Abstract
Fruit colour is a crucial factor influencing both the marketability and quality of pepper (Capsicum annuum), particularly ornamental varieties. Fruit colour is a complex multigenic trait in plant species. Previously, the Arabidopsis pseudo-response regulator 2 (APRR2) gene, one of the regulators that control fruit chlorophyll content and chloroplast development, thereby influencing fruit colour at the green immature stage in tomato (Solanum lycopersicum), cucumber (Cucumis sativus) and pepper, was reported. Functional molecular markers associated with this homologous gene in pepper could be employed to discern fruit colour at the seedling stage, thus improving the efficiency of green/white-fruited pepper breeding. In this study, a derived cleaved amplified polymorphic sequences (dCAPS) marker was developed based on the mutation site in the sequence of a CaAPRR2-like gene that was cloned from the inbred line B1-2, which exhibits milky white fruit at the green immature stage. The marker was subsequently validated in a CaAPRR2-like progeny segregation population. After digestion with the restriction endonuclease TaqI, the amplification product exhibited evident polymorphic bands, enabling it to distinguish between peppers with milky white and green fruit colours. The developed molecular marker displayed remarkable stability and repeatability, thus offering a straightforward and effective tool for enhancing fruit colour in pepper breeding programs.
Introduction
Pepper (Capsicum spp.) is native to the tropical and subtropical regions of Central and South America, and is one of the world’s oldest cultivated crops [Citation1]. It was introduced to China in the late sixteenth century and has since evolved into one of the main vegetable crops in China’s ‘vegetable basket’ [Citation2]. Peppers exhibit wide colour variation in both the mature and immature periods [Citation3]. Such colour variation displayed by peppers represents a crucial quality trait, because the pigments that impart colour contribute to the fruit’s flavour, nutrition and potential health benefits [Citation4,Citation5]. The fruit colour is a critical quality that greatly impacts consumer preferences, making it a central focus of interest for breeders [Citation5,Citation6].
During the maturation of pepper fruit, metabolic fluctuations in various pigments (i.e. chlorophyll, anthocyanin, carotenoid and flavonoid) lead to changes in the fruit’s colour. The most common fruit colour for peppers at the green immature stage is green, while variations include light green, white, yellow, purple and black [Citation5,Citation7,Citation8]. As peppers reach the red mature stage, the dominant fruit colours shift to red and yellow, along with other shades and colours such as white, orange, olive green and brown [Citation9–12]. The intensity of green is closely linked to the chlorophyll content and is also affected by specific carotenoids, mainly β-carotene and xanthophyll. Purple and black fruit colours are mainly determined by anthocyanin and chlorophyll levels, with black fruit exhibiting significantly higher concentrations than purple fruit of both anthocyanin and chlorophyll. White pepper fruit displays minimal chlorophyll and carotenoid content, while yellow fruit has lower contents of chlorophyll and anthocyanin yet a higher carotenoid content [Citation13,Citation14]. At the red mature stage, the diversity of pepper colours is closely related to the type and proportional concentration of carotenoids present in the pepper fruit [Citation7]. For instance, carotenoids found in red pepper fruit at the red mature stage mainly include ketolutein varieties such as capsanthin and capsorubin, along with xanthophylls such as zeaxanthin and violaxanthin [Citation15,Citation16]. Conversely, yellow and orange pepper fruits at the red mature stage contain violaxanthin, lutein and other xanthophylls, rather than capsanthin and capsorubin [Citation17,Citation18]. Carotenoids found in olive green pepper fruit at the red mature stage primarily consist of yellow carotenoids, while brown pepper fruit during the red mature stage predominantly contains red carotenoids, such as capsanthin and capsorubin.
