citation9–11].
The use of biological control agents (BCA) as an alternative to the detrimental use of chemical pesticides has attracted tremendous scientific interest as an environmentally friendly strategy for controlling plant diseases and insect pest damage in agriculture, forestry and fruit tree production [Citation12–14]. Unlike most chemical pesticides that often cause environmental contamination and the indiscriminate toxicity against non-target organisms [Citation10,Citation11,Citation15,Citation16], BCAs are considered safe and environmentally friendly since they do not leave toxic residuals in the plant and the environment [Citation13,Citation14]. While some fungal BCAs which can spread, stabilize and alter the soil microbiota [Citation17], the bacterial BCAs are both non-toxic and they quickly drop to natural levels after application because of the limited nutrient supply [Citation18] and due to the biological buffering of the environment [Citation19]. Various bacterial species, including Bacillus sp., Pseudomonas sp., Streptomyces sp., Lysobacter sp. and Seratia sp., produce antimicrobial and entomopathogenic metabolites of potential application in crop protection [Citation20–23]. However, more research is required to develop efficient and cost-effective utilization techniques and to discover and categorize new BCAs with a wide range of beneficial properties [Citation12,Citation20–24]. These BCAs have several bioactive properties that facilitate phytopathogenic antagonism, entomopathogenic effects and other plant-microbial interactions that are necessary for survival, competition and niche colonization [Citation21,Citation25,Citation26]. Some BCAs, especially Bacillus sp. produce metabolites that regulate plant-microbial interactions, including elicitor molecules for induced systemic resistance (ISR) to stimulate plant defence responses against diseases and insect pest damage [Citation27–32]. The biocidal activity of Bacillus sp. against phytopathogens and insect pests is attributed to the prolific production of a wide range of organic compounds such as lipopeptides (LPs) [Citation33–35], polyketides (PKs) [Citation36–38], bacterial volatile compounds (BVCs) [Citation39–41], hydrolytic enzymes [Citation39,Citation42–47] and other organic macromolecules such as protein toxins [Citation48,Citation49]. Besides the production of antimicrobial metabolites, Bacillus species have unique ecological adaptability that enhances their survival, colonization and successful competition against other competing microbes. According to Bonaterra et al. [Citation21], the efficacy of bacterial biocontrol agents depends on the mechanisms of action, the environmental (abiotic) factors (such as chemical residues, nutrient availability, temperature and moisture), the rate and methods of application and biotic factors such as the target phytopathogen susceptibility, physical and chemical properties of the host plant (including potential hypersensitivity, plant microbial interactions and niche modifications). The successfulness of Bacillus species as a biocontrol agent is based not only on the potential to produce a diverse range of antimicrobial arsenal but also on the successful adaptation to diverse environments, rapid proliferation, colonization and secretion of stable and effective antimicrobial/insecticidal metabolites under various conditions [Citation21]. For instance, Bacillus species produce spores that are highly resistant to adverse environmental conditions such as heat, pressure, salinity and pH stress, which eases their handling and processing; they suppress competing microbes through siderophores and biofilm formation and have a special ability to solubilize essential nutrients which enhances their survival and symbiotic relations [Citation50–53]. Consequently, Bacillus species have been widely studied and reviewed. However, most of the earlier research was focused on the role of insecticidal toxins from entomopathogenic Bacillus thuringiensis (Bt), which also takes the biggest share of biocontrol products on the market [Citation54]. Recently, research attention has been extended to other Bacillus species and their role in plant-microbial interaction of potential benefit in agriculture, forestry and fruit tree production. However, there is still limited and unconsolidated knowledge of their potential applications in the field of forestry and fruit tree production. This review provides insights into such Bacillus species and the potential for field application, especially in forestry and fruit tree production based on their biocontrol efficacy through the production of lipopeptides, polyketides, volatile compounds and potential elicitation of ISR that has been demonstrated against phytopathogens and insect pests in a wide range of economically important agricultural crops, forestry and fruit trees.
The prospect of antimicrobial lipopeptides from Bacillus species as biopesticides
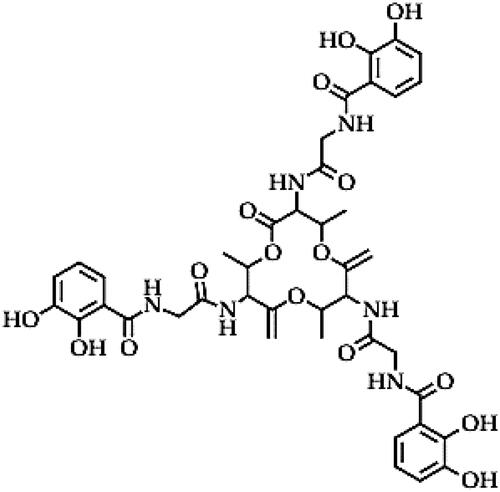
Surfactin (C53H93N7O13)
Surfactins are amphiphilic cyclic lipopeptides (), which are synthesized by non-ribosomal peptide synthetases (NRPSs) mega-enzymes, composed of heptapeptides of various amino acids interlinked with a β-hydroxy fatty acid to form diverse congeners [Citation55–58]. The surfactin family includes compounds such as surfactin, lichenysin and pumilacidin, which have strong antibiotic activity against numerous plant pathogens and pests [Citation35,Citation55,Citation59–62]. They have amphiphilic properties that facilitate them to act on the surface of the susceptible host cells, causing pore formation, disruption of cellular organelles and solubilization of the lipid bilayer in the cell membranes (through cation-carrier effect), resulting in swelling, roughness, apical outgrowths, distortions and protrusions of the cells to inhibit normal phytopathogenic growth [Citation59,Citation61]. Surfactins from Bacillus sp. play a vital role in the biocontrol of plant bacterial pathogens (), not only through direct antibiosis, but also via biofilm formation and facilitating the colonization of the phyllosphere and rhizosphere to suppress pathogenic infection. The long-held theory for the bactericidal/bacteriostatic activity of Surfactins was based on the suppressed production of exopolysaccharides and amyloid-like TasA protein in the extracellular matrix of the target bacterial pathogen [Citation59,Citation63]. The poor development and thus the disfunction of exopolysaccharides and amyloid-like TasA protein in the target bacteria makes the membrane highly porous, leading to loss of cellular contents (organelles) and the general loss of function, including the inability to reproduce and loss of pathogenicity. In their theory, Zeriouh et al. [Citation59] propose that biofilm formation facilitates effective colonization of the biocontrol strain (Bacillus sp.) on the surface of the host plant, which then ensure a relatively sustained production of other lipopeptides that have a more direct antimicrobial effect. However, this has recently been contradicted by another laboratory-based model which hypothesized that the biocontrol role of surfactins from Bacillus subtilis could be independent of biofilm formation but rather surfactins could play an important role in colony expansion of Bacillus sp., which enables them to exert their biocontrol effect [Citation63]. Despite the contrasting mechanisms, the role of surfactins produced by Bacillus sp. in the biocontrol of phytopathogens has not been disputed and their production has been demonstrated to be vital for the biocontrol activity of various Bacillus sp., especially B. subtilis strains.
Table 1. Summary of antimicrobial peptides from Bacillus strains for potential application in agriculture, forestry, and fruit tree management.
For example, B. subtilis strains NH-100 and NH-217 were reported to produce surfactin A which exhibited strong antifungal activity against fungal pathogens such as Fusarium oxysporum, Fusarium moniliforme and Fusarium solani that cause bakanae disease in rice [Citation64]. In their study, Sarwar et al. [Citation64] reported a mycelial growth inhibition rate of more than 80% at a surfactin concentration of 2000 ppm, but the inhibition rate dropped to less than 30% at a concentration of 200 ppm. This indicates that the antifungal activity of surfactins could be more reliable if high concentrations of the purified lipopeptide are applied. Park et al. [Citation61] described the chemical structure of surfactins with C15-lipid chain from Bacillus velezensis strain GH1-13, which exhibited strong antagonistic activity against Colletotrichum gloeosporioides fungal pathogen that causes anthracnose disease in various commercial crops. The surfactins from B. velezensis strain GH1-13 caused the disruption of chitin, glucans and glycoproteins components in the fungal cell wall, leading to loss of structural integrity in the cell wall [Citation61], while Bacillus pumilus strain PTB180 was reported for exclusive secretion of surfactins which effectively inhibited the mycelial growth and conidial germination of phytopathogenic fungi, Botrytis cinerea and subsequently suppressed grey mould disease in tomato [Citation65]. Other examples include the surfactins from B. velezensis strain SH-B 74, which exhibited strong antagonistic activity against Magnaporthe oryzae that causes rice blast disease. The surfactins from strain SH-B 74 caused the inhibition of appressoria formation on the fungal germ tubes, which consequently suppresses the pathogen virulence to prevent plant infection [Citation66]. Surfactin C from B. velezensis strain 1B-23 showed antagonistic activity against numerous phytopathogenic fungi such as Cochliobolus carbonum that causes northern leaf spot in maize, Cylindrocarpon destructans that causes root rot in ginseng, Fusarium sp., that cause Fusarium wilt, Monilinia fructicola causing brown rot in stone fruits and Rhizoctonia solani which causes stem and root rot (and dumping off) in various plants [Citation67]. Surfactin B and surfactin C from B. velezensis strain 9D-6 demonstrated antibacterial activity against Clavibacter michiganensis that causes ring rot/bacterial canker in tomatoes, Pseudomonas syringae that causes bacterial cankers in numerous fruit trees such as kiwifruit and exhibited antifungal activity against C. carbonum that causes fruit spots in pear [Citation68]. Surfactins have been reported to cause membrane depolarization of the producing bacteria (mainly B. sabtilis) to improve cell viability under oxygen-depleted conditions, which could be important for the continued survival endophytically or under anoxic environments [Citation69,Citation70]. Moreover, under normal oxygen conditions, surfactins also exhibit antibacterial activity through cytoplasmic membrane depolarization that causes cell membrane disintegration and osmotic pressure imbalance, preventing cell reproduction, and inhibiting pathogenic enzyme activity and protein synthesis, which could consequently prevent the normal cell metabolism of the target bacterial pathogens [Citation61,Citation71]. Some strains of B. velezensis such as BBC023 and BBC047 [Citation60] and MS20 [Citation27] have demonstrated multiple strategies of protecting plants from phytopathogens. Besides their role in biofilm formation which helps in the control of bacterial infections, the surfactins from these B. velezensis strains also exerted effective antifungal activity against phytopathogens such as R. solani that causes leaf/sheath blight in maize and B. cinerea which is known to affect numerous plants including tomatoes and grapes. They are proposed to be activated elicitors for induced systemic resistance in plants by enhancing plant defence enzymes [Citation27,Citation60]. The inhibition of mycelial and appressorial growth, as well as the induction of systemic resistance in plants by surfactins from Bacillus sp., lowers the rate of fungal and bacterial phytopathogenic infections [Citation27,Citation57,Citation59–61]. Some studies have demonstrated the role of surfactins as elicitors of induced systemic resistance against phytopathogenic infection in several plants which is presented in later section about induced systemic resistance [Citation32].
Iturins (C48H73N11O15)
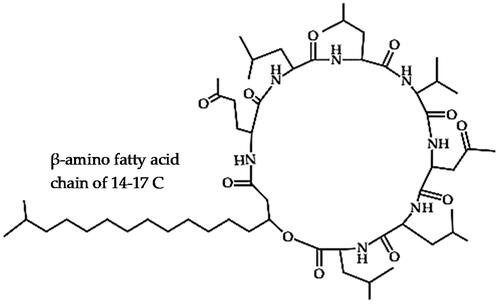
Iturins are heptapeptides linked to a β-amino fatty acid chain of 14–17 carbon atoms () and are a major family of antimicrobial lipopeptides secreted by different strains of Bacillus species that have potential applications in the biocontrol of numerous phytopathogens [Citation35,Citation72]. Iturins display a strong fungistatic activity by causing severe morphological distortions and conglobation in the hyphae and conidia, mainly due to their membrane permeabilization properties that lead to pore formation [Citation35,Citation73,Citation74]. Besides iturin bacillomycins, mycosubtilins and more recently mojavensin A are also categorized as members of the iturin family due to the structural similarities [Citation56,Citation75,Citation76].
For instance, iturin A purified from B. subtilis strain Z-14 was reported to induce the disappearance of the cell wall, degeneration of the cell membrane and the shrinkage of organelles and fragmentation of the hyphae, which consequently led to growth inhibition and suppressed the infection of Gaeumannomyces graminis var. tritici (Ggt), which causes wheat take-all disease [Citation77]. Iturin A from B. amyloliquefaciens strain S76-3 caused severe morphological changes in the conidial spores, distortion of the hyphae, as well as leakage and inactivation of cellular contents which resulted in a strong fungicidal effect against Fusarium graminearum, a notorious pathogen in various plants [Citation73]. Other strains include B. velezensis C16 which produced iturins that demonstrated effective inhibition of Alternaria solani by causing leakage of cytoplasmic components, which could indicate the formation of ion-conducting pores in the cell wall [Citation78]. This caused shrinkage of the protoplast, disrupted the cell morphology, inhibited the growth of hyphae and suppressed conidial germination of A. solani, which ultimately lowered early blight disease in potato leaves [Citation78]. Another strain, B. velezensis Jt84, produced iturins that effectively controlled M. oryzae, which causes rice blast disease, by inhibiting spore germination and mycelial growth, while the iturin mutant strain exhibited a considerable loss of antifungal properties compared to the wild type [Citation74]. Another strain, B. velezensis ND produced iturin-A which inhibited Verticillium dahlia that causes verticillium wilt in cotton [Citation79]. Iturin A from B. amyloliquefaciens strain PPCB004 was identified as the major factor for the antifungal activity against post-harvest pathogens such as Alternaria citri, C. gloeosporioides and Penicillium crustosum by altering conidial germination and germ tube development and consequently suppressed their respective disease incidence in citrus fruits [Citation80]. Iturins C15 and AC15 from B. amyloliquefaciens strain ARP23 and ME218, caused alterations in the mycelial morphology and disrupted sclerotial germination of Sclerotia sclerotiorum, Sclerotinia minor and Sclerotium rolfsii and foliar application of the bacteria reduced stem rot diseases in soybean [Citation81]. Iturin A5 from B. amyloliquefaciens strain S185 isolated from banana rhizosphere caused severe morphological alterations and subsequently inhibited spore germination and hyphae growth of F. oxysporum f. sp. cubense that causes Panama disease/Fusarium wilt disease of banana [Citation82]. Iturin A (C14) from B. amyloliquefaciens strain NCPSJ7, isolated from a ginger field, antagonized mycelial growth and conidia germination (EC50 of 60 µg/mL) of F. oxysporum f. sp. niveum that causes Fusarium wilt in watermelon [Citation83]. Treatment with iturin A caused swelling of the mitochondria, increased vacuolation and increased fungal wall and membrane permeability (based on propidium iodide (PI) staining and conductivity measurements) and effectively inhibited F. oxysporum f. sp. niveum [Citation83]. Iturins from B. subtilis strains UMAF6614 and UMAF6639 demonstrated effective antibacterial activity against phytopathogenic Xanthomonas campestris pv. cucurbitae and Pectobacterium carotovorum subsp. carotovorum that causes melon/cucurbits bacterial leaf spot and soft rot diseases, respectively [Citation84]. Several other strains of B. subtilis, B. amyloliquefaciens and B. velezensis have been reported to produce iturins and optimization studies under various media and culturing techniques have been documented [Citation85,Citation86]. However, iturins tend to show high antimicrobial efficacy only at higher concentrations and their efficacy substantially declines at high dilution rates (for example below 10 µg/mL against X. campestris pv. cucurbitae) [Citation84]. Moreover, iturins have demonstrated a rapid degradation rate in the soil to less than half the concentration in less than 5 days after inoculation and were barely detectable after 20 days [Citation87,Citation88]. For example, iturin A from B. subtilis strain, RB14-CS exhibited the potential to inhibit A. solani and reduced damping-off disease in tomatoes to 39.9% compared to 87% in the control, with an estimated iturin concentration of 949 µg/g-dry soil, corresponding with at least > 20 mL/pot of culture medium, but no significant effect was observed at 21.7 µg/g-dry soil [Citation87]. This would thus require the application of high concentrations of the purified product at very short intervals to control such highly prolific plant fungal pathogens.