In recent years, researchers have conducted localization and functional studies on key regulatory genes responsible for pepper fruit colour formation, aided by the advancement of high-throughput molecular biological techniques and the availability of pepper germplasm resources with varying fruit colours. Brand et al. [Citation19], using an F2 population derived from two parental lines (one with dark green fruit and the other with light green fruit), identified pc8.1 (i.e. pc1) and pc10.1 as two prominent quantitative trait loci (QTL) responsible for chlorophyll content in pepper fruit at the green immature stage [Citation19]. The candidate gene for the pc10.1 locus was identified as CaGLK2 (Golden2-like), a member of the GARP family [Citation20]. Borovsky et al. [Citation21] confirmed that the zinc finger transcription factor LSD one like1 (CcLOL1) in pepper serves as the target gene of QTL pc8.1. This gene is responsible for the regulation of chloroplast size and chlorophyll content in a fruit-specific way, and its homeotic gene was conservative in tomato fruit [Citation21]. The colour of mature pepper fruit is proposed to be controlled by three independent loci: C1, C2 and Y. The genes at the C1, C2 and Y loci, respectively, encode pseudo-response regulator 2-like (PRR2), phytoene synthase (PSY1) and capsanthin-capsorubin synthase (CCS) proteins [Citation22–25]. The observed white fruit in AC08-201 line is attributed to mutations in all three of these genes [Citation26].
The Arabidopsis pseudo-response regulator 2 gene (APRR2), belonging to the pseudo-response regulators (PRR) subfamily that originated from response regulators (RRs), has been partially characterized as a novel ripening related transcription factor in pepper and tomato [Citation27]. Several lines of evidence have confirmed the involvement of the APRR2 gene in promoting pigment accumulation and chloroplasts development in fruit tissues [Citation27,Citation28]. In cucumber, APRR2 is the candidate responsible for determining the green colour of immature fruit, and its allele aprr2, which governs the white colour, displays a notable 101-amino acid residue deletion at the C-terminus when compared to APRR2 [Citation29]. Additionally, Pan et al. [Citation27] cloned an APRR2-like gene in sweet pepper (Capsicum annuum) and observed that this gene was in the same position as pc8.1 on the genetic map. Alignment of this gene sequence between wild-type and white-fruited parental lines from a mapping population revealed a G-to-A substitution, resulting in premature termination of the protein translation [Citation27].
Functional molecular markers are molecular markers developed based on nucleotide polymorphic sites within functional genes closely associated with specific phenotypic traits [Citation30]. They enable researchers to select individuals with the desired functional characteristics independent of phenotype. Due to the dominance of the green colour trait over white, naturally occurring white-fruited pepper germplasms are scarce, highlighting the necessity for their development in the breeding field. However, the absence of molecular markers associated with white colour has impeded the efficiency of breeding white-fruited peppers. In the present study, we aimed to develop an accurate, efficient and convenient dCAPS (derived cleaved amplified polymorphic sequences) marker targeting the variation sites of the CaAPRR2-like gene cloned from the inbred line B1-2, known for its milky white fruit at the green immature stage. This marker is intended to perform effectively in assisted selection.
Materials and methods
Plant materials
In total, 36 inbred lines were employed in this study (Supplemental Figure S1 and Table S1). These materials were all landrances collected from various regions of China. Among these, B1-2 (fruit with milky white colour at the green immature stage) and D50 (fruit with dark green colour at the green immature stage) were used to develop a segregating F2 population (Supplemental Figure S1), where B1-2 was used as the female parent. To establish the F2 population, eight plants from each parental line were crossed to generate F1 seeds. Subsequently, an F2 population consisting 3,000 individuals was successfully obtained through the self-crossing of 20 F1 individuals. From this population, 344 F2 individuals were randomly chosen and employed to evaluate the reliability of the developed molecular marker. Another 34 inbred lines that produce fruits with varying degrees of white colour at the green immature stage were used to assess the reliability of the developed molecular marker (Supplemental Table S1). Six plants from these 34 lines were cultivated for collection of DNA samples. The seeds of all the used 36 lines were kept by the Institute of Vegetables at Quzhou Academy of Agricultural and Forestry Sciences. The plants were grown in a plastic tunnel under natural sunlight at an experimental farm (29°10′′N, 119°03′E) in Quzhou, China.
Genomic DNA and cDNA preparation
The genomic DNA from pepper leaf tissues was extracted using a modified CTAB method [Citation31]. In brief, 0.2 g of leaf tissue was ground in liquid nitrogen and gently dispersed in 1 mL of extraction buffer [0.7 mol/L NaCl, 1% CTAB, 50 mmol/L Tris-HCl (pH 8.0), 10 mmol/L EDTA, and 1% β-mercaptoethanol]. The mixture was then incubated for 20 min at 55 °C with occasional gentle mixing. Subsequently, 1 mL of chloroform/octanol (24:1) was added and mixed. After centrifugation at 13,000 g for 10 min, the clear aqueous phase was transferred to a new 1.5 mL centrifuge tube and mixed with 50 μL 3 mol/L NaAC and 500 μL ice-cold anhydrous ethanol. Following incubation at −20 °C for 1 h, the mixture was centrifuged at 13,000 g for 3 min, and the supernatant was discarded. The white precipitate at the bottom, which consisted of DNA, was washed twice using 500 μL of 70% ice-cold ethanol. Finally, the DNA was disovled in 50 μL ddH2O.
Total RNA was extracted from pepper leaves using an E.Z.N.A®Plant RNA Kit (Omega, GA, USA, R6827-01, https://us.vwr.com/store/product/9693339/e-z-n-a-plant-rna-kit-omega-bio-tek) and the first strand of cDNA was synthesized using a FastKing cDNA RT Kit (with gDNase) (TIANGEN, Beijing, China, KR116-02, https://en.tiangen.com/content/details_71_4498.html). A total of 500 ng RNA, with the genomic DNA removed, was reverse-transcribed into first-strand cDNA. The reaction procedure consisted of an incubation at 42 °C for 35 min, followed by denaturation at 95 °C for 3 min. The concentrations of the obtained DNA and cDNA were analyzed using a spectrophotometer (NanoReady Touch, Life Real, Hangzhou, China), and the samples were diluted to 30–50 ng/μL.
PCR amplification and detection
The gDNA and CDS sequences of CaAPRR2 (Capana01g000809) were derived from the Capsicum annuum Zunla-1 cultivar genome, v2.0 (https://plantgarden.jp/en/list/t4072/genome/t4072.G002). The specific amplification primers (CaAPRR2-F: ATGATTTGCATTGAGGATGAATTATTGGGTTG; CaAPRR2-R: TCATCTCCAACATCGAGAGCCATT) were designed using Primer5.0 software, and checked using the NCBI Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). PCR amplification was performed using gDNA and cDNA as templates, and the reaction system comprised the following: KOD OneTM PCR Master Mix (Toyobo, Osaka, Japan, KMM-101, https://www.toyobo-global.com/seihin/xr/lifescience/products/pcr_020.html) 25.0 μL, ddH2O 20 μL, 1.5 μL each for forward and reverse primers (10 μmol/L), 2 μL DNA or cDNA template at a concentration of 30–50 ng/μL. The PCR procedure was set as the following: 95 °C for 3 min; 98 °C for 10 s, 55 °C for 15 s, 68 °C for 35 cycles depending on fragment size (1 kb/min); 72 °C for 10 min. A thermocycler (Eppendorf, AG 22331, Hamburg, Germany) was used. The PCR products were visualized via 2% agarose gel electrophoresis (voltage 110 V, around 20–30 min, 1x TAE buffer). Goldview Nucleic Acid Gel Stain (Biohao, Wuhan, China, D0125, http://www.biohao.com/ProductShow.asp?ID=970), DL5000 DNA marker (Baiaolaibo, Beijing, China, GS0028, https://www.bjbalb.com/html/Nucleic-Acid-Electrophoresis/GS0028.html) and DL2000 DNA marker (Baiaolaibo, Beijing, China, BTN70503R, https://www.bjbalb.com/html/Nucleic-Acid-Electrophoresis/BTN70503R.html) were used. After electrophoresis, the gel was placed and visualized on a UV transilluminator (BioRed, GelDoc XR+, Hercules, CA, USA).
Gene cloning and sequence analysis
In this experiment, a pEASY®-T5 Zero Cloning Kit (Transgen Biotech, Beijing, China, CT501-01, https://www.transgenbiotech.com/cloning_vector/peasy_t5_zero_cloning_kit.html) was used for gene cloning and transformation. After successful transformation, three positive clones were selected and sent for sequencing (Sangon Biotech, Shanghai, China). BioEdit and BioXM software were employed for sequence alignment and protein translation. Additionally, the structure of the CaAPRR2-like gene was determined using the Gene Structure Display Server (GSDS) 2.0 (http://gsds.gao-lab.org).