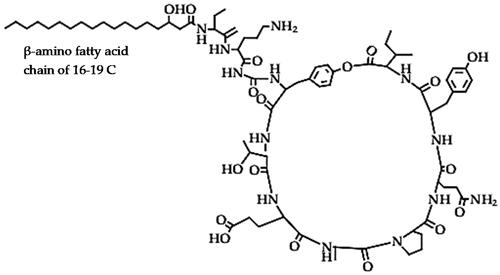
Fengycins (C72H110N12O)
The fengycin family is a class of cyclic lipopeptides with different subtypes (Fengycin A, fengycin B, fengycin S and plipastatin), which are partially cyclic decapeptide lipopeptides with a β-hydroxy fatty acid with a side-chain length of 16–19 carbon atoms () [Citation56,Citation89]. They are another important group of antimicrobial lipopeptides secreted by the different strains of Bacillus species and have an effective antagonistic activity against various fungal phytopathogens [Citation35]. Fengycins have been reported to destroy the internal cell structure of susceptible pathogens by degrading the cytoplasm and cellular organelles, which leads to the shrinkage and distortion of the hyphae [Citation77]. Plipastatin A, a member of the fengycin family from B. amyloliquefaciens strain S76-3 caused vacuolation, which resulted in conglobation in young hyphae and branch tips, and the plasma membranes were severely damaged and separated from cell walls [Citation73]. The biocontrol activity of fengycins as a major antifungal lipopeptide has been reported in some biocontrol strains such as B. subtilis strain CPA-8. For instance, the fenB gene mutant strain of B. subtilis CPA-8 had a suppressed antifungal activity regardless of the presence of the other lipopeptides, confirming that fengycin B was the major biocontrol factor of strain CPA-8 against Monilinia sp. that causes brown rot in peach [Citation90]. Moreover, other fengycin-type lipopeptides were also reported as the main antifungal compounds from B. subtilis strain NCD-2, active against R. solani that causes damping-off in cotton [Citation91]. Other examples include B. velezensis strain BA-26 which secretes fengycin that inhibited B, cinerea pathogen, which causes fruit rot in strawberries [Citation92], while fengycins from B. velezensis strain FJAT were identified as the major lipopeptides responsible the antibacterial activity against Ralstonia solanacearum which causes bacterial wilt in tomato [Citation93]. Fengycin A and B from B. velezensis strain PW192 exhibited emulsifying power, decreased the surface tension, and inhibited the growth of Colletotrichum sp. which causes anthracnose diseases in various plants [Citation94]. Another biocontrol agent, B. velezensis strain HC6 secreted three LPs, but fengycins were the only group of lipopeptides that demonstrated antifungal activity against Aspergillus sp. and Fusarium sp. and their associated mycotoxins in corn seeds [Citation95]. The fengycin from B. amyloliquefaciens strain FZB42 caused deformations in the hyphae of F. graminearum, suppressed its pathogenicity in wheat heads/kernels, and reduced aflatoxin production in infected grains [Citation96].
Bacillomycins (C45H68N10O15)
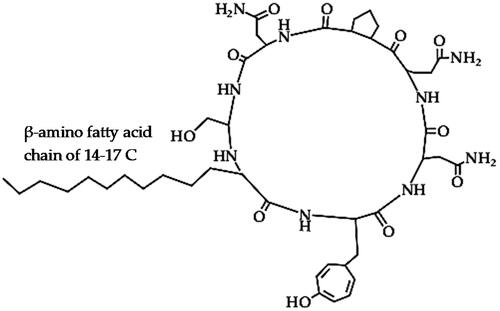
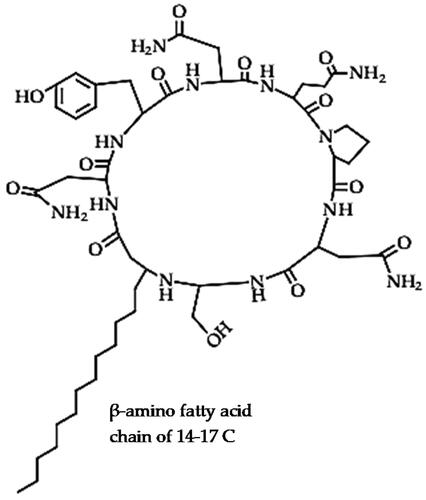
Bacillomycins are cyclic lipopeptides (), which are further classified into bacillomycin D, F, L and Lc based on the positions of the 7 amino acids in the peptide moiety, with a β- β-amino fatty acid chain of 14–17 C atoms and an acetyl chain, which plays a vital role in the antifungal activity [Citation97–99]. Several classes of bacillomycin, including bacillomycin D, F, L and Lc have been reported in Xiao et al. [Citation100]. Bacillomycins are cyclic lipopeptides that are produced by Bacillus sp. and have strong antimicrobial activity against numerous phytopathogenic fungi [Citation97,Citation101].
Bacillomycins exert antifungal activity by injuring the cell wall and cell membrane of phytopathogenic fungal hyphae and spores to discharge the cytoplasm and cell organelles as observed with bacillomycin D from B. velezensis strain HN-2 against C. gloeosporioides that cause anthracnose disease in mango [Citation101]. In another study, bacillomycin D from B. subtilis fmbJ caused hyphal and spore cell wall and cell membrane perforation leading to exudation of cytoplasm and cell organelles, leading to the formation of hollow bodies [Citation102]. This led to the inhibition of sporulation, spore germination and mycelial growth of Aspergillus flavus, a phytopathogen that causes grey mould and aflatoxins in corn [Citation102]. Bacillomycin was also reported among the major lipopeptides responsible for the biocontrol efficacy of B. velezensis strain FZB42 against phytopathogenic bacteria, Xanthomonas campestris pv. campestris that causes black rot in cabbages [Citation103]. The bacillomycin D from B. amyloliquefaciens strain NJN-6 showed antagonistic activity against F. oxysporum f. sp. cubense, creating an inhibition zone between the wells and the fungal culture in each panel that contained the antibiotic [Citation104], while bacillomycin D (and fengycin) from B. amyloliquefaciens SQR9 was antagonistic against F. oxysporum [Citation105]. However, the bacillomycin D mutant strain of B. amyloliquefaciens SQR9 lost its antifungal activity against F. oxysporum, while the fengycin mutant strain was as effective as the wild type, indicating that bacillomycin D was the main antifungal weapon deployed by strain SQR9 [Citation105]. In addition, bacillomycin D from B. amyloliquefaciens strain SQR9 was also reported to play a vital role in biofilm formation, which could be yet another mode for its antimicrobial activity [Citation105]. In a similar study, the bacillomycin D mutant strain of B. amyloliquefaciens YN201732 (which produces both bacillomycin D and fengycin lipoptides), displayed lower antifungal efficacy (spore germination inhibition) against both F. solani and Erysiphe cichoracearum, which cause powdery mildew in tobacco [Citation106]. The bacillomycin D mutant strain of B. amyloliquefaciens YN201732 also lost biofilm-forming potential compared to the wild type or the fengycin mutant strain, which compromises their colonization and antagonistic activity [Citation106]. Another bacillomycin D from B. amyloliquefaciens strain FZB42 effectively antagonized F. graminearum in corn and wheat by inducing the accumulation of oxygen reactive species (ROS) and causing morphological deformations in the plasma membrane and cell wall leading to cell death of fungal hyphae and conidial [Citation107]. The homologues of bacillomycin D from B. amyloliquefaciens strain 83 were effective against spore germination and mycelial growth of C. gloeosporioides by damaging the cell membrane (which caused cytoplasm leakage and plasmolysis) and consequently lowered cell viability [Citation108]. According to Luna-Bulbarela et al. [Citation108], the fatty acid chain length of bacillomycin D homologues (C14 − 16, which is related to the hydrophobicity) affected their efficacy against spore germination of C. gloeosporioides, with C16 homologues having the lowest minimum inhibitory concentrations (MIC100) but all the homologues effectively inhibited the mycelial growth at much lower concentrations. They attributed the difference between the spores and mycelia of C. gloeosporioides in response to bacillomycin treatment to the double cell wall that makes spores more resistant [Citation108]. This is a particularly important aspect in the biocontrol of fungal phytopathogens since the inhibition of spore germination could effectively prevent proliferation and infection than mycelial growth inhibition. Biomolecules with higher efficacy against the germination of spores could be more suitable biocontrol weapons. Another bacillomycin-L from B. velezensis G341 from Korean ginseng was reported to contribute to the diverse antifungal/anti-oomycete activity of the strain against various phytopathogens (diseases) such as B. cinerea (grey mould in tomato), Colletotrichum coccodes (anthracnose in red pepper, M. oryzae (rice blast), Phytophthora infestans (late blight in tomato) and R. solani (sheath blight in rice) [Citation99]. Bacillomycin-L from B. velezensis Bs916 was proposed to act as a turning signal of swarming motility and biofilm formation which is important for the antagonism of phytopathogens and plant growth-promoting efficacy [Citation109]. On the other hand, bacillomycin D was also hypothesized to be involved in multiple bioactivities including iron acquisition by regulating the transcription of iron transport gene feuABC, which in turn facilitates biofilm formation since the high iron concentration activates KinB-Spo0A-SinI-SinR pathway [Citation110].
Cyclic tetrapeptides
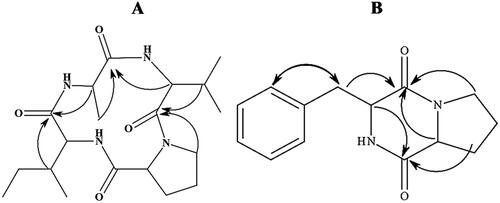
Cyclic tetrapeptides (CTPs) are a group of small cyclic peptides that often contain turn-inducing residues such as proline, D-amino acids, or α-amino iso-butyric acid (Aib) and unusual amino acids with multiple stereocenters and functional groups [Citation111]. For example, Sarojini et al. [Citation111], demonstrated the stereocenters and functional groups several CTPs such as AM-toxins. Some CTPs such as cyclo-(prolyl-valyl-alanyl-isoleucyl) () and cereusitin A () have been reported to antagonize plant fungal pathogens, but the potential for application could be limited by low concentrations under natural production. For instance, B. velezensis strain CE 100 produced a cyclic tetrapeptide, cyclo-(prolyl-valyl-alanyl-isoleucyl) which exhibited antifungal activity against C. gloeosporioides, which causes anthracnose disease in walnut and jujube orchards [Citation112]. The cyclic tetrapeptide from B. velezensis strain CE 100 was reported to cause cell wall degradation which led to the inhibition of spore germination and mycelial deformations [Citation112]. The cyclic tetrapeptide cereusitin A (1), (a cyclo-(L-phenylalanyl-trans-4-hydroxy-L-prolyl-L-leucyl-trans-4-hydroxy-L-proline) from B. cereus caused a mild antifungal activity against C. gloeosporoides, which causes yam anthracnose [Citation113]. This mild effect indicates that CTPs have less potential as stand-alone antifungal compounds but could cause a synergic inhibition of fungal phytopathogens along with other bioactive compounds. However, these tetrapeptides have been reported to exhibit anticancer activity and may have other potential biological applications [Citation111]. There are limited studies about the bioactivity of cyclic tetrapeptides from Bacillus sp. that have focused on the biocontrol of plant diseases, which could either be attributed to their novelty in this field of research or their limited efficacy as biocontrol metabolites.
Figure 5. Illustration of the structures of cyclic tetrapeptides: cyclo-(prolyl-valyl-alanyl-isoleucyl) (A) and cyclo-(D-phenylalanyl-D-prolyl) (B).
There are also other small-sized cyclic peptides from Bacillus sp. with potential for application in the biocontrol of plant diseases including; a cyclic dipeptide, cyclo-(D-phenylalanyl-D-prolyl) from B. velezensis strain CE 100, which demonstrated antifungal activity against various strains of Colletotrichum sp., which causes anthracnose diseases in various plants [Citation114]. Benzoic acid (C6H5COOH) from B. licheniformis strain MH48 was reported to effectively degrade and inhibit the mycelial growth of R. solani at a concentration of 128 μg/mL and antagonized the conidial germination of C. gloeosporides at 100 μg/mL, which demonstrates their potential for application in the management of plant phytopathogens [Citation115].
Bacillibactin (C26H30N4O13)
Bacillibactin is a microbial catecholate siderophore, which is a non-ribosomal peptide synthesized through the condensation of three 2,3-dihydroxybenzoate-glycine-threonine units that are attached to a core of cyclic amino acid () [Citation116]. Bacillibactin peptides are vital weapons for the ecological adaptations of the producing Bacillus strains [Citation116,Citation117]. Bacillibactin plays an important role in the antimicrobial activity of Bacillus sp. against plant pathogens, both through iron scavenging and antibiosis [Citation50]. Irion scavenging occurs when siderophores bind with iron (III) ion to form siderophore–iron complexes which are then transported via siderophore-binding proteins (and the associated enzymes such as siderophore-permeases and ATPases) in the cell membrane into bacterial cells, making it unavailable for the growth of pathogenic microbes. Once in the bacterial cells, iron (III) is reduced to iron (II) and is then utilized for the growth of the biocontrol agent, especially under iron-limiting conditions [Citation118,Citation119]. This scavenging mechanism inhibits the growth of phytopathogens, including non-susceptible microbes (since it is independent of direct antibiosis mechanisms). For instance, bacillibactin from B. amyloliquefaciens strain MBI600 was only produced under iron-limiting conditions and exerted bacteriostatic activity against a non-susceptible phytopathogen, P. syringae pv. tomato under iron starvation [Citation50]. Similarly, bacillibactin from strain MBI600 inhibited the growth of non-susceptible phytopathogens or phytopathogens with low susceptibility such as F. oxysporum f. sp. radicis-lycopersici, R. solani, A. flavus and V. dahliae under iron starvation, in a dose-dependent manner [Citation50]. The findings of Dimopoulou et al. [Citation50] demonstrate the antimicrobial role of bacillibactin through the iron scavenging mechanism. The production of siderophores was also proposed to be among the mechanisms by which B. velezensis strain FKM10 inhibited F. verticillioides and several other plant phytopathogens of Malus hupehensis Rehd trees (used as apple rootstock in China) and consequently improved rootstock growth [Citation120]. Other Bacillus sp. such as Bacillus niabensis strain PT-32-1, B. subtilis strain SWI16b, B. subtilis strain HPC21, B. mojavensis strain JCEN3 and B. subtilis strain HPC24 were reported to produce different types of siderophores (catecholate and carboxylate) and showed antimicrobial activity against some banana wilt pathogens (unspecified) based on the halazone formation [Citation121]. Some studies have been based on the presence of bacillibactin-siderophore biosynthetic genes in several Bacillus sp. to deduce their biocontrol potential and to predict the involvement of siderophore (in iron scavenging, biofilm formation and direct antibiosis activity) in the biocontrol of plant diseases [Citation50,Citation122–124]. Other studies have suggested that the siderophore activity of Bacillus sp. not only suppresses phytopathogenic growth through iron deprivation but could also be involved in other plant/microbial interactive mechanisms that improve plant growth [Citation117,Citation120,Citation122]. The mechanism by which iron (II) inside the bacterial cell becomes available for plant growth is not yet fully understood, but signalling molecules between root exudates and Bacillus strains could be involved in the conversion of siderophore–iron complexes into plant-available forms. The other positive applications of iron-siderophore bacillibactin from different Bacillus sp. include the inhibition of drug-resistant pathogens via the iron-scavenging mechanism as observed in B. velezensis strain MBTDLP1 and B. amyloliquefaciens strains MTCC 12713 which were reported in clinical studies, but these mechanisms indicate the potential application of these strains against phytopathogens of economic importance [Citation125,Citation126].
Bacteriocins (20–90 kDa)
Bacteriocins () are ribosomal-synthesized peptide toxins with heterogeneous biochemical and structural groups that cause bactericidal effects by adsorbing specific receptors on the surface of susceptible bacterial pathogens, altering the metabolic, biological and morphological functions [Citation127,Citation128]. In Bacillus species, lantibiotics are the main biocidal bacteriocin group, though other bacteriocins such as nisin, subtilin, ericin, lichenicidin, subtilosin, cerein and thuricin have been reported [Citation129]. Bacteriocins such as plantazolicin (Microcin, 1.3 kDa) protein toxin [Citation130] and amylocyclicin (6.4 kDa) toxin [Citation131] from B. amyloliquefaciens FZB42 have been reported for antibacterial effect against closely related bacteria of pathogenic potential in plants. Another bacteriocin, bacitracin was among the bioactive toxins from Bacillus sp. strain LU 2184 that showed antifungal activity against B. cinerea that causes grey mould in various crops [Citation132]. In the same study, another endosymbiotic (from arthropods) strain Bacillus sp. LU 7314 was also reported to produce amicoumacin B, which demonstrated a herbicidal effect against duckweed (Lemna minor) that grows in freshwater [Citation132], while another bacteriocin (5 kDa) from B. pumilus strains ZED17 and DFAR8 demonstrated antifungal activity against R. solani but also inhibited seed germination in some herbs [Citation133], which indicates potential bioherbicidal effect. Thuricin 17 bacteriocin produced by an endophytic B. thuringiensis strain NEB17, inhibited various bacterial species, including some closely related strains [Citation134]. While a 21 kDa bacteriocin from B. subtilis strain 14B reduced the percentage of crown gall infections caused by Agrobacterium tumefaciens pathogen in weeping fig tree [Citation135]. Other bacteriocins such as amylolysin (lantibiotic) from B. amyloliquefaciens strain GA1 have been reported for bactericidal activities against food-borne pathogens such as Listeria monocytogenes, Staphylococcus aureus and B. cereus and are used in food preservation, but these bactericidal properties could potentially be applied against phytopathogenic bacteria [Citation136]. Generally, bacteriocins could be more important in enhancing niche colonization by the biocontrol agent as they eliminate or suppress other related microbes, including some important phytopathogens. Moreover, the bacteriocins such as thuricin 17 from other bacterial strains play a vital role in alleviating plant stress [Citation137]. Thus, the role of bacteriocins from Bacillus sp. in plant stress alleviation should be a subject of research interest, especially as a climate change adaptation strategy.
Antimicrobial polyketides from Bacillus species
Besides the well-known medical applications, some polyketides like bacillaene, macrolactins, bacilysin and difficidin from Bacillus sp. have recently attracted research attention for their potential biocidal activity against various phytopathogens [Citation36,Citation138], as shown in .
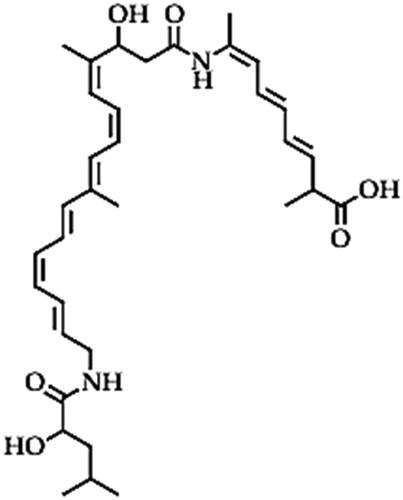
Bacillaene (C34H48N2O6)
Bacillaene is a non-ribosomal linear polyketide (PK/NRP hybrid) from Bacillus sp. (mainly B. subtilis), which is synthesized by the trans-acyltransferase polyketide synthetase (trans-AT PKSs) enzymes [Citation37,Citation139]. The bacillaene structure () has two amide bonds: one linking the α-hydroxy carboxylic acid to the ω-amino carboxylic acid (with conjugated hexaene) and another that links the hexaene-containing carboxylic acid to (ω-1) amino carboxylic acid (with conjugated triene) [Citation139]. This makes bacillaene a highly effective antibiotic that could potentially be applied in the control of pathogenic microbes [Citation37,Citation139]. Even though most reports about the antimicrobial role of bacillaene have been in the field of biomedicine, some studies have demonstrated the potential of bacillaene from Bacillus species for application in crop protection. Bacillaene from Bacillus species have demonstrated antifungal properties against phytopathogens that cause devastating economic losses in fruit production, pre- and post-harvest. For instance, the bacillaene from B. amyloliquefaciens strain DH-4 exerted some degree of fungistatic activity against Penicillium digitatum phytopathogen, which causes post-harvest rot disease in citrus fruit [Citation140]. Bacillaene caused reduced the rate of spore germination and antagonized mycelial growth by disrupting the mitochondria, causing vacuolation and cell leakage [Citation140]. This could prolong the shelf life of fruits during storage and transportation. Another biocontrol strain, Bacillus sp. W176, produced a heat-stable bacillaene that effectively inhibited the growth of P. digitatum by degrading the mitochondria and causing vacuolation and consequently reduced green mould decay in citrus (Satsuma mandarin) fruits [Citation141]. In another study, the genomic analysis of B. velezensis strain TSA32-1 indicated the presence of a bacillaene biosynthesis gene cluster that inhibits protein biosynthesis in phytopathogens such as F. graminearum and Pythium ultimum that infect corn and pepper seeds [Citation142]. Another interesting scenario is the symbiotic association of Macrotermes natalensis (a fungus-growing termite) with B. subtilis strains which produce bacillaene A (1) as the only major antibiotic. Bacillaene A (1) selectively inhibits the entomopathogenic fungi of Termitomyces, enabling the termite to survive in a potentially dangerous environment [Citation143]. However, some contradictions about the role of bacillaene have also been reported. For instance, B. amyloliquefaciens strain SQR9 was reported to antagonize F. oxysporum f. sp. cucumerinum that causes cucumber vascular wilt disease through a stepwise phosphorylation of DegU (DegU ∼ P) promoter gene [Citation144]. Yet in their report, Xu et al. [Citation141] proposed that the downregulation of this gene (degQ mutation) increased the production of bacillaene (and macrolactin), which then impaired the biofilm formation, colonization and lowered the biocontrol efficiency of strain SQR9. The overexpression of degQ and degSU increases other bioactive lipopeptides such as bacillomycin D and difficidin, that enhance the biocontrol efficacy. This could mean that bacillaene was not the bioactive peptide responsible for the biocontrol activity of strain SQR9, but its production could be vital for the K-state transformability of the bacterial strain, which is also important for its adaptability [Citation145]. The impairment of biofilm formation by bacillaene produced by Bacillus species has also been confirmed in other studies and has even been utilized to inhibit the colonization and spread of other pathogenic bacterial species that rely on biofilms to establish themselves. For instance, bacillaene from B. subtilis PS-216 has been positively applied to suppress harmful bacterial growth through the inhibition of biofilm formation, suppressing the colonization and pathogenicity as demonstrated in the case of Campylobacter jejuni that causes food borne-infections [Citation146]. Based on genomic analysis, bacillaene from B. amyloliquefaciens FZB42 was suggested to moderate the antibacterial activity against Erwinia amylovora, a pathogen that causes fire blight in blossoms of apples and pears [Citation147]. This provides prospects for the potential application of bacillaene in the field of forestry to control phytopathogenic bacteria by disrupting their colonization on plant surfaces since biofilm formation is a vital property for the successful establishment and host infection in most bacterial pathogens.
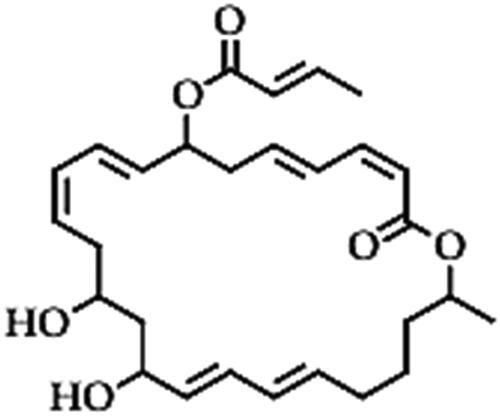
Macrolactins (C24H34O5)
Macrolactins are a group of six 24-C ring lactones of highly unsaturated macrolides (), which are mainly secreted by deep-sea bacteria [Citation148,Citation149]. Macrolactins from Bacillus species have been mainly reported as clinical antibiotics but have potential for application in the biocontrol of fungal and bacterial phytopathogens [Citation148]. Macrolactins such as macrolactin A and 7-O-2′E-butenoyl macrolactin A from the marine (deep sea sediments) strain B. subtilis B5 were reported to exhibit antifungal activity against Pestalotiopsis theae and C. gloeosporioides, which cause grey tea blight and anthracnose in tea leaves (Camellia sinensis) [Citation148]. Macrolactin A and 7-O-succinyl macrolactin A from B. amyloliquefaciens strain ELI149 isolated from the soil showed antifungal activity against F. oxysporum, a notorious soil-borne phytopathogen that causes wilt disease in numerous plants, Rhizoctonia sp. which causes several rots and damping off diseases in fruits and vegetables and Moniliophthora roreri, which causes frosty pod rot disease in cacao (Theobroma cacao) [Citation38]. The fungi exhibited reduced microconidia due to plasmolysis and vacuolation of the conidial cells and curling/deformation (with loss septa) of the hyphae [Citation38]. Macrolactin W from Bacillus siamensis strain LZ88 isolated from tobacco rhizosphere was also reported as one of the non-volatile compounds (together with iturin) that exerted antifungal activity against A. alternata, which causes tobacco brown spot disease [Citation150]. The macrolactin W caused rupturing and shrinking of conidia, twisting of hyphae and loss of conidial attachment on the hyphae, which resulted in the inhibition of spore germination (IC50 of approximately 29 µg/mL) and reduced mycelial growth (IC50 − 24 µg/mL) against A. alternata, in vitro [Citation150]. This indicates a great potential for the application of macrolactin W, but the precise efficacy of the purified compound is necessary. Macrolactin R from B. siamensis strain NJ08-3 has also been reported to inhibit spore germination and germ tube elongation (IC50 − 1.93 mg/L) and mycelial growth (IC50 − 2.88 mg/L) of B. cinerea that caused grey mould in strawberry by altering membrane permeability, leading to cell leakage of proteins and nucleic acids (organelles), which resulted in cell death [Citation151]. The biocontrol strain B. amyloliquefaciens NJN-6 isolated from the banana rhizosphere also produced macrolactin A, 7-O-malonyl macrolactin A and 7-O-succinyl macrolactin A (in addition to iturin and bacillomycin D lipopeptides) and these three polyketides demonstrated an in vitro fungicidal effect against F. oxysporum f. sp. cubensein that causes Fusarium wilt disease of banana and a bacteriostatic effect against R. solanacearum that causes bacterial wilt in various plants such as tomato [Citation104]. However, the antifungal activity was less compared to that of iturins, bacillomycin D and the bacterial volatile compounds (BVCs) from the same bacteria [Citation152,Citation153]. This indicates that these polyketides are not the main mode of antifungal activity for B. amyloliquefaciens NJN-6 but could certainly contribute to its biocontrol efficacy through a synergic effect with the other lipopeptides and BVCs [Citation104].
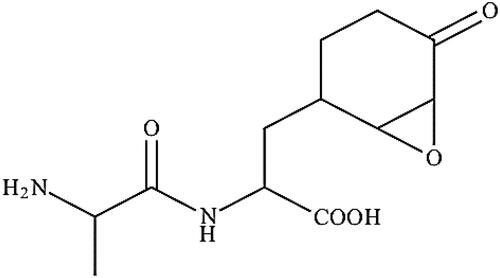
Bacilysin (C12H18N2O5)
Bacilysin (l-alanine-[2,3-epoxycyclohexano-4]-l-alanine) polyketides secreted by Bacillus sp. are non-ribosomal simple peptide (NRP) antibiotics containing an L-alanine residue and the nonproteinogenic amino acid L-Anticapsin [Citation154]. Bacilysin has a very simple structure (), which is highly active against a broad range of important phytopathogen after activation (depending on environmental conditions) [Citation155,Citation156]. The predicted model of bacilysin activation follows the cytoplasmic peptidase hydrolysis of dihydroanticapsin and dihydrobacilysin into non-proteinogenic anticapsin, which is further processed by the removal of N-terminal L-alanin to produce antimicrobial bacilysins [Citation155–158]. The strong antimicrobial activity of bacilysin against phytopathogenic bacteria and fungi is related to the irreversible interference with glucosamine synthetase, which consequently disrupts peptidoglycan synthesis in bacterial pathogens or mannoprotein biosynthesis in fungal pathogens, making it a lethal weapon against plant pathogens [Citation157,Citation158].
There are various examples of potential application of bacilysin in the field of crop protection just as the case for pharmacological applications. Bacilysin D from B. velezensis strain FZB42 has been reported to show anti-oomycete activity by severely damaging hyphal structures and causing loss of intracellular contents against Phytophthora sojae which causes root and stem rot in soybeans and showed potential to antagonize other phytopathogenic strains of Phytophthora sp. [Citation159]. In addition, the bacilysin from B. amyloliquefaciens FZB42 was also identified as the antimicrobial peptide responsible for the inhibition of E. amylovora pathogen which causes fire blight disease of pear, while the mutant strain devoid of bacilysin lost its antibacterial activity [Citation36]. The bacilysin from B. velezensis strain RC218 was also proposed to be responsible for antifungal activity against F. graminearum that causes head blight in wheat and consequently reduced disease severity and mycotoxin accumulation [Citation160]. However, their research mainly relied on the presence of gene clusters responsible for bacilysin biosynthesis in the genome of strain RC218 than the specific anti-oomycete activity of bacilysin and this may not be sufficient since these in silico data from the same study also reveal the presence of other potential antimicrobial compounds [Citation160]. The bacilysin from B. subtilis UTB1 was proposed to be the main antagonistic antimicrobial peptide against A. flavus [Citation161]. The fungicidal activity of bacilysin against A. flavus could be of significant application in the control of ear rot diseases in various cereal grains, nuts and legumes and preventing the accumulation of some of the most dangerous aflatoxins produced by the fungus in human food and animal feeds [Citation161]. Based on genomic and specific antifungal and anti-aflatoxin analysis of B. subtilis UTB1, Afsharmanesh et al. [Citation161] reported that the bacC gene, which is responsible for bacilysin biosynthesis, also directs the synthesis of BacC oxidoreductase enzyme that degrades aflatoxins in pistachio nuts. Thus, the production of bacilysin antibiotic by B. subtilis strain UTB1 controls aflatoxins by inhibiting fungal growth as well as via the direct degradation of the aflatoxins [Citation161]. The bacilysin from B. amyloliquefaciens FZB42 demonstrated antibacterial activity against Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola, the respective pathogens for bacterial blight and bacterial leaf streak in rice [Citation162]. Their study revealed that bacilysin not only caused deformations in the cell wall structure (making it loose and porous, with morphological distortions and rupturing) but also down-regulated the expression of the genes responsible for virulence, as well as extracellular polysaccharide and cell wall protein synthesis in Xanthomonas pathogens [Citation162]. Moreover, the derivatives of strain FZB42 expressed higher bacilysin production and effective control of C. michiganense subsp. sepedonicum, and substantially reduced the severity of potato ring rot disease [Citation163]. Rabbee et al. [Citation155] discussed the other antibacterial evidence and applications of bacilysin against harmful algae bloom bacteria and their mode of action, which involves the disruption of biosynthetic enzyme activity, glucosamine synthase, leading to the deformations in the cell wall peptidoglycans in the target bacteria [Citation155].