dCAPS marker development
After comparation of amplified sequences of the CaAPRR2-like gene from B1-2 and D50, a dCAPS marker APR2-D was developed based on the mutation site in the CaAPRR2-like gene in B1-2. The dCAPS primers containing the recognition site of TaqI (dCAPS-F: AGTGATGGTTCATCTCCTCATCAAAAGTCG; dCAPS-R: CGGAACCAGTAGACAAAACATAATGGC) were designed using the dCAPS Finder 2.0 program (http://helix.wustl.edu/dcaps/dcaps.html). The developed marker APR2-D was used for PCR amplification within the templets of B1-2, D50 and the constructed F1 populations. The PCR products were digested using TaqI enzyme (Thermo Scientific, MA, USA, ER0671, https://www.thermofisher.cn/order/catalog/product/ER0671). The DNA was digested at 65 °C for 5 h using the following digestion system: PCR products 25 μL, TaqI 1 μL, buffer 3 μL. For the detection of products following TaqI digestion, 2% agarose gel electrophoresis (110 V, around 20–30 min, 1x TAE buffer) was used. Goldview Nucleic Acid Gel Stain (Biohao, Wuhan, China, D0125, http://www.biohao.com/ProductShow.asp?ID=970) and DL2000 DNA marker (Baiaolaibo, Beijing, China, BTN70503R, https://www.bjbalb.com/html/Nucleic-Acid-Electrophoresis/BTN70503R.html) were used.
dCAPS marker validation
For validation of the dCAPS marker reliability, DNA samples extracted from 344 individuals that were randomly selected from an F2 population consisting of 3,000 plants and 34 inbred lines with varying degrees of white colour fruits were used for PCR amplification, where the dCAPS primers were used. After TaqI digestion, the products were detected using silver staining post 8% polyacryamide gel electrophoresis [Citation32]. Polyacryamide gel (8%) electrophoresis was conducted using 0.5x TBE buffer at 200 V, 300 mA, around 1.5–2 h. DL500 DNA marker (Takara, Beijing, China, 3590Q, https://www.takarabiomed.com.cn/DownLoad/3590Q.pdf) and DL2000 DNA marker (Baiaolaibo, Beijing, China, BTN70503R, https://www.bjbalb.com/html/Nucleic-Acid-Electrophoresis/BTN70503R.html) were used.
Results
Cloning of the CaAPRR2-like gene in pepper
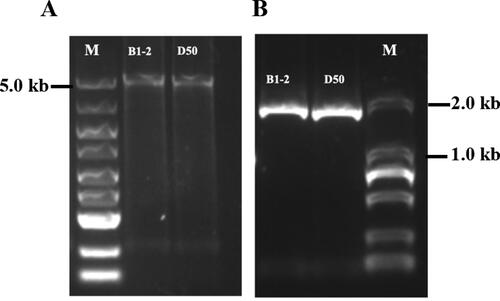
The gDNA and RNA of B1-2 (fruit with milky white colour at the green immature stage) and D50 (fruit with dark green colour at the green immature stage) lines were extracted, and RNA was used as a template for cDNA reverse transcription. Using the obtained gDNA and cDNA as templates, and CaAPRR2-F and CaAPRR2-R as primers, the CaAPRR2-like genes from B1-2 and D50 were amplified and detected by 2% agarose gel electrophoresis. The PCR detections, respectively, generated approximately 5.5 and 1.7 kb products (). The size of the PCR products was consistent with the target band size, confirming the successful amplification of the CaAPRR2-like genes from B1-2 and D50.
Multiple sequence alignment analysis
The cloned gDNA and cDNA sequences of CaPPR2-like gene from B1-2 and D50 were subjected to sequencing, and the resulting cDNA sequences were translated into amino acid (AA) sequences using BioXM software. The results showed that the cDNA cloned from B1-2 had a total length of 1,763 bp, encoding 343 amino acids, and displaying a predicted molecular weight of 37.58 kDa. In contrast, the cDNA cloned from D50 had a total length of 1,764 bp, encoding 587 amino acids, with a predicted molecular weight of 65.26 kDa.
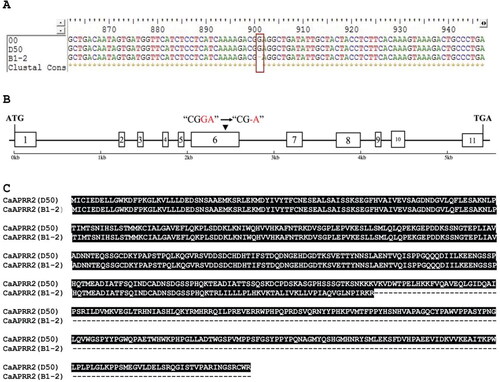
Multiple sequence alignment was performed to compare the CaAPRR2-like nucleotide sequences obtained from B1-2 and D50 with the reference genome. The results of cDNA sequence alignment showed a single base deletion (G) at site 901 of the B1-2 cDNA sequence, leading to the premature termination of the B1-2 coding amino acid sequence (). This variation site was also identified in the gDNA sequence of B1-2. The CaAPRR2 gene was identified to possess 11 exons. The single base mutation site was in the sixth encoding region of the CaAPRR2-like gene ().