Difficidin (C31H45O6P) and oxydifficidin (C31H45O7P)
Difficidin and oxydifficidin are another class of polyketides produced by Bacillus sp. and have demonstrable antimicrobial activity against important plant bacterial pathogens [Citation36,Citation155,Citation162,Citation164–166]. Difficidin and oxydifficidin are highly unsaturated macrolide phosphates with almost identical 22 C cyclical structure (lactones), only differentiated by a H atom in the difficidin structure (), which is replaced by hydroxyl group in oxydifficidin ((B)). Difficidin and oxydifficidin have a broad range of antibacterial activity against important phytopathogens, where the antibiotics cause cell lysis, loosening and rupturing of the cell membrane, distorting the cells and making them highly porous and partially vesiculated, plasmolysed due to the efflux cell organelles [Citation162,Citation166]. Based on the bacterial model (Escherichia coli), the antibacterial activity of difficidin and oxydifficidin is premised on the inhibition of protein synthesis, which consequently disrupts the synthesis of bacterial cell wall and nucleic acids, leading to loss of cell viability [Citation167]. Some examples of the bactericidal activity of these polyketides, especially in forestry and fruit tree production, include the biocontrol of fire blight in orchards, where difficidins produced by B. amyloliquefaciens FZB42 showed strong antibacterial activity against E. amylovora, a pathogen that causes fire blight in apple blossoms [Citation36]. However, in their study, Chen et al. [Citation36] demonstrated that a combination of polyketides: difficidin and bacilysin were necessary to attain the maximum antibacterial effect of strain FCB42. This indicates that the efforts dedicated to the purification and commercialization of single bioactive compounds could be rather devoted to optimization studies that seek to enhance the production of different bioactive metabolites in the bacterial culture for effective biocontrol of bacterial diseases. In another study, these polyketides demonstrated a strong bactericidal effect against X. oryzae pv. oryzae and X. oryzae pv. oryzicola, which cause bacterial blight and bacterial leaf streak in rice, respectively [Citation162]. Difficidin and oxydifficidin from Bacillus methylotrophicus strain DR-08, were also effective against various phytopathogenic bacteria, with the most notable antagonism against R. solanacearum (with a minimum inhibitory concentration (MIC) of 10 μg/mL), which causes tomato bacterial wilt [Citation164]. The phytopathogenic bacteria that exhibited high susceptibility to difficidin and oxydifficidin include Xanthomonas arboricola pv. pruni, Xanthomonas citri, Xanthomonas euvesicatoria, A. tumefaciens, A. tumefaciens, Burkholderia glumae, and C. michiganensis subsp. michiganensis (at MIC of 10 μg/mL), X. oryzae pv. oryzae (at MIC of 1.1 μg/mL), while the inhibition of Pseudomonas required a higher concentration (MIC of 30 μg/mL) [Citation164]. Consequently, the application of strain DR-08 was reported to effectively control tomato bacterial wilt in potted plants and reduced the development of bacterial leaf spot disease in peach and red pepper [Citation164]. Therefore, whereas various antimicrobial peptides such as macrolactin, bacilysin and bacillaene have potential bactericidal effects [Citation160], difficidin and oxydifficidin could be the most reliable antibacterial peptides from Bacillus sp. The limited studies on the antibacterial effect of difficidin and oxydifficidin could be indicative of their rare production by most biocontrol strains but also highlights the need for extensive screening of bacterial agents and the elucidation of their antibacterial effects, the bioactive metabolites and the mechanisms of their antimicrobial activity.
Bacterial volatile compounds (BVCs)
Bacterial volatile compounds (BVCs) are a diverse group (with structural and functional differences) of bacterial metabolites with a high vapour pressure at room temperature and are thus emitted or quickly vaporized into gaseous form with low water solubility. Most BVCs are species-specific and greatly influenced by environmental conditions where they play different roles in plant-microbial interactions as signal molecules and have diverse antimicrobial and pesticidal properties ( and ), though their application in open environments is still not feasible [Citation168–170]. A few groups of BVCs secreted by Bacillus species have exhibited antimicrobial activity against plant pathogens [Citation39,Citation78,Citation171,Citation172]. BVCs exert toxicity against the phytopathogenic fungal mycelia by causing structural damage and deformations, including cell wall lysis, cell leakage, plasmolysis, protoplasm retraction and swelling of cellular organelles [Citation172,Citation173]. These structural deformations alter the normal cell processes of the phytopathogen, such spore germination, cell growth, environmental signalling and host infection, which ultimately reduces disease incidence and severity. However, the effects of BVCs seem to be temporary (microbiostatic), and the phytopathogenic cells tend to recover and regain their pathogenicity when the concentration of the toxic volatiles declines [Citation152,Citation172]. Moreover, most microbiostatic BVCs from Bacillus sp. are often produced along with other non-bioactive BVCs (or BVCs with unknown bioactive activity) [Citation39,Citation171,Citation173]. The complexity involved in the purification of the antimicrobial BVCs to highly effective concentrations could limit the investigation of individual compounds and their commercialization. Nonetheless, some examples of antifungal BVCs include acetophenone from B. velezensis strain C16, which inhibited A. solani, a phytopathogen that causes early blight in potatoes [Citation78]. However, the BVC was produced alongside other known antifungal lipopeptides, and therefore, acetophenone could play a role in the antimicrobial activity of strain C16, but is unlikely to be the major antifungal arsenal that controlled early blight disease [Citation78]. Two BVCs from B. velezensis strain CE 100, 5-nonylamine and 3-methylbutanoic acid demonstrated reduced mycelial growth and spore germination of C. gloeosporioides that causes anthracnose in fruit trees under laboratory conditions [Citation39]. Another strain, B. velezensis ZSY-1 produced phenol (4-chloro-3-methyl), pyrazine (2,5-dimethyl), benzothiazole and phenol-2,4-bis (1,1-dimethyl ethyl), which effectively inhibited the growth and sporulation of A. solani that causes early blight disease and B. cinerea that causes grey mould in tomatoes [Citation172]. The antifungal activity of the BVCs produced by strain ZSY-1 against the phytopathogens was rather temporally since A. solani recovered its reproductive potential once the bacterial treatment was withdrawn, indicating that the effect of the volatile compounds was rather suppressive than inhibitory, since they did not cause complete cell death [Citation172]. Numerous BVCs from B. amyloliquefaciens strain NJN-6, including benzenes, benzothiazoles, phenols, 2-undecanol, 2-nonanone, 2-decanone, nonanal and naphthyl compounds demonstrated a very strong antifungal effect (with more 90% inhibition rate) against F. oxysporum f. sp. cubense that causes Panama disease [Citation152]. Unlike many other pathogens that infect the shoots and fruits, Fusarium sp. is mainly soil-borne and thus fumigation with BVCs could reduce the fungal density in the soil, but the reliability of such a control strategy against these soil-borne pathogens needs a better investigation.
Table 2. Summary of Bacillus species reported for eliciting induced systemic resistance (ISR).
Since BVC production by bacterial strains is heavily dependent on environmental conditions [Citation168], a more specific study regarding the method of application, the persistence of BVCs in the soil and the signalling pathways that stimulate their production and interaction with rhizosphere biota under field or greenhouse simulated conditions could shed better light on their reliability [Citation170]. When BVCs from B. amyloliquefaciens strain NJN-6 were applied in the soil, a reduction of F. oxysporum f. sp. cubense from 104 g−1 of soil in the control group to 102 g−1 in treated soil after 45 days was reported [Citation152]. However, the BVCs from strain NJN-6 were only fungistatic and not fungicidal [Citation152] and therefore, it is likely that this bacterium mainly used other antimicrobial peptides in biocontrol activity [Citation104]. Thus, BVCs could be particularly important if supplemented with other biocontrol strategies such as treatment with antimicrobial lipopeptides with a strong fungicidal effect through contact interactions but have limited potential to spread and saturate the soil. The BVCs would suppress the germination of surviving pathogenic conidia and keep the fungal density below threshold levels after the initial fungicidal effect of the lipopeptides [Citation152]. The prospect for soil fumigation using BVCs could be more feasible in potted soils or bed soils under greenhouse conditions, or further still if the soils are subsequently covered with a polythene mulching sheet. The BVCs from B. subtilis strain PPCB001 and B. amyloliquefaciens strain PPCB004 showed a fungistatic effect against the spore germination and mycelial growth of Penicillium sp. that causes fruit decay in citrus, and the BVC mixture subsequently reduced the disease incidence and severity in citrus fruit cv. Valencia that were artificially infected with P. crustosum [Citation174]. In another study, several strains of B. amyloliquefaciens subsp. plantarum (strains UCMB5033, UCMB5036, UCMB5113 and FZB42) produced diverse and varying BVCs depending on media conditions, which antagonized fungal growth of various phytopathogens such as B. cinerea, Alternaria brassicicola, Alternaria brassicae, S. sclerotiorum and to a lesser extent Verticillium longisporum, but also promoted root and shoot growth of Arabidopsis thaliana Col-0 through the interaction with root exudates at low BVC concentrations [Citation175]. The results of Asari et al. [Citation175] could be particularly invaluable in promoting forest seedling growth and increasing seedling survival by suppressing the proliferation of fungal pathogens. Various BVCs from B. velezensis strains (BUZ-14, I3 and I5), especially benzaldehyde and diacetyl and to a lesser extent, isoamyl alcohol were highly effective against fungal phytopathogens such as B. cinerea, M. fructicola, Monilinia laxa, Penicillium italicum, P. digitatum and Penicillium expansum based on in vitro antifungal assay [Citation171]. Even though their phyto toxicological evaluations, as well as their active doses were yet to be determined, Calvo et al. [Citation171] demonstrated the potential for applying benzaldehyde and diacetyl from B. velezensis BUZ-14, I3 and I5 in the post-harvest control of grey mould disease in table grapes (caused by B. cinerea) and blue rot disease in mandarins (caused by Penicillium species) during storage and transportation. Dimethylsulphoxide, 1-butanol and 3-hydroxy-2-butanone from B. velezensis strain G341 demonstrated an effective antifungal activity against various phytopathogens, especially R. solani, B. cinerea and S. sclerotiorum by inhibiting mycelial growth in vitro [Citation99]. However, the efficacy of the individual volatile compounds was not established. Moreover, other well established antimicrobial lipopeptides like bacillomycin L and fengycin A were also produced by strain G341, which could be a major antifungal arsenal of the bacterium [Citation99]. Thus, the antifungal activity of the BVCs produced by strain G341 might have only played a supplementary role in its biocontrol efficacy against the fungal pathogens [Citation99]. The BVCs from B. subtilis strain BL02 and B. pumilus strains BSH-4 and ZB13 effectively inhibited the growth of phytopathogenic fungi, including B. cinerea, Ascochyta citrullina, A. solani and A. brassicae by causing morphological abnormalities in the mycelia, interrupting pigment formation and decreasing the sclerotoid production (in S. sclerotiorum) [Citation176]. The BVCs from the Bacillus strains also antagonized the germination of fungal spores by causing cracking and melanization of spores (which oxidative and toxic intermediates that cause fungicidal effect) in B. cinerea [Citation176]. The total headspace BVCs from B. methylotrophicus strain BCN2 also demonstrated an effective antifungal activity against postharvest pathogens; F. oxysporum, Botryosphaeria sp. and C. gloeosporioides, while the BVCs from B. thuringiensis strain BCN10 were effective against Botryosphaeria sp., P. expansum and Trichoderma atroviride based on in vitro mycelial growth inhibition assay [Citation177]. The BVCs from the two strains BCN2 and BCN10 demonstrated a synergic effect on all the five post-harvest fungal pathogens and consequently reduced fruit rot disease incidence and severity in loquat fruit in vivo, which extended the fruit shelf life for at least 10 days during storage, despite the optimal conditions (at 25 °C and 85% RH) for fungal growth [Citation177]. The total BVCs (mainly composed of 2,3,5-trimethyl pyrazine, 3-amino-1,3-oxazolidin-2-one and 6,10-dimethyl-5,9-undecadien-2-one) from Bacillus acidiceler isolated from avocado rhizosphere demonstrated anti-oomycete activity by causing morphological alterations such as shrivelling of the hyphae and inhibited mycelial growth of Phytophthora cinnamomi by more than 70%, which was higher than the antagonistic effect under dual culture assay and also promoted the in vitro growth of A. thaliana [Citation178]. In another study, the volatile fraction of B. velezensis strain UCD10614 demonstrated partial antifungal activity against Neofusicoccum parvum and Diplodia seriata that cause grapevines trunk diseases [Citation179], while a complex range of volatile compounds produced by B. atrophaeus strain CAB-1 also displayed a strong antifungal activity against some of the notorious plant pathogenic fungi [Citation180]. For instance, O-anisaldehyde, the most abundant volatile compound demonstrated the highest inhibition rate against the mycelial growth of B. cinerea that causes tomato grey mould diseases [Citation180]. Another antifungal BVC, 2,4-di-tert-butylphenol produced by B. subtilis strain CF-3 inhibited the growth of C. gloeosporioides which causes anthracnose disease in harvested litchi fruits [Citation40]. The BVCs from strain CF-3 inhibited the germination of spores and disrupted hyphal growth of C. gloeosporioides by causing morphological deformations and decreasing cell membrane fluidity and integrity [Citation40]. In their study, Zhao et al. [Citation40] reported interesting results where the 2,4-di-tert-butylphenol compound suppressed the fungal pathogenicity (fruit decomposition) by inhibiting the activity of the pathogenic enzymes of C. gloeosporioides such as pectinase and cellulase. The BVC also reduced fruit cell damage by eliminating excessive ROS through the activation of antioxidative enzymes such as peroxidase, polyphenol oxidase, catalase and superoxide dismutase and enhanced disease resistance by increasing the production of pathogenesis-related (PR) enzymes such as phenylalanine ammonia-lyase, chitinases, β-1,3-glucanase enzymes in litchi fruits [Citation40]. In general, there are tremendous application prospects for BVCs from Bacillus sp., such as in post-harvest preservation, soil fumigation and stimulating plant defence and growth, potentially by serving as signals for plant-microbial interaction. The major offset for the application of these compounds lies in their very volatile nature and low water solubility, which renders their use under field conditions in both forestry and fruit tree production less feasible.
Induced systemic resistance (ISR) in plant hosts by Bacillus species
Several Bacillus species have been reported to produce elicitor molecules or compounds that facilitate the development of induced systemic resistance of host plants against diseases and pests (). For instance, Enebak and Carey [Citation181] studied two strains of B. pumilus strain SE34 and INR7 for the ability to induce systemic resistance based on the suppression of fusiform rust (fusoid swelling/galls) caused by Cronartium quercuum f. sp. fusiforme in Pinus taeda for two years. The application of the bacterial agents by seed treatment substantially suppressed fusiform galls in pine seedlings within six months after fungal infection [Citation181]. Since the bacterial agents were applied by seed treatment and suppressed the disease symptoms after six months, the effect could be attributed to the elicitation of ISR rather than antibiosis (since the treatments had no direct contact with the fungal pathogen). However, there is still lack of molecular and physiological evidence to concretely demonstrate the mechanism by which these strains caused ISR in pine.
Several other studies have demonstrated the elicitation of plant defence proteins and pathogen-related proteins (PRP) by Bacillus species which could be exploited to protect forest and fruit trees from phytopathogenic infections. However, most forest and fruit trees have a slow growth rate and are highly lignified, making it difficult to readily obtain the soft plant tissues for the extraction of proteins used to analyse ISR. To obtain accurate results, researchers often take samples from young plant tissues to analyse the accumulation of defence proteins and genes related to ISR, especially in fast-growing plants such as Arabidopsis. Yet in perennial plants with a slow rate of growth, most researchers rely on the observation of disease symptoms to draw conclusions on ISR.
In this review, we highlight studies that reported the elicitation of ISR based on molecular evidence (expression of defence genes and accumulation of PR proteins) or based on disease symptom analysis after priming the host plant with Bacillus sp. and the phytopathogen. For instance, Niu et al. [Citation182] reported the elicitation of ISR by B. cereus strain AR156 against P. syringae pv. tomato strain DC3000 in Arabidopsis ecotype Col-0 plants. The primed plants showed an increase in biomass production, reduced disease severity and reduced pathogen density in the leaves [Citation182]. In addition, the primed plants expressed defence-related genes (PR1, PR2, PR5 and PDF1.2) for salicylic acid (SA)- dependent, jasmonic acid (JA)-dependent and ethylene (ET)-dependent signalling pathways [Citation182]. Even though P. syringae pv. tomato is a known pathogen for tomato plants, it was used as a model pathogen in fast-growth model Col-0 plants for the purpose of confirming the elicitation of ISR by B. cereus strain AR156. This evidence could indicate the potential of Bacillus strains AR156 to induce defence responses against plant pathogens such as P. syringae in other plants such as Solanum lycopersicum, and similar models should be studied in forest seddlings and fruit trees.