Figure 2. Multiple sequence alignment analysis of CaAPRR2-like cDNA from B1-2, D50 and the reference genome. (A) The mutation site in cDNA sequence extracted from B1-2. The red box represents a single base deletion (G) at site 901 of the B1-2 sequence, and 00 represents the reference genome sequence. (B) Structure and variation site pattern of CaAPRR2-like gene. The boxes represent the exon regions; the black line represents the intron region; the numbers 1–11 indicate the position of the exon region; the black arrow indicates the site of the single base deletion in the B-12 sequence. (C) Alignment analysis of AA sequences from B1-2 and D50.
dCAPS marker development
Based on the single base deletion (G) at site 901 in the B1-2 cDNA sequence, the dCAPS marker APR2-D was developed using the dCAPS Finder 2.0 program. By introducing a mismatched base T, a TaqI restriction site (TCGA) was successfully created. Consequently, TaqI would cleave exclusively at site A, leaving other bases intact. The specific amplification primers were then designed.
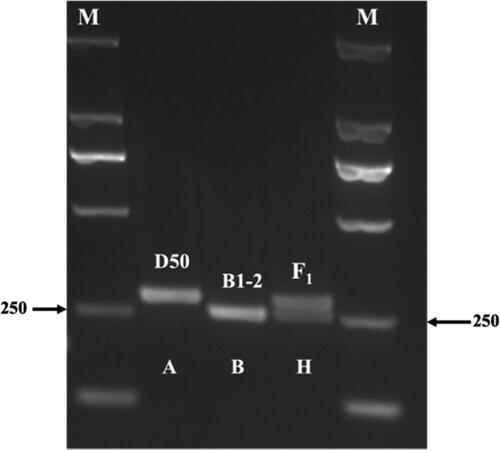
Using B1-2, D50 and F1 (B1-2 × D50) as templates, amplification was performed using the primers of APR2-D marker (dCAPS-F, dCAPS-R). After digestion using a TaqI restriction endonuclease enzyme, the PCR products exhibited three distinct bands (). The target D50 sequence had no TaqI restriction site, and the resulting DNA product had a length of 255 bp (A-band type). The target B1-2 sequence with two restriction sites could be cut into 28 and 227 bp (B-band type) by TaqI restriction endonuclease. Since the 28 bp band moved outside the gel during electrophoresis, only the 227 bp (B-band type) was visible on the gel. As the F1 (B1-2 × D50) target sequence had a single restriction site, after TaqI digestion, half of its amplification products could be cut into 28 and 227 bp fragments. As shown in , the products of F1 ultimately showed 227 and 255 bp bands (H-band type). These results affirm the polymorphic nature of the dCAPS marker between the parental lines.
Validation of dCAPS marker reliability
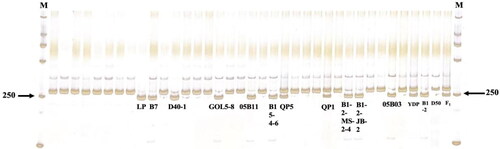
The reliability of the developed dCAPS molecular marker was validated in 344 individuals that were randomly selected from an F2 population consisting of 3,000 plants, and the practicability and reliability of the dCAPS marker were confirmed. As shown in , there were three types of band patterns in the gel electrophoretic map. There are 93 F2 individuals displaying milky white fruit, in agreement with the phenotypes of B1-2, showed an A-type pattern. In contrast, the F2 individuals with varying degrees of green fruit, had a B-type pattern (169 individuals) or a C-type pattern (82 individuals). The F2 individuals indicated that the accuracy of marker assisted selection based on fruit colour genes was 100%. Therefore, this molecular marker can successfully perform genotyping and phenotype prediction in samples, and thus can be used for subsequent molecular assisted breeding.