Another bacterium, B. velezensis strain CLA178 induced ISR through the SA- or ET- signalling pathways, stimulating the production of plant defence-related genes and hormones, reactive oxygen species (ROS) and antioxidants in Arabidopsis plants infected with Agrobacterium tumefaciens strain C58 [Citation29]. The elicitation of ISR by strain CLA178 suppressed the infection of A. tumefaciens and consequently reduced the symptoms of crown gall disease (crown gall tumour biomass), improved plant biomass and increased the chlorophyll content/photosynthetic rate in Rosa multiflora plants [Citation29]. The methodology of Chen et al. [Citation29] is of particular interest in studying ISR in perennial plants like forest and fruit trees, by investigating the presence of elicitor molecules using model plants such as Arabidopsis and evaluating the disease symptoms and plant growth improvement in the host plant.
Ongena et al. [Citation32] demonstrated that B. subtilis strain Bs168 produced surfactins and fengycins as antimicrobial lipopeptides. The overexpression of either or both lipopeptides (Bs2500 for overexpressed surfactins and Bs2508 for overexpressed surfactins and fengycins) were reported to cause ISR-mediated protection in tomato and bean plants against Botrytis infection [Citation32]. The plants pre-inoculated with strains Bs168, Bs2500 and Bs2508 stimulated the production of antifungal proteins such as lipoxygenase and lipid hydroperoxidase in the leaves of tomato plants [Citation32]. Li et al. [Citation183] reported that B. amyloliquefaciens strain LJ02 elicited ISR in cucumber against powdery mildew disease caused by Sphaerotheca fuliginea. The plants treated with strain LJ02 showed reduced disease incidence, expressed PR-1 gene and produced plant defence enzymes such as superoxide dismutase, peroxidase, polyphenol oxidase and phenylalanine ammonia lyase [Citation183]. The inoculated plants also produced pathogen-related (PR) hormone, salicylic acid (SA) in cucumber leaves and released these defence chemicals into the rhizosphere to inhibit fungal spore germination [Citation183]. Bargabus et al. [Citation184] reported that B. pumilus strains 203-6 and 203-7 elicited the production of chitinase and β-1,3-glucanase and biphasic hydrogen peroxide as pathogen related proteins and subsequently reduced the severity of Cercospora leaf spot caused by Cercospora beticola Sacc in sugar beet leaves. The level of PR protein elicitation in sugar beet by strain 203-6 and 203-7 was comparable to that caused by Bacillus mycoides strain Bac J, which also primed for ISR through chitinase, β-1,3-glucanase and chemical inducer acibenzolar-S-methyl [Citation28,Citation184]. In another study, Kim et al. [Citation185] demonstrated that Bacillus sp. strain JS inhibited the growth of R. solani (leaf spot disease) and P. nicotianae (black shank disease) and controlled disease symptoms in tobacco. The pre-treatment of tobacco leaves with the BVCs emitted by Bacillus sp. strain JS up-regulated antifungal enzyme genes such as PR-2 gene (encoding β-1,3-glucanase), acidic PR-3 and PR-4 genes (chitinase), acidic PR-9 (peroxidase) and PR-14 (lipid transfer protein) [Citation185]. Tahir et al. [Citation186] reported three major antifungal BVCs (benzaldehyde, 1,2-benzisothiazol-3(2 H)-one and 1,3-butadiene) from B. amyloliquefaciens strain FZB42 and Bacillus artrophaeus strain LSSC22. Priming the tobacco plants with the BVCs from strains FZB42 and LSSC22 decreased bacterial wilt disease symptoms by regulating the expression of genes related to wilt resistance and pathogen defence such as EDS1 and NPR1 (SA pathway of ISR) [Citation186]. Moreover, the BVCs from strains FZB42 and LSSC22 altered the virulance-related genes in R. solanacearum such as global virulence regulator (PhcA), type III and IV secretion system, extracellular polysaccharides and chemotaxis-related genes [Citation186]. In another related study, B. cereus strain C1L produced dimethyl disulphide, which protected tobacco plants against B. cinerea and corn plants against Cochliobolus heterostrophus through the elicitation of ISR, but no analysis of defence proteins was confirmed [Citation187]. Other BVC elicitors of ISR produced by Bacillus strains include; 2,3-Butanediol from B. subtilis strain GB03 and B. amyloliquefaciens strain IN 937a which suppressed bacterial wilt caused by Erwinia carotovora subsp. carotovora through the ET-, SA-, and JA- signalling pathways in Arabidopsis [Citation188].
Two other strains of B. pumilus, SE34 (via the SA-signalling pathway) and T4 (undetermined pathway), elicited ISR in Arabidopsis, and both strains effectively suppressed bacterial leaf spots disease severity in plants infected with P. syringae pv. maculicola [Citation189]. In another study, the pre-treatment of tobacco plants with B. pumilus strain SE34 elicited ISR in via the SA-signalling pathway only after the plants were infected with Peronospora tabacina, leading to the suppression of blue mould disease severity [Citation190].
Despite the important role of SA in stimulating the production of PRP, some phytopathogens such as Pseudomonas, Xanthomonas, Ralstonia and Erwinia species have been demonstrated to encode hypersensitive response and pathogenicity (hrp) genes. These hrp genes regulate the secretion of effector proteins directly into the host cells via a type III secretion system (TTSS) to overcome the SA-induced defence mechanisms and subsequently infect the host cells [Citation191]. Moreover, the elicitation of the SA-, JA- and ET-signalling pathway of defence induction by Bacillus sp. tend to occur only after pathogen challenge [Citation190]. This weak point could aid the TTSS pathogenicity mechanism unless the biocontrol agent deploys other antifungal mechanisms such as antibiosis and competition to suppress the pathogen density and infection.
Interestingly, some Bacillus sp. display multiple biocontrol mechanisms against phytopathogens, including antibiosis, competition and the elicitation of ISR. For instance, B. velezensis strain RHF4.1–25 controlled rice blast disease caused by Pyricularia oryzae by eliciting ISR via root application and by direct antagonism through the production of cyclic lipopeptides (fengycin and iturin) [Citation31]. In this case, the inhibition of spore germination and mycelial growth by fengycin and iturin (antibiosis) lowers the pathogen density, which would in turn suppress the TTSS pathogenicity pathway while simultaneously priming for the production of plant defence proteins. Another strain, B. amyloliquefaciens 41B-1, controlled Verticillium wilt in cotton caused by V. dahliae through direct antibiosis by iturins which suppress microsclerotia germination [Citation192]. At the same time, treatment with iturin isoforms enhanced plant defence responses by inducing pathogen-associated molecular pattern-triggered immunity in the cotton plant and suppressed disease severity by more than 50% [Citation192]. This dual effect is perhaps the model that best explains the role of Bacillus sp. in plant protection. Moreover, most antimicrobial metabolites have a faster rate of dissociation in the soil [Citation87], and the microbial population of Bacillus sp. tend to drop back to natural levels in a relatively short period after inoculation due to nutrient limitation and competition with other native microbes [Citation193,Citation194]. This indicates that direct antibiosis alone would not be sustainable to suppress phytopathogenic populations, but when coupled with the elicitation of ISR, Bacillus sp. becomes a lethal arsenal against plant diseases over a long period. Other mechanisms include plant-microbial association, where some Bacillus strains colonize the rhizosphere or live endophytically in the host plant where they reproduce and continue secreting antimicrobial metabolites to suppress phytopathogenic infections [Citation195–197]. The mechanisms of endophytic and rhizospheric association and the elicitation of ISR should be given more research attention while studying plant-microbial interaction in forest and fruit tree production and nursery seedlings.
Similarly, studies have shown the Bacillus species have reported to elicit plant defence response against herbivorous activity of phytophagous insects such as sap-sucking Hemiptera pests like aphids. For example, Veselova et al. [Citation198] reported an effective suppression of Schizaphis graminum Rond (greenbug/wheat aphid) through direct aphicidal activity and indirectly through the elicitation of ISR in wheat plants treated with B. subtilis Cohn. strain 26 D (via the SA-signalling pathway) and B. thuringiensis Berliner strains V-6066 and V-5689 (via the JA-signalling pathway). This was evidenced by the increased expression of NADPH oxidases, PR-6 and PR-9 defence-related genes, the regeneration of hydrogen peroxide and peroxidase activity [Citation198]. Another endophytic B. velezensis strain YC7010 was previously reported to elicit ISR against bacterial panicle blight (Burkholderia glumae) and bacterial blight (X. oryzae pv. oryzae) and fungal pathogens in rice [Citation30]. Strain YC7010 was also independently demonstrated to elicit ISR against Myzus persicae (green peach aphid) by inducing hydrogen peroxide accumulation, cell death and callose deposition in leaves of Arabidopsis plants [Citation199].
The elicitation of ISR substantially reduced settling, feeding and reproduction of M. persicae on Arabidopsis leaves through hypersensitive responses such as increasing the expression of the PHYTOALEXIN DEFICIENT4 (PAD4) gene, which promotes senescence as a means of regulating herbivorous activity [Citation199]. Strain YC7010 also suppressed the BOTRYTIS-INDUCED KINASE1 (BIK1) gene, which causes cell death as a negative response to block Botrytis infection, and its down-regulation could reduce have reduced the hypersensitivity [Citation199]. In another study, the inoculation of B. subtilis strain BEB-DN in tomato rhizosphere elicited long-term JA-dependent and JA-independent ISR against Bemisia tabaci which suppressed the number of pupae that moulted into adults and caused growth retardation [Citation200]. Strain BEB-DN mediated pest resistance response in the tomato plants through phenylpropanoid and terpenoid biosynthetic pathways, heat shock protein 90 chaperonin and the down-regulation of pathogenesis and hypersensitive response genes [Citation200]. Endophytic B. subtilis strains EPCO 102 and EPCO 16 also elicited ISR against the aphid infestation in cotton [Citation201]. The pest resistance effect was increased by the addition of chitin substrate in the bacterial medium, leading to reduced pest infestation and induced the activity of peroxidase, polyphenol oxidase, phenylalanine ammonia-lyase, chitinase, β-1.3-glucanase and phenol accumulation in cotton [Citation201]. Herman et al. [Citation202] reported a slightly different phenomenon where treatment with B. subtilis and B. amyloliquefaciens and their combinations did not reduce the population of M. persicae Sulzer in bell pepper plants but rather improved the plant tolerance to aphids. Unfortunately, there is no molecular or physiochemical data in their study that could be used to assess the possible mode of interaction. It is plausible that the increased crop growth observed in the bacterial treatment compensated for the aphid feeding activity and led to higher crop performance compared to conventional (imidacloprid-treated plants) treatment despite the reverse observation in the aphid population [Citation202]. Zehnder et al. [Citation203] reported that field inoculation of B. pumilus strain INR-7 elicited ISR in cucumber plants against Diabrotica undecimpunctata howardi Barber (spotted cucumber beetle) and the Acalymma vittatum (F.) (striped cucumber beetle). The results of Zehnder et al. [Citation203] demonstrated effective control of the beetle population in cucumber (more than in the chemical insecticide treatment when the treatments were applied at peak infestation levels), which consequently improved productivity [Citation203]. Most studies that have demonstrated the elicitation of ISR by several Bacillus species against different insect pests have reported almost similar modes of action (signalling pathways and defence proteins), with only a few intriguing exceptions that require further investigations, such as in Herman et al. [Citation202]. The insecticidal role of Bacillus sp. (excluding the well-established role of B. thuringiensis insecticidal toxins) have not been fully studied, yet they hold great prospects in plant protection as effective biopesticides.
Conclusions
Bacillus species interact with their environment by producing a highly diverse and versatile array of metabolites that enable them to survive competition in a complex soil environment and establish successful beneficial plant-microbial associations with their host plant. Bacillus species are perhaps the most prospective biocontrol agents for the future development of commercial products in the field of agricultural crops, forestry and fruit tree production. Among their various beneficial interactions with plants, the role of antimicrobial metabolites has been widely reported by researchers as biological control strategies that are highly efficient and eco-friendly in the management of phytopathogens, insect pests and plant parasitic and free-living nematodes and elicitation of ISR. While a lot of research has been done in the general area of biological control using Bacillus species, there are still gaps that demand considerable research attention to enhance the application of these promising bacterial agents. Some researchers have reported intriguing results with unknown modes or mechanisms of action. For instance, the elicitation of ISR through the Bacillus-plant-pathogen interaction has been relatively well reported as a biocontrol strategy for suppressing phytopathogens or insect pest populations to reduce plant damage. However, the ISR eliciting factors and signalling pathways, such as the salicylic acid, jasmonic acid and ethylene pathways, and the resulting molecular indicators (such as the expression of plant defence genes) and phytochemical indicators such as plant defence proteins (chitinase, β-1,3-glucanase, POD, PPO, PAL and biphasic hydrogen peroxide etc.), have been variedly reported and some are still unknown. Even though the impact of ISR such as reduced disease severity has been relatively well reported, more research is required to elucidate the involved mechanisms and how they can be optimized for maximum plant protection. There is also an intriguing notion that Bacillus species could elicit plant tolerance to the herbivorous activity of insect pests without necessarily inducing plant defence responses. This could be a biologically inexpensive strategy under low levels of infestation and requires further investigation to elucidate the potential of using Bacillus species to promote disease or pest tolerance in agricultural crops, forestry and fruit trees. A large body of research has been devoted to the biocontrol potential of Bacillus sp. to control fungal and bacterial phytopathogens in agricultural crops, forestry and fruit trees through the production of lipopeptide compounds such as iturins, fengycin, surfactins, bacillomycins etc., polyketides such as macrolactins, bacilysin and difficidin and several BVCs. These biocidal metabolites and signalling molecules control fungal and bacterial phytopathogens by direct antagonism mainly through antibiosis and cell wall/membrane perforation and indirectly through competition, biofilm formation, iron scavenging and the elicitation of ISR. There is a need for optimization studies to produce these bioactive compounds in effective formulations and enhance their application in agricultural crops, forestry and fruit tree production. Some Bacillus species may not sufficiently control plant pests and diseases, and more research would be necessary to study their co-inoculation with other strains with varying modes of action or their application in an integrated pest/disease management system. Future research should also continue to elucidate the unknown molecular mechanisms of some of the biocontrol weapons produced by Bacillus sp., including the elicitor molecules for ISR, polyketides and BVCs to improve our understanding and facilitate innovative applications of these effective microbes in plant protection.
Author contributions
Conceptualization, Y.S.A., H.A. and H-I.L.; writing—original draft preparation, Y.S.A., H.A. and H-I.L.; writing—Critical review and editing, H.A., H-I.L., J-H.M., S-J.W., C.V., S-I.C., J-Y.Y. and Y.S.A.; supervision, Y.S.A.; project administration, Y.S.A.; and funding acquisition, Y.S.A. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The article reviewed is available in the public domain and other data has been used in this article.
Additional information
Funding
References
- Cerda R, Avelino J, Gary C, et al. Primary and secondary yield losses caused by pests and diseases: assessment and modeling in coffee. PLoS One. 2017;12(1):1. doi: 10.1371/journal.pone.0169133.
- Brown B, Wylie F. Diseases and pests of Australian Forest nurseries: past and present. In: Proceedings of the First Meeting of IUFRO Working Party S.2.07–09 (Diseases and Insects in Forest Nurseries), Sutherland, J.R., Glover,S.G. (Eds). August 23-30, 1990, Victoria, British Colombia, Canada. The Pacific Forestry Centre (Forestry Canada, Pacific and Yukon Region), Victoria, British Colombia, Canada, 1991. p. 3–31.
- Pimentel D. Green revolution agriculture and chemical hazards. Sci Total Environ. 1996;188: S86–S98. doi: 10.1016/0048-9697(96)05280-1.