Figure 4. Polymorphic bands obtained from TaqI restriction endonuclease digestion in F2 segregation population. M: DL500 DNA marker; a: polymorphic band of B1-2; b: polymorphic band of D50; c: polymorphic band of F1.
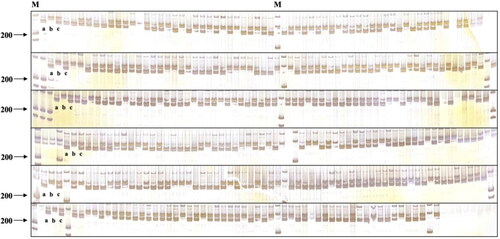
To further illustrate the practicability and reliability of the APR2-D marker, 34 inbred lines with varying degrees of white-coloured fruit were also subjected to testing (). Among these 34 pepper lines, nine showed the band type B, and except for 05B11, the remaining eight lines also showed the same gene allelic variation points. These results indicate that the CaAPRR2 genes in these nine inbred lines (LP, B7, D40-1, GOL5-8, 05B11, B15-4-6, B1-2-EMS-2-4, B1-2-JB-2 and 05B03) were all mutated, and the mutation sites were consistent with B1-2. Taken together, the results affirm the reliability and practicality of theAPR2-D marker.
Discussion
As one of the most significant quality traits of peppers, fruit colour not only influences visual presentation, but also plays a pivotal role in modulating the nutritional value, health benefits and overall flavor of peppers [Citation4,Citation5]. Therefore, the colour of pepper fruit stands as one of the most essential quality traits influencing breeder preferences during the cultivation process [Citation5,Citation6]. Since the 1930s, researchers have directed their attention towards exploring the the diversity of fruit colour in pepper, its primary pigment components and the genetic and regulatory mechanisms of fruit colouration during both the green immature and red mature stages [Citation5,Citation10,Citation15]. A thorough comprehension has been attained concerning the complex biosynthetic pathways and regulatory networks of chlorophyll, carotenoid, anthocyanin and other pigments. Additionally, essential metabolic enzymes and genes contributing to these pathways have been studied [Citation5,Citation33].
APRR2, much like LOL1, GLK2 and SGR genes, are related to the chlorophyll synthesis and degradation processes in pepper fruits [Citation20,Citation21,Citation34]. In 2013, an APRR2-like gene in sweet pepper was cloned and was found in the same location as pc8.1 on the genetic map [Citation27]. The mutation of this gene contributed to the formation of white colour fruit of white-fruited parental lines at the green immature stage. In the present study, the inbred line B1-2 had milky white colour fruit at the green immature stage. To confirm whether the APRR2-like gene contributes to this trait, we amplified the cDNA and gDNA sequences of a CaAPRR2-like gene from B1-2 and a green-fruited inbred line D50. Comparison of these sequences to the reference genome shows a single base deletion (G) at site 901 of the B1-2 cDNA sequence, leading to the premature termination of the protein translation. This mutation is also observed in the gDNA sequence, and in the sixth expression region of CaAPRR2-like gene. These results indicate that the trait of milky white fruit colour of B1-2 was controlled at least by the CaAPRR2-like gene.
There are several lines of evidence confirming the role of APRR2-like gene in promoting pigment accumulation in fruit, yet little effort has been made to develop molecular markers based on this gene. In this study, based on the single mutation of a CaAPRR2-like gene in the B1-2 parent line, a dCAPS marker was developed. The reliability of the dCAPS marker was validated in F2 populations resulting from the cross between B1-2 and D50. This marker can accurately distinguish milky white fruited lines from green fruited lines, thus significantly improving screening efficiency and facilitating the utilization of this variation for crop improvement.
Substantial progress has been made in understanding the formation of fruit colour in pepper during the red mature stage. However, there has been little research regarding fruit colour traits such as white during the green immature stage. Further exploration and analysis are required to identify the functional genes and regulatory genes that control white, light green and dark green fruit colour at this stage.
Conclusions
In this study, the cDNA sequence of the CaAPRR2-like gene from the milky white-fruited line B1-2 exhibited a single base deletion (G) at site 901 in comparison to the dark green-fruited line D50. Then, based on this mutation, a dCAPS marker with a TaqI restriction site was developed using the dCAPS Finder 2.0 program. Following digestion with the TaqI restriction endonuclease enzyme, distinct bands were observed in the PCR products of B1-2, D50 and F1 (B1-2 × D50) plants, confirming the polymorphic nature of the marker. The reliability of the developed dCAPS molecular marker was successfully validated in the F2 population and 34 pepper inbred lines with white colour fruit at the green immature stage.