- Dhaliwal G, Jindal V, Dhawan A. Insect pest problems and crop losses: changing trends. Indian J Ecol. 2010;37:1–7.
- Abubakar Y, Tijjani H, Egbuna C, et al. Pesticides, history, and classification. In: Egbuna C, Sawicka B, editors. Natural remedies for pest, disease and weed control. Cambridge (MA): Academic Press; 2020. p. 29–42.
- Kole R, Roy K, Panja B, et al. Use of pesticides in agriculture and emergence of resistant pests. IJAH. 2019;58(2-SPL):53–70. doi: 10.36062/ijah.58.2SPL.2019.53-70.
- Hawkins NJ, Bass C, Dixon A, et al. The evolutionary origins of pesticide resistance. Biol Rev Camb Philos Soc. 2019;94(1):135–155. doi: 10.1111/brv.12440.
- Guedes RNC, Walse SS, Throne JE. Sublethal exposure, insecticide resistance, and community stress. Curr Opin Insect Sci. 2017;21:47–53. doi: 10.1016/j.cois.2017.04.010.
- Thakore Y. The biopesticide market for global agricultural use. Ind Biotechnol. 2006;2(3):194–208. doi: 10.1089/ind.2006.2.194.
- Zaller JG. Daily poison: pesticides-an underestimated danger. Springer Nature: Cham, Switzerland; 2020, 315.
- Kumar V, Kumar P. Pesticides in agriculture and environment: impacts on human health. In: Kumar V, Kumar R, Singh J, Kumar P. editors. Contaminants in agriculture and environment: health risks and remediation. Haridwar, India: Agriculture and Environmental Science Academy. 2019. p. 76.
- Berini F, Katz C, Gruzdev N, et al. Microbial and viral chitinases: attractive biopesticides for integrated pest management. Biotechnol Adv. 2018;36(3):818–838. doi: 10.1016/j.biotechadv.2018.01.002.
- Copping LG, Menn JJ. Biopesticides: a review of their action, applications and efficacy. Pest Manag Sci. 2000;56(8):651–676. doi: 10.1002/1526-4998(200008)56:8<651::AID-PS201>3.0.CO;2-U.
- Gupta S, Dikshit A. Biopesticides: an ecofriendly approach for pest control. J Biopest. 2010;3:186.
- Ferreira VB, Estrella LF, Alves MGR, et al. Residues of legacy organochlorine pesticides and DDT metabolites in highly consumed fish from the polluted Guanabara Bay, Brazil: distribution and assessment of human health risk. J Environ Sci Health B. 2020;55(1):30–41. doi: 10.1080/03601234.2019.1654808.
- Bempah CK, Asomaning J, Boateng J. Market basket survey for some pesticides residues in fruits and vegetables from Ghana. J Microbiol Biotechnol Food Sci. 2020;9:850–871.
- Scheepmaker J, Butt T. Natural and released inoculum levels of entomopathogenic fungal biocontrol agents in soil in relation to risk assessment and in accordance with EU regulations. Biocontrol Sci Technol. 2010;20(5):503–552. doi: 10.1080/09583150903545035.
- Bren A, Hart Y, Dekel E, et al. The last generation of bacterial growth in limiting nutrient. BMC Syst Biol. 2013;7(1):27. doi: 10.1186/1752-0509-7-27.
- Köhl J, Booij K, Kolnaar R, et al. Ecological arguments to reconsider data requirements regarding the environmental fate of microbial biocontrol agents in the registration procedure in the European Union. BioControl. 2019;64(5):469–487. doi: 10.1007/s10526-019-09964-y.
- Ajuna HB, Kim I, Han YS, et al. Aphicidal activity of Bacillus thuringiensis strain AH-2 against cotton aphid (Aphis gossypii). Entomol Res. 2021;51(4):151–160. doi: 10.1111/1748-5967.12481.
- Bonaterra A, Badosa E, Daranas N, et al. Bacteria as biological control agents of plant diseases. Microorganisms. 2022;10(9):1759. doi: 10.3390/microorganisms10091759.
- Lee J, Kim S, Jung H, et al. Exploiting bacterial genera as biocontrol agents: mechanisms, interactions and applications in sustainable agriculture. J Plant Biol. 2023;66(6):485–498. doi: 10.1007/s12374-023-09404-6.
- Moon J-H, Won S-J, Maung CEH, et al. The role of Lysobacter antibioticus HS124 on the control of fall webworm (Hyphantria cunea drury) and growth promotion of Canadian poplar (Populus canadensis moench) at Saemangeum reclaimed land in Korea. Microorganisms. 2021;9(8):1580. doi: 10.3390/microorganisms9081580.
- Kwon J-H, Won S-J, Moon J-H, et al. Control of fungal diseases and increase in yields of a cultivated jujube fruit (Zizyphus jujuba miller var. Inermis rehder) orchard by employing Lysobacter antibioticus HS124. Forests. 2019;10(12):1146. doi: 10.3390/f10121146.
- Simon J-C, Marchesi JR, Mougel C, et al. Host-microbiota interactions: from holobiont theory to analysis. Microbiome. 2019;7(1):5. doi: 10.1186/s40168-019-0619-4.
- Dolatabadian A. Plant–microbe interaction. Biology. 2020;10(1):15. doi: 10.3390/biology10010015.
- Ali SAM, Sayyed R, Mir MI, et al. Induction of systemic resistance in maize and antibiofilm activity of surfactin from Bacillus velezensis MS20. Front Microbiol. 2022;13:879739. doi: 10.3389/fmicb.2022.879739.
- Bargabus R, Zidack N, Sherwood J, et al. Characterisation of systemic resistance in sugar beet elicited by a non-pathogenic, phyllosphere-colonizing Bacillus mycoides, biological control agent. Physiol Mol Plant Pathol. 2002;61(5):289–298. doi: 10.1006/pmpp.2003.0443.
- Chen L, Wang X, Ma Q, et al. Bacillus velezensis CLA178-induced systemic resistance of rosa multiflora against crown gall disease. Front Microbiol. 2020;11:587667. doi: 10.3389/fmicb.2020.587667.
- Chung EJ, Hossain MT, Khan A, et al. Bacillus oryzicola sp. nov., an endophytic bacterium isolated from the roots of rice with antimicrobial, plant growth promoting, and systemic resistance inducing activities in rice. Plant Pathol J. 2015;31(2):152–164. doi: 10.5423/PPJ.OA.12.2014.0136.
- Lam VB, Meyer T, Arias AA, et al. Bacillus cyclic lipopeptides iturin and fengycin control rice blast caused by Pyricularia oryzae in potting and acid sulfate soils by direct antagonism and induced systemic resistance. Microorganisms. 2021;9(7):1441. doi: 10.3390/microorganisms9071441.
- Ongena M, Jourdan E, Adam A, et al. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol. 2007;9(4):1084–1090. doi: 10.1111/j.1462-2920.2006.01202.x.
- Zhao H, Shao D, Jiang C, et al. Biological activity of lipopeptides from Bacillus. Appl Microbiol Biotechnol. 2017;101(15):5951–5960. doi: 10.1007/s00253-017-8396-0.
- Villegas-Escobar V, González-Jaramillo LM, Ramírez M, et al. Lipopeptides from Bacillus sp. EA-CB0959: active metabolites responsible for in vitro and in vivo control of Ralstonia solanacearum. Biol Control. 2018;125:20–28. doi: 10.1016/j.biocontrol.2018.06.005.
- Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16(3):115–125. doi: 10.1016/j.tim.2007.12.009.
- Chen X-H, Scholz R, Borriss M, et al. Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J Biotechnol. 2009;140(1-2):38–44. doi: 10.1016/j.jbiotec.2008.10.015.
- Miao S, Liang J, Xu Y, et al. Bacillaene, sharp objects consist in the arsenal of antibiotics produced by Bacillus. J Cell Physiol. 2023:1–15. doi: 10.1002/jcp.30974.
- Salazar F, Ortiz A, Sansinenea E. A strong antifungal activity of 7-O-succinyl macrolactin a vs macrolactin a from Bacillus amyloliquefaciens ELI149. Curr Microbiol. 2020;77(11):3409–3413. doi: 10.1007/s00284-020-02200-2.
- Choub V, Won S-J, Ajuna HB, et al. Antifungal activity of volatile organic compounds from Bacillus velezensis CE 100 against Colletotrichum gloeosporioides. Horticulturae. 2022;8(6):557. doi: 10.3390/horticulturae8060557.
- Zhao P, Li P, Wu S, et al. Volatile organic compounds (VOCs) from Bacillus subtilis CF-3 reduce anthracnose and elicit active defense responses in harvested litchi fruits. AMB Exp. 2019;9(1):119. doi: 10.1186/s13568-019-0841-2.
- Li X-Y, Mao Z-C, Wu Y-X, et al. Comprehensive volatile organic compounds profiling of Bacillus species with biocontrol properties by head space solid phase microextraction with gas chromatography-mass spectrometry. Biocontrol Sci Technol. 2015;25(2):132–143. doi: 10.1080/09583157.2014.960809.
- Saxena AK, Kumar M, Chakdar H, et al. Bacillus species in soil as a natural resource for plant health and nutrition. J Appl Microbiol. 2020;128(6):1583–1594. doi: 10.1111/jam.14506.
- Won S-J, Moon J-H, Ajuna HB, et al. Biological control of leaf blight disease caused by Pestalotiopsis maculans and growth promotion of Quercus acutissima Carruth container seedlings using Bacillus velezensis CE 100. Int J Mol Sci. 2021;22(20):11296. doi: 10.3390/ijms222011296.
- Moon J-H, Won S-J, Maung CEH, et al. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes growth of Japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms. 2021;9(4):821. doi: 10.3390/microorganisms9040821.
- Hong S, Kim TY, Won S-J, et al. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms. 2022;10(2):365. doi: 10.3390/microorganisms10020365.
- Moon J-H, Ajuna HB, Won S-J, et al. Entomopathogenic potential of Bacillus velezensis CE 100 for the biological control of termite damage in wooden architectural buildings of Korean cultural heritage. Int J Mol Sci. 2023;24(9):8189. doi: 10.3390/ijms24098189.
- Moon J-H, Ajuna HB, Won S-J, et al. The anti-termite activity of Bacillus licheniformis PR2 against the subterranean termite, Reticulitermes speratus kyushuensis Morimoto (Isoptera: Rhinotermitidae). Forests. 2023;14(5):1000. doi: 10.3390/f14051000.
- Syed T, Askari M, Meng Z, et al. Current insights on vegetative insecticidal proteins (Vip) as next generation pest killers. Toxins. 2020;12(8):522. doi: 10.3390/toxins12080522.
- Palma L, Muñoz D, Berry C, et al. Molecular and insecticidal characterization of a novel cry-related protein from Bacillus thuringiensis toxic against Myzus persicae. Toxins. 2014;6(11):3144–3156. doi: 10.3390/toxins6113144.
- Dimopoulou A, Theologidis I, Benaki D, et al. Direct antibiotic activity of bacillibactin broadens the biocontrol range of Bacillus amyloliquefaciens MBI600. Msphere. 2021;6(4):e0037621. doi: 10.1128/mSphere.00376-21.
- Soni R, Keharia H. Phytostimulation and biocontrol potential of gram-positive endospore-forming Bacilli. Planta. 2021;254(3):49. doi: 10.1007/s00425-021-03695-0.
- Yánez-Mendizabal V, Viñas I, Usall J, et al. Endospore production allows using spray-drying as a possible formulation system of the biocontrol agent Bacillus subtilis CPA-8. Biotechnol Lett. 2012;34(4):729–735. doi: 10.1007/s10529-011-0834-y.
- Sadfi N, Cherif M, Fliss I, et al. Evaluation of bacterial isolates from salty soils and Bacillus thuringiensis strains for the biocontrol of Fusarium dry rot of potato tubers. J Plant Pathol. 2001; 83, 101–117.
- Devi PV, Duraimurugan P, Chandrika K. Bacillus thuringiensis-based nanopesticides for crop protection. In: Koul O, editor. Nano-biopesticides today and future perspectives. Cambridge, MA, USA: Academic Press; 2019. p. 249–260.
- Fira D, Dimkić I, Berić T, et al. Biological control of plant pathogens by Bacillus species. J Biotechnol. 2018;285:44–55. doi: 10.1016/j.jbiotec.2018.07.044.
- Geissler M, Heravi KM, Henkel M, et al. Lipopeptide biosurfactants from Bacillus species. In: Douglas GH, Daniel KYS, Ashby RD, editors. Biobased surfactants. 2nd ed. Urbana (IL): AOCS Press; 2019. p. 205–240.
- Théatre A, Cano-Prieto C, Bartolini M, et al. The surfactin-like lipopeptides from bacillus spp.: natural biodiversity and synthetic biology for a broader application range. Front Bioeng Biotechnol. 2021;9:623701. doi: 10.3389/fbioe.2021.623701.
- Liu X, Ren B, Gao H, et al. Optimization for the production of surfactin with a new synergistic antifungal activity. PLoS One. 2012;7(5):e34430. doi: 10.1371/journal.pone.0034430.
- Zeriouh H, de Vicente A, Pérez-García A, et al. Surfactin triggers biofilm formation of Bacillus subtilis in melon phylloplane and contributes to the biocontrol activity. Environ Microbiol. 2014;16(7):2196–2211. doi: 10.1111/1462-2920.12271.
- Stoll A, Salvatierra-Martínez R, González M, et al. The role of surfactin production by Bacillus velezensis on colonization, biofilm formation on tomato root and leaf surfaces and subsequent protection (ISR) against Botrytis cinerea. Microorganisms. 2021;9(11):2251. doi: 10.3390/microorganisms9112251.
- Park G, Nam J, Kim J, et al. Structure and mechanism of surfactin peptide from Bacillus velezensis antagonistic to fungi plant pathogens. Bull Korean Chem Soc. 2019;40(7):704–709. doi: 10.1002/bkcs.11757.
- Meena KR, Kanwar SS. Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. Biomed Res Int. 2015;2015:473050–473059. doi: 10.1155/2015/473050.
- Thérien M, Kiesewalter HT, Auria E, et al. Surfactin production is not essential for pellicle and root-associated biofilm development of Bacillus subtilis. Biofilm. 2020;2:100021. doi: 10.1016/j.bioflm.2020.100021.
- Sarwar A, Hassan MN, Imran M, et al. Biocontrol activity of surfactin a purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol Res. 2018;209:1–13. doi: 10.1016/j.micres.2018.01.006.
- Bouchard-Rochette M, Machrafi Y, Cossus L, et al. Bacillus pumilus PTB180 and Bacillus subtilis PTB185: production of lipopeptides, antifungal activity, and biocontrol ability against Botrytis cinerea. Biol Control. 2022;170:104925. doi: 10.1016/j.biocontrol.2022.104925.
- Ma Z, Zhang S, Zhang S, et al. Isolation and characterization of a new cyclic lipopeptide surfactin from a marine-derived Bacillus velezensis SH-B74. J Antibiot. 2020;73(12):863–867. doi: 10.1038/s41429-020-0347-9.
- Li MS, Piccoli DA, McDowell T, et al. Evaluating the biocontrol potential of Canadian strain Bacillus velezensis 1B-23 via its surfactin production at various pHs and temperatures. BMC Biotechnol. 2021;21(1):31. doi: 10.1186/s12896-021-00690-x.
- Grady EN, MacDonald J, Ho MT, et al. Characterization and complete genome analysis of the surfactin-producing, plant-protecting bacterium Bacillus velezensis 9D-6. BMC Microbiol. 2019;19(1):5. doi: 10.1186/s12866-018-1380-8.
- Arjes HA, Vo L, Dunn CM, et al. Biosurfactant-mediated membrane depolarization maintains viability during oxygen depletion in Bacillus subtilis. Curr Biol. 2020;30(6):1011.e1016–1022.e1016. doi: 10.1016/j.cub.2020.01.073.
- Arjes HA, Vo L, Dunn CM, et al. Biosurfactant production maintains viability in anoxic conditions by depolarizing the membrane in Bacillus subtilis. bioRxiv. 2019;720532.