Author contributions
Conceptualization, P.F. and Q.G.; Supervision, P.F and H.L.; Data analysis, T.Z., Q.G., and W.C.; Methodology, Q.G., C.L. and H.L.; Validation, T.Z. and J.W.; Investigation, Q.G, W.C, J.W., X.W., D.Z. and X.X.; Writing—original draft preparation, J.W. and P.F.; Writing—review and editing, P.F.; Funding acquisition, H.L., X.W. and T.Z. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (200.7 KB)Disclosure statement
The authors declare no conflict of interest.
Data availability statement
Data that support the findings reported in this study will be available upon request.
Additional information
Funding
References
- Vázquez-Espinosa M, Álvarez-Romero M, González-de-Peredo AV, et al. Capsaicinoid content in the pericarp and placenta of bolilla peppers (Capsicum annuum L.) throughout the ripening of the fruit at two different stages of plant maturation. Agronomy. 2023;13(2):1. doi:10.3390/agronomy13020435.
- Zou Z, Zou X. Geographical and ecological differences in pepper cultivation and consumption in China. Front Nutr. 2021;8:718517. doi:10.3389/fnut.2021.718517.
- Song Z, Zhong J, Dong JC, et al. Mapping immature fruit colour‐related genes via bulked segregant analysis combined with whole‐genome re‐sequencing in pepper (Capsicum annuum). Plant Breed. 2022;2:141.
- Jiménez-Viveros Y, Valiente-Banuet JI. Colored shading nets differentially affect the phytochemical profile, antioxidant capacity, and fruit quality of piquin peppers (Capsicum annuum L. var. glabriusculum). Horticulturae. 2023;9(11):1240.
- Wang L, Zhong Y, Liu J, et al. Pigment biosynthesis and molecular genetics of fruit color in pepper. Plants. 2023;12(11):2156. doi:10.3390/plants12112156.
- Baranski R, Goldman I, Nothnagel T, et al. Chapter 22 - Improving color sources by plant breeding and cultivation. In: Schweiggert R, editor. Woodhead publishing series in food science, technology and nutrition, Handbook on natural pigments in food and beverages. 2nd ed. Sawston, Cambridge, England: Woodhead Publishing; 2024. p. 507–10.
- Jeong HB, Jang SJ, Kang MY, et al. Candidate gene analysis reveals that the fruit color locus C1 corresponds to PRR2 in pepper (Capsicum frutescens). Front Plant Sci. 2020;11:399. doi:10.3389/fpls.2020.00399.
- Guo Q, Zhang T, Li C, et al. A new pepper F1 hybrid - ‘Qujiao no. 5’. China Veg. 2023;10:106–108.
- Lee SY, Jang SJ, Jeong HB, et al. A mutation in zeaxanthin epoxidase contributes to orange coloration and alters carotenoid contents in pepper fruit (Capsicum annuum). Plant J. 2021;106(6):1692–1707. doi:10.1111/tpj.15264.
- Banerjee S, Bhattacharjee T, Maurya PK, et al. Genetic control of qualitative and quantitative traits in bell pepper crosses involving varied fruit colors and shapes. Int J Veg Sci. 2022;28(5):477–492. doi:10.1080/19315260.2021.2025186.
- Li QH, Yang SH, Yu YN, et al. Comprehensive transcriptome-based characterization of differentially expressed genes involved in carotenoid biosynthesis of different ripening stages of capsicum. Sci Hortic. 2021;288:110311. doi:10.1016/j.scienta.2021.110311.
- Liu Y, Lv J, Liu Z, et al. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.). Food Chem. 2020;306:125629. doi:10.1016/j.foodchem.2019.125629.
- Wu XX, Xue LB, Chen JL, et al. EBB and flow of pigment of colour sweet pepper fruit in colour-changed period. J Changjiang Veg. 2005;5:38–40.
- Wu XX, Xue LB, Zha DS, et al. Changes of major pigments of color sweet pepper during growth periods. Southwest China J Agric Sci. 2008;4:1040–1044.
- Venkatesh J, Lee SY, Back S, et al. Update on the genetic and molecular regulation of the biosynthetic pathways underlying pepper fruit color and pungency. Curr Plant Biol. 2023;35–36:100303.