- Chen X, Lu Y, Shan M, et al. A mini-review: mechanism of antimicrobial action and application of surfactin. World J Microbiol Biotechnol. 2022;38(8):143. doi: 10.1007/s11274-022-03323-3.
- Khan N, Maymon M, Hirsch AM. Combating Fusarium infection using Bacillus-based antimicrobials. Microorganisms. 2017;5(4):75. doi: 10.3390/microorganisms5040075.
- Gong A-D, Li H-P, Yuan Q-S, et al. Antagonistic mechanism of iturin a and plipastatin a from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS One. 2015;10(2):e0116871. doi: 10.1371/journal.pone.0116871.
- Zhang R-S, Wang F-G, Qi Z-Q, et al. Iturins produced by Bacillus velezensis Jt84 play a key role in the biocontrol of rice blast disease. Biol Control. 2022;174:105001. doi: 10.1016/j.biocontrol.2022.105001.
- Dunlap CA, Bowman MJ, Rooney AP. Iturinic lipopeptide diversity in the Bacillus subtilis species group–important antifungals for plant disease biocontrol applications. Front Microbiol. 2019;10:1794. doi: 10.3389/fmicb.2019.01794.
- Ma Z, Wang N, Hu J, et al. Isolation and characterization of a new iturinic lipopeptide, mojavensin a produced by a marine-derived bacterium Bacillus mojavensis B0621A. J Antibiot. 2012;65(6):317–322. doi: 10.1038/ja.2012.19.
- Xiao J, Guo X, Qiao X, et al. Activity of fengycin and iturin a isolated from Bacillus subtilis Z-14 on Gaeumannomyces graminis var. tritici and soil microbial diversity. Front Microbiol. 2021;12:682437. doi: 10.3389/fmicb.2021.682437.
- Zhang D, Yu S, Zhao D, et al. Inhibitory effects of non-volatiles lipopeptides and volatiles ketones metabolites secreted by Bacillus velezensis C16 against alternaria solani. Biol Control. 2021;152:104421. doi: 10.1016/j.biocontrol.2020.104421.
- Tang Z, Cao X, Zhang H. Production of iturin a by Bacillus velezensis ND and its biological control characteristics. J Basic Microbiol. 2023;63(2):179–189. doi: 10.1002/jobm.202200473.
- Arrebola E, Jacobs R, Korsten L. Iturin a is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J Appl Microbiol. 2010;108(2):386–395. doi: 10.1111/j.1365-2672.2009.04438.x.
- Alvarez F, Castro M, Príncipe A, et al. The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J Appl Microbiol. 2012;112(1):159–174. doi: 10.1111/j.1365-2672.2011.05182.x.
- Singh P, Xie J, Qi Y, et al. A thermotolerant marine Bacillus amyloliquefaciens S185 producing iturin A5 for antifungal activity against Fusarium oxysporum f. sp. cubense. Mar Drugs. 2021;19(9):516. doi: 10.3390/md19090516.
- Wang J, Qiu J, Yang X, et al. Identification of lipopeptide iturin a produced by Bacillus amyloliquefaciens NCPSJ7 and its antifungal activities against Fusarium oxysporum f. sp. niveum. Foods. 2022;11(19):2996. doi: 10.3390/foods11192996.
- Zeriouh H, Romero D, Garcia-Gutierrez L, et al. The iturin-like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Mol Plant Microbe Interact. 2011;24(12):1540–1552. doi: 10.1094/MPMI-06-11-0162.
- Dang Y, Zhao F, Liu X, et al. Enhanced production of antifungal lipopeptide iturin a by Bacillus amyloliquefaciens LL3 through metabolic engineering and culture conditions optimization. Microb Cell Fact. 2019;18(1):68. doi: 10.1186/s12934-019-1121-1.
- Yue H, Zhong J, Li Z, et al. Optimization of iturin a production from Bacillus subtilis ZK-H2 in submerge fermentation by response surface methodology. 3 Biotech. 2021;11(2):36. doi: 10.1007/s13205-020-02540-7.
- Asaka O, Shoda M. Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl Environ Microbiol. 1996;62(11):4081–4085. doi: 10.1128/aem.62.11.4081-4085.1996.
- Mizumoto S, Hirai M, Shoda M. Enhanced iturin a production by Bacillus subtilis and its effect on suppression of the plant pathogen Rhizoctonia solani. Appl Microbiol Biotechnol. 2007;75(6):1267–1274. doi: 10.1007/s00253-007-0973-1.
- Steller S, Vater J. Purification of the fengycin synthetase multienzyme system from Bacillus subtilis b213. J Chromatogr B Biomed Sci Appl. 2000;737(1-2):267–275. doi: 10.1016/s0378-4347(99)00481-8.
- Yánez-Mendizábal V, Zeriouh H, Viñas I, et al. Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur J Plant Pathol. 2012;132(4):609–619. doi: 10.1007/s10658-011-9905-0.
- Guo Q, Dong W, Li S, et al. Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiol Res. 2014;169(7-8):533–540. doi: 10.1016/j.micres.2013.12.001.
- Wang F, Xiao J, Zhang Y, et al. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mould in postharvest strawberry fruit. Postharvest Biol Technol. 2021;174:111456. doi: 10.1016/j.postharvbio.2020.111456.
- Chen M, Wang J, Liu B, et al. Biocontrol of tomato bacterial wilt by the new strain Bacillus velezensis FJAT-46737 and its lipopeptides. BMC Microbiol. 2020;20(1):160. doi: 10.1186/s12866-020-01851-2.
- Jumpathong W, Intra B, Euanorasetr J, et al. Biosurfactant-producing Bacillus velezensis PW192 as an anti-fungal biocontrol agent against Colletotrichum gloeosporioides and Colletotrichum musae. Microorganisms. 2022;10(5):1017. doi: 10.3390/microorganisms10051017.
- Liu Y, Teng K, Wang T, et al. Antimicrobial Bacillus velezensis HC6: production of three kinds of lipopeptides and biocontrol potential in maize. J Appl Microbiol. 2020;128(1):242–254. doi: 10.1111/jam.14459.
- Hanif A, Zhang F, Li P, et al. Fengycin produced by Bacillus amyloliquefaciens FZB42 inhibits Fusarium graminearum growth and mycotoxins biosynthesis. Toxins. 2019;11(5):295. doi: 10.3390/toxins11050295.
- Baindara P, Korpole S. Lipopeptides: status and strategies to control fungal infection. In: Basak A, Chakraborty R, Mandal S. editors. Recent trends in antifungal agents and antifungal therapy. New Delhi, India: Springer Nature, 2016. p. 97–121.
- Suneeta P, Aiyanathan KEA, Nakkeeran S. Bacillomycins–the effective molecules in plant disease management. Int J Curr Microbiol Appl Sci. 2018;7(2):823–835. doi: 10.20546/ijcmas.2018.702.104.
- Lim SM, Yoon M-Y, Choi GJ, et al. Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant Pathol J. 2017;33(5):488–498. doi: 10.5423/PPJ.OA.04.2017.0073.
- Xiao S, Chen N, Chai Z, et al. Secondary metabolites from marine-derived Bacillus: a comprehensive review of origins, structures, and bioactivities. Mar Drugs. 2022;20(9):567. doi: 10.3390/md20090567.
- Jin P, Wang H, Tan Z, et al. Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic Biochem Physiol. 2020;163:102–107. doi: 10.1016/j.pestbp.2019.11.004.
- Gong Q, Zhang C, Lu F, et al. Identification of bacillomycin D from Bacillus subtilis fmbJ and its inhibition effects against Aspergillus flavus. Food Control. 2014;36(1):8–14. doi: 10.1016/j.foodcont.2013.07.034.
- Mácha H, Marešová H, Juříková T, et al. Killing effect of Bacillus velezensis FZB42 on a Xanthomonas campestris pv. Campestris (Xcc) strain newly isolated from cabbage Brassica oleracea Convar. capitata (L.): a metabolomic study. Microorganisms. 2021;9(7):1410. doi: 10.3390/microorganisms9071410.
- Yuan J, Li B, Zhang N, et al. Production of bacillomycin-and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J Agric Food Chem. 2012;60(12):2976–2981. doi: 10.1021/jf204868z.
- Xu Z, Shao J, Li B, et al. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl Environ Microbiol. 2013;79(3):808–815. doi: 10.1128/AEM.02645-12.
- Jiao R, Cai Y, He P, et al. Bacillus amyloliquefaciens YN201732 produces lipopeptides with promising biocontrol activity against fungal pathogen Erysiphe cichoracearum. Front Cell Infect Microbiol. 2021;11:598999. doi: 10.3389/fcimb.2021.598999.
- Gu Q, Yang Y, Yuan Q, et al. Bacillomycin D produced by Bacillus amyloliquefaciens is involved in the antagonistic interaction with the plant-pathogenic fungus Fusarium graminearum. Appl Environ Microbiol. 2017;83(19):e01075–01017. doi: 10.1128/AEM.01075-17.
- Luna-Bulbarela A, Tinoco-Valencia R, Corzo G, et al. Effects of bacillomycin D homologues produced by Bacillus amyloliquefaciens 83 on growth and viability of Colletotrichum gloeosporioides at different physiological stages. Biol Control. 2018;127:145–154. doi: 10.1016/j.biocontrol.2018.08.004.
- Luo C, Liu J, Bilal M, et al. Extracellular lipopeptide bacillomycin L regulates serial expression of genes for modulating multicellular behavior in Bacillus velezensis Bs916. Appl Microbiol Biotechnol. 2021;105(18):6853–6870. doi: 10.1007/s00253-021-11524-3.
- Xu Z, Mandic-Mulec I, Zhang H, et al. Antibiotic bacillomycin D affects iron acquisition and biofilm formation in Bacillus velezensis through a Btr-mediated FeuABC-dependent pathway. Cell Rep. 2019;29(5):1192.e1195–1202.e1195. doi: 10.1016/j.celrep.2019.09.061.
- Sarojini V, Cameron AJ, Varnava KG, et al. Cyclic tetrapeptides from nature and design: a review of synthetic methodologies, structure, and function. Chem Rev. 2019;119(17):10318–10359. doi: 10.1021/acs.chemrev.8b00737.
- Choub V, Maung CEH, Won S-J, et al. Antifungal activity of cyclic tetrapeptide from Bacillus velezensis CE 100 against plant pathogen Colletotrichum gloeosporioides. Pathogens. 2021;10(2):209. doi: 10.3390/pathogens10020209.
- Pinzón-Espinosa A, Martinez-Matamoros D, Castellanos L, et al. Cereusitin A, a cyclic tetrapeptide from a Bacillus cereus strain isolated from the soft coral Antillogorgia (syn. Pseudopterogorgia) elisabethae. Tetrahedron Lett. 2017;58(7):634–637. doi: 10.1016/j.tetlet.2017.01.002.
- Kim TY, Hwang SH, Noh JS, et al. Antifungal potential of Bacillus velezensis CE 100 for the control of different Colletotrichum species through isolation of active dipeptide, cyclo-(D-phenylalanyl-D-prolyl). Int J Mol Sci. 2022;23(14):7786. doi: 10.3390/ijms23147786.
- Jeong M-H, Lee Y-S, Cho J-Y, et al. Isolation and characterization of metabolites from Bacillus licheniformis MH48 with antifungal activity against plant pathogens. Microb Pathog. 2017;110:645–653. doi: 10.1016/j.micpath.2017.07.027.
- May JJ, Wendrich TM, Marahiel MA. The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2, 3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin. J Biol Chem. 2001;276(10):7209–7217. doi: 10.1074/jbc.M009140200.
- Nithyapriya S, Lalitha S, Sayyed R, et al. Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability. 2021;13(10):5394. doi: 10.3390/su13105394.
- Fukushima T, Allred BE, Sia AK, et al. Gram-positive siderophore-shuttle with iron-exchange from Fe-siderophore to apo-siderophore by Bacillus cereus YxeB. Proc Natl Acad Sci U S A. 2013;110(34):13821–13826. doi: 10.1073/pnas.1304235110.
- Rabbee MF, Ali MS, Choi J, et al. Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules. 2019;24(6):1046. doi: 10.3390/molecules24061046.
- Wang C, Zhao D, Qi G, et al. Effects of Bacillus velezensis FKM10 for promoting the growth of Malus hupehensis Rehd. and inhibiting Fusarium verticillioides. Front Microbiol. 2019;10:2889. doi: 10.3389/fmicb.2019.02889.
- Kesaulya H, Hasinu J, Tuhumury GN. Potential of Bacillus spp produces siderophores insuppressing thewilt disease of banana plants. IOP Conf Ser Earth Environ Sci. 2018;102:012016. doi: 10.1088/1755-1315/102/1/012016.
- Shin J-H, Park B-S, Kim H-Y, et al. Antagonistic and plant growth-promoting effects of Bacillus velezensis BS1 isolated from rhizosphere soil in a pepper field. Plant Pathol J. 2021;37(3):307–314. doi: 10.5423/PPJ.NT.03.2021.0053.
- Chen XH, Koumoutsi A, Scholz R, et al. Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol. 2007;25(9):1007–1014. doi: 10.1038/nbt1325.
- Dunyashev T, Laptev GY, Yildirim E, et al. Identification of genes associated with the synthesis of siderophores by the Bacillus subtilis. J Livest Sci. 2021;12(4):287–291. doi: 10.33259/JLivestSci.2021.287-291.
- Chakraborty K, Francis A, Chakraborty RD, et al. Marine macroalga-associated heterotrophic Bacillus velezensis: a novel antimicrobial agent with siderophore mode of action against drug-resistant nosocomial pathogens. Arch Microbiol. 2021;203(9):5561–5575. doi: 10.1007/s00203-021-02513-1.
- Chakraborty K, Kizhakkekalam VK, Joy M, et al. Bacillibactin class of siderophore antibiotics from a marine symbiotic Bacillus as promising antibacterial agents. Appl Microbiol Biotechnol. 2022;106(1):329–340. doi: 10.1007/s00253-021-11632-0.
- Daw MA, Falkiner FR. Bacteriocins: nature, function and structure. Micron. 1996;27(6):467–479. doi: 10.1016/s0968-4328(96)00028-5.
- Grinter R, Milner J, Walker D. Bacteriocins active against plant pathogenic bacteria. Biochem Soc Trans. 2012;40(6):1498–1502. doi: 10.1042/BST20120206.
- Abriouel H, Franz CM, Omar NB, et al. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev. 2011;35(1):201–232. doi: 10.1111/j.1574-6976.2010.00244.x.
- Scholz R, Molohon KJ, Nachtigall J, et al. Plantazolicin, a novel microcin B17/streptolysin S-like natural product from Bacillus amyloliquefaciens FZB42. J Bacteriol. 2011;193(1):215–224. doi: 10.1128/JB.00784-10.
- Scholz R, Vater J, Budiharjo A, et al. Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. J Bacteriol. 2014;196(10):1842–1852. doi: 10.1128/JB.01474-14.
- Gebhardt K, Schimana J, Müller J, et al. Screening for biologically active metabolites with endosymbiotic Bacilli isolated from arthropods. FEMS Microbiol Lett. 2002;217(2):199–205. doi: 10.1111/j.1574-6968.2002.tb11475.x.
- Dehghanifar S, Keyhanfar M, Emtiazi G. Production and partial purification of thermostable bacteriocins from Bacillus pumilus ZED17 and DFAR8 strains with antifungal activity. Mol Biol Res Commun. 2019;8:41.
- Gray E, Lee K, Souleimanov A, et al. A novel bacteriocin, thuricin 17, produced by plant growth promoting rhizobacteria strain Bacillus thuringiensis NEB17: isolation and classification. J Appl Microbiol. 2006;100(3):545–554. doi: 10.1111/j.1365-2672.2006.02822.x.
- Hammami I, Rhouma A, Jaouadi B, et al. Optimization and biochemical characterization of a bacteriocin from a newly isolated Bacillus subtilis strain 14B for biocontrol of Agrobacterium spp. strains. Lett Appl Microbiol. 2009;48(2):253–260. doi: 10.1111/j.1472-765X.2008.02524.x.
- Arguelles Arias A, Ongena M, Devreese B, et al. Characterization of amylolysin, a novel lantibiotic from Bacillus amyloliquefaciens GA1. PLoS One. 2013;8(12):e83037. doi: 10.1371/journal.pone.0083037.
- Nazari M, Smith DL. A PGPR-produced bacteriocin for sustainable agriculture: a review of thuricin 17 characteristics and applications. Front Plant Sci. 2020;11:916. doi: 10.3389/fpls.2020.00916.
- Tareq FS, Kim JH, Lee MA, et al. Antimicrobial gageomacrolactins characterized from the fermentation of the marine-derived bacterium Bacillus subtilis under optimum growth conditions. J Agric Food Chem. 2013;61(14):3428–3434. doi: 10.1021/jf4009229.
- Rath CM, Scaglione JB, Kittendorf JD, et al. NRPS/PKS hybrid enzymes and their natural products. Vol. 1. Mander H-WBLL, editor. Amsterdam (Netherlands): Elsevier; 2010. p. 453–492.
- Chen K, Tian Z, Luo Y, et al. Antagonistic activity and the mechanism of Bacillus amyloliquefaciens DH-4 against citrus green mold. Phytopathology. 2018;108(11):1253–1262. doi: 10.1094/PHYTO-01-17-0032-R.
- Tian Z, Chen C, Chen K, et al. Biocontrol and the mechanisms of Bacillus sp. w176 against postharvest green mold in citrus. Postharvest Biol Technol. 2020;159:111022. doi: 10.1016/j.postharvbio.2019.111022.
- Kim J-A, Song J-S, Kim PI, et al. Bacillus velezensis TSA32-1 as a promising agent for biocontrol of plant pathogenic fungi. JoF. 2022;8(10):1053. doi: 10.3390/jof8101053.
- Um S, Fraimout A, Sapountzis P, et al. The fungus-growing termite Macrotermes natalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi. Sci Rep. 2013;3(1):3250. doi: 10.1038/srep03250.
- Xu Z, Zhang R, Wang D, et al. Enhanced control of cucumber wilt disease by Bacillus amyloliquefaciens SQR9 by altering the regulation of its DegU phosphorylation. Appl Environ Microbiol. 2014;80(9):2941–2950. doi: 10.1128/AEM.03943-13.
- Miras M, Dubnau D. A DegU-P and DegQ-dependent regulatory pathway for the K-state in Bacillus subtilis. Front Microbiol. 2016;7:1868. doi: 10.3389/fmicb.2016.01868.
- Erega A, Stefanic P, Dogsa I, et al. Bacillaene mediates the inhibitory effect of Bacillus subtilis on Campylobacter jejuni biofilms. Appl Environ Microbiol. 2021;87(12):e02955–e02920. doi: 10.1128/AEM.02955-20.
- Chen X, Koumoutsi A, Scholz R, et al. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol. 2009;140(1-2):27–37. doi: 10.1016/j.jbiotec.2008.10.011.
- Li W, Tang X-X, Yan X, et al. A new macrolactin antibiotic from deep sea-derived bacteria Bacillus subtilis B5. Nat Prod Res. 2016;30(24):2777–2782. doi: 10.1080/14786419.2016.1155576.
- Smith AB, Ott GR. Total synthesis of (−)-macrolactin A. J Am Chem Soc. 1996;118(51):13095–13096. doi: 10.1021/ja963543a.
- Wang D, Li Y, Yuan Y, et al. Identification of non-volatile and volatile organic compounds produced by Bacillus siamensis LZ88 and their antifungal activity against Alternaria alternata. Biol Control. 2022;169:104901. doi: 10.1016/j.biocontrol.2022.104901.
- Ni J, Yu L, Li F, et al. Macrolactin R from Bacillus siamensis and its antifungal activity against Botrytis cinerea. World J Microbiol Biotechnol. 2023;39(5):117. doi: 10.1007/s11274-023-03563-x.
- Yuan J, Raza W, Shen Q, et al. Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl Environ Microbiol. 2012;78(16):5942–5944. doi: 10.1128/AEM.01357-12.
- Yuan J, Zhang F, Wu Y, et al. Recovery of several cell pellet-associated antibiotics produced by Bacillus amyloliquefaciens NJN-6. Lett Appl Microbiol. 2014;59(2):169–176. doi: 10.1111/lam.12260.
- Tojo S, Kim J-Y, Tanaka Y, et al. The mthA mutation conferring low-level resistance to streptomycin enhances antibiotic production in Bacillus subtilis by increasing the S-adenosylmethionine Pool size. J Bacteriol. 2014;196(8):1514–1524. doi: 10.1128/JB.01441-13.
- Fazle Rabbee M, Baek K-H. Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules. 2020;25(21):4973. doi: 10.3390/molecules25214973.
- Parker JB, Walsh CT. Action and timing of BacC and BacD in the late stages of biosynthesis of the dipeptide antibiotic bacilysin. Biochemistry. 2013;52(5):889–901. doi: 10.1021/bi3016229.
- Rajavel M, Mitra A, Gopal B. Role of Bacillus subtilis BacB in the synthesis of bacilysin. J Biol Chem. 2009;284(46):31882–31892. doi: 10.1074/jbc.M109.014522.
- Wu L, Li X, Wu H, et al. Research advances on Bacilysin from Bacillus. J Nanjing Agric Univ. 2018;41:778–783.
- Han X, Shen D, Xiong Q, et al. The plant-beneficial rhizobacterium Bacillus velezensis FZB42 controls the soybean pathogen Phytophthora sojae due to bacilysin production. Appl Environ Microbiol. 2021;87(23):e01601–e01621. doi: 10.1128/AEM.01601-21.
- Palazzini JM, Dunlap CA, Bowman MJ, et al. Bacillus velezensis RC 218 as a biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation: genome sequencing and secondary metabolite cluster profiles. Microbiol Res. 2016;192:30–36. doi: 10.1016/j.micres.2016.06.002.
- Afsharmanesh H, Perez-Garcia A, Zeriouh H, et al. Aflatoxin degradation by Bacillus subtilis UTB1 is based on production of an oxidoreductase involved in bacilysin biosynthesis. Food Control. 2018;94:48–55. doi: 10.1016/j.foodcont.2018.03.002.
- Wu L, Wu H, Chen L, et al. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci Rep. 2015;5:12975. doi: 10.1038/srep12975.
- Wu L, Wu H, Chen L, et al. Bacilysin overproduction in Bacillus amyloliquefaciens FZB42 markerless derivative strains FZBREP and FZBSPA enhances antibacterial activity. Appl Microbiol Biotechnol. 2015;99(10):4255–4263. doi: 10.1007/s00253-014-6251-0.
- Im SM, Yu NH, Joen HW, et al. Biological control of tomato bacterial wilt by oxydifficidin and difficidin-producing Bacillus methylotrophicus DR-08. Pestic Biochem Physiol. 2020;163:130–137. doi: 10.1016/j.pestbp.2019.11.007.
- Mondol MAM, Shin HJ, Islam MT. Diversity of secondary metabolites from marine bacillus species: chemistry and biological activity. Mar Drugs. 2013;11(8):2846–2872. doi: 10.3390/md11082846.
- Olishevska S, Nickzad A, Restieri C, et al. Bacillus velezensis and Paenibacillus peoriae strains effective as biocontrol agents against Xanthomonas bacterial spot. Appl Microbiol. 2023;3(3):1101–1119. doi: 10.3390/applmicrobiol3030076.
- Zweerink MM, Edison A. Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis III. Mode of action of difficidin. J Antibiot. 1987;40(12):1692–1697. doi: 10.7164/antibiotics.40.1692.
- Rani A, Rana A, Dhaka RK, et al. Bacterial volatile organic compounds as biopesticides, growth promoters and plant-defense elicitors: current understanding and future scope. Biotechnol Adv. 2023;63:108078. doi: 10.1016/j.biotechadv.2022.108078.
- Almeida OAC, de Araujo NO, Mulato ATN, et al. Bacterial volatile organic compounds (VOCs) promote growth and induce metabolic changes in rice. Front Plant Sci. 2022;13:1056082. doi: 10.3389/fpls.2022.1056082.
- Heenan-Daly D, Coughlan S, Dillane E, et al. Volatile compounds from Bacillus, Serratia, and Pseudomonas promote growth and alter the transcriptional landscape of Solanum tuberosum in a passively ventilated growth system. Front Microbiol. 2021;12:628437. doi: 10.3389/fmicb.2021.628437.
- Calvo H, Mendiara I, Arias E, et al. Antifungal activity of the volatile organic compounds produced by Bacillus velezensis strains against postharvest fungal pathogens. Postharvest Biol Technol. 2020;166:111208. doi: 10.1016/j.postharvbio.2020.111208.
- Gao Z, Zhang B, Liu H, et al. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol Control. 2017;105:27–39. doi: 10.1016/j.biocontrol.2016.11.007.
- Chen H, Xiao X, Wang J, et al. Antagonistic effects of volatiles generated by Bacillus subtilis on spore germination and hyphal growth of the plant pathogen, Botrytis cinerea. Biotechnol Lett. 2008;30(5):919–923. doi: 10.1007/s10529-007-9626-9.
- Arrebola E, Sivakumar D, Korsten L. Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol Control. 2010;53(1):122–128. doi: 10.1016/j.biocontrol.2009.11.010.
- Asari S, Matzén S, Petersen MA, et al. Multiple effects of Bacillus amyloliquefaciens volatile compounds: plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol Ecol. 2016;92(6):fiw070. doi: 10.1093/femsec/fiw070.
- Liu W-W, Wei M, Zhu B-Y, et al. Antagonistic activities of volatiles from four strains of Bacillus spp. and Paenibacillus spp. against soil-borne plant pathogens. Agric Sci China. 2008;7(9):1104–1114. doi: 10.1016/S1671-2927(08)60153-4.
- He C-N, Ye W-Q, Zhu Y-Y, et al. Antifungal activity of volatile organic compounds produced by Bacillus methylotrophicus and Bacillus thuringiensis against five common spoilage fungi on loquats. Molecules. 2020;25(15):3360. doi: 10.3390/molecules25153360.
- Méndez-Bravo A, Cortazar-Murillo EM, Guevara-Avendaño E, et al. Plant growth-promoting rhizobacteria associated with avocado display antagonistic activity against Phytophthora cinnamomi through volatile emissions. PLoS One. 2018;13(3):e0194665. doi: 10.1371/journal.pone.0194665.
- Bustamante MI, Elfar K, Eskalen A. Evaluation of the antifungal activity of endophytic and rhizospheric bacteria against grapevine trunk pathogens. Microorganisms. 2022;10(10):2035. doi: 10.3390/microorganisms10102035.
- Zhang X, Li B, Wang Y, et al. Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-1. Appl Microbiol Biotechnol. 2013;97(21):9525–9534. doi: 10.1007/s00253-013-5198-x.
- Enebak S, Carey W. Evidence for induced systemic protection to fusiform rust in loblolly pine by plant growth-promoting rhizobacteria. Plant Dis. 2000;84(3):306–308. doi: 10.1094/PDIS.2000.84.3.306.
- Niu D-D, Liu H-X, Jiang C-H, et al. The plant growth–promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate-and jasmonate/ethylene-dependent signaling pathways. Mol Plant Microbe Interact. 2011;24(5):533–542. doi: 10.1094/MPMI-09-10-0213.
- Li Y, Gu Y, Li J, et al. Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew. Front Microbiol. 2015;6:883. doi: 10.3389/fmicb.2015.00883.
- Bargabus R, Zidack N, Sherwood J, et al. Screening for the identification of potential biological control agents that induce systemic acquired resistance in sugar beet. Biol Control. 2004;30(2):342–350. doi: 10.1016/j.biocontrol.2003.11.005.
- Kim J-S, Lee J, Lee C-h, et al. Activation of pathogenesis-related genes by the rhizobacterium, Bacillus sp. JS, which induces systemic resistance in tobacco plants. Plant Pathol J. 2015;31(2):195–201. doi: 10.5423/PPJ.NT.11.2014.0122.
- Tahir HAS, Gu Q, Wu H, et al. Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci Rep. 2017;7(1):40481. doi: 10.1038/srep40481.
- Huang CJ, Tsay JF, Chang SY, et al. Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag Sci. 2012;68(9):1306–1310. doi: 10.1002/ps.3301.
- Ryu C-M, Farag MA, Hu C-H, et al. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004;134(3):1017–1026. doi: 10.1104/pp.103.026583.
- Ryu CM, Hu CH, Reddy M, et al. Different signaling pathways of induced resistance by rhizobacteria in Arabidopsis thaliana against two pathovars of Pseudomonas syringae. New Phytol. 2003;160(2):413–420. doi: 10.1046/j.1469-8137.2003.00883.x.
- Zhang S, Moyne A-L, Reddy M, et al. The role of salicylic acid in induced systemic resistance elicited by plant growth-promoting rhizobacteria against blue mold of tobacco. Biol Control. 2002;25(3):288–296. doi: 10.1016/S1049-9644(02)00108-1.
- Beth Mudgett M. New insights to the function of phytopathogenic bacterial type III effectors in plants. Annu Rev Plant Biol. 2005;56(1):509–531. doi: 10.1146/annurev.arplant.56.032604.144218.
- Han Q, Wu F, Wang X, et al. The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ Microbiol. 2015;17(4):1166–1188. doi: 10.1111/1462-2920.12538.
- Cakmakci R, Dönmez MF, Erdoğan Ü. The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk J Agric For. 2007;31:189–199.
- Bever JD, Platt TG, Morton ER. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu Rev Microbiol. 2012;66(1):265–283. doi: 10.1146/annurev-micro-092611-150107.
- Szczech M, Shoda M. The effect of mode of application of Bacillus subtilis RB14-C on its efficacy as a biocontrol agent against Rhizoctonia solani. J Phytopathol. 2006;154(6):370–377. doi: 10.1111/j.1439-0434.2006.01107.x.
- Perea-Molina PA, Pedraza-Herrera LA, Beauregard PB, et al. A biocontrol Bacillus velezensis strain decreases pathogen Burkholderia glumae population and occupies a similar niche in rice plants. Biol Control. 2022;176:105067. doi: 10.1016/j.biocontrol.2022.105067.
- Rojas-Ruiz NE, Sansinenea-Royano E, Cedillo-Ramirez ML, et al. Analysis of Bacillus thuringiensis population dynamics and its interaction with Pseudomonas fluorescens in soil. Jundishapur J Microbiol. 2015;8(9):e27953. doi: 10.5812/jjm.27953.
- Veselova S, Burkhanova G, Rumyantsev S, et al. Strains of Bacillus spp. regulate wheat resistance to greenbug aphid Schizaphis graminum Rond. Appl Biochem Microbiol. 2019;55(1):41–47. doi: 10.1134/S0003683819010186.
- Rashid MH-O, Khan A, Hossain MT, et al. Induction of systemic resistance against aphids by endophytic Bacillus velezensis YC7010 via expressing PHYTOALEXIN DEFICIENT4 in Arabidopsis. Front Plant Sci. 2017;8:211. doi: 10.3389/fpls.2017.00211.
- Valenzuela-Soto JH, Estrada-Hernández MG, Ibarra-Laclette E, et al. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta. 2010;231(2):397–410. doi: 10.1007/s00425-009-1061-9.
- Rajendran L, Ramanathan A, Durairaj C, et al. Endophytic Bacillus subtilis enriched with chitin offer induced systemic resistance in cotton against aphid infestation. Arch Phytopathol Plant Protect. 2011;44(14):1375–1389. doi: 10.1080/03235408.2010.499719.
- Herman M, Nault B, Smart C. Effects of plant growth-promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Protect. 2008;27(6):996–1002. doi: 10.1016/j.cropro.2007.12.004.
- Zehnder G, Kloepper J, Yao C, et al. Induction of systemic resistance in cucumber against cucumber beetles (Coleoptera: Chrysomelidae) by plant growth-promoting rhizobacteria. J Econ Entomol. 1997;90(2):391–396. doi: 10.1093/jee/90.2.391.