- Kapoor L, Simkin AJ, George Priya Doss C, et al. Fruit ripening: dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022;22(1):27. doi:10.1186/s12870-021-03411-w.
- Wahyuni Y, Ballester AR, Sudarmonowati E, et al. Metabolite biodiversity in pepper (capsicum) fruits of thirty-two diverse accessions: variation in health-related compounds and implications for breeding. Phytochemistry. 2011;72(11–12):1358–1370. doi:10.1016/j.phytochem.2011.03.016.
- Matus Z, Deli J, Szabolcs J. Carotenoid composition of yellow pepper during ripening: isolation of beta-cryptoxanthin 5,6-epoxide. J Agric Food Chem. 1991;39:1907–1914.
- Brand A, Borovsky Y, Meir S, et al. Paran, I. pc8.1, a major QTL for pigment content in pepper fruit, is associated with variation in plastid compartment size. Planta. 2012;235(3):579–588. doi:10.1007/s00425-011-1530-9.
- Brand A, Borovsky Y, Hill T, et al. CaGLK2 regulates natural variation of chlorophyll content and fruit color in pepper fruit. Theor Appl Genet. 2014;127(10):2139–2148. doi:10.1007/s00122-014-2367-y.
- Borovsky Y, Monsonego N, Mohan V, et al. The zinc-finger transcription factor CcLOL1 controls chloroplast development and immature pepper fruit color in Capsicum chinense and its function is conserved in tomato. Plant J. 2019;99(1):41–55.
- Hurtado-Hernandez H, Smith PG. Inheritance of mature fruit color in Capsicum annuum L. J Heredity. 1985;76(3):211–213. doi:10.1093/oxfordjournals.jhered.a110070.
- Popovsky S, Paran I. Molecular genetics of the y locus in pepper: its relation to capsanthin-capsorubin synthase and to fruit color. Theor Appl Genet. 2000;10:86–89.
- Lefebvre V, Kuntz M, Camara B, et al. The capsanthin-capsorubin synthase gene: a candidate gene for the y locus controlling the red fruit colour in pepper. Plant Mol Biol. 1998;36(5):785–789. doi:10.1023/A:1005966313415.
- Huh JH, Kang BC, Nahm SH, et al. A candidate gene approach identified phytoene synthase as the locus for mature fruit color in red pepper (Capsicum spp.). Theor Appl Genet. 2001;102(4):524–530. doi:10.1007/s001220051677.
- Jeong HB, Jang SJ, Kang MY, et al. Candidate gene analysis reveals that the fruit color locus C1 corresponds to PRR2 in pepper (capsicum frutescens). Frontiers in Plant Sience. 2020;11:399.
- Pan Y, Bradley G, Pyke K, et al. Network inference analysis identifies an APRR2-like gene linked to pigment accumulation in tomato and pepper fruits. Plant Physiol. 2013;161(3):1476–1485. doi:10.1104/pp.112.212654.
- Liu HQ, Jiao JQ, Liang XJ, et al. Map-based cloning, identification and characterization of the w gene controlling white immature fruit color in cucumber (Cucumis sativus L.). Theor Appl Genet. 2016;129(7):1247–1256. doi:10.1007/s00122-016-2700-8.
- Jiao JQ, Liu HQ, Liu J, et al. Identification and functional characterization of APRR2 controlling green immature fruit color in cucumber (Cucumis sativus L.). Plant Growth Regul. 2017;83(2):233–243. doi:10.1007/s10725-017-0304-1.
- Jeppe R. Functional markers in plants. Trends Plant Sci. 2003;8:554–560.
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8(19):4321–4325. doi:10.1093/nar/8.19.4321.
- Bassam BJ, Caetano-Anollés G, Gresshoff PM. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem. 1991;196(1):80–83. doi:10.1016/0003-2697(91)90120-i.
- Jiang S, Kim GW, Han K, et al. Investigation of genetic factors regulating chlorophyll and carotenoid biosynthesis in red pepper fruit. Front Plant Sci. 2022;13:922963.
- Borovsky Y, Paran I. Chlorophyll breakdown during pepper fruit ripening in the chlorophyll retainer mutation is impaired at the homolog of the senescence-inducible stay-green gene. Theor Appl Genet. 2008;117(2):235–240. doi:10.1007/s00122-008-0768-5.