Abstract
Uterine smooth muscle tumors are common neoplasms of smooth muscle in the uterus. They range from benign leiomyomas and their variants to malignant leiomyosarcomas. Although there are many subtypes of leiomyoma, mitotically active leiomyoma and leiomyoma with bizarre nuclei are sometimes difficult to differentiate from leiomyosarcoma. Our aim in this study was to investigate whether EBP50 and Merlin expression can be used in the differential diagnosis of uterine smooth muscle tumors and to determine their prognostic significance. Our study analyzed 80 cases diagnosed with uterine smooth muscle tumors from the Pathology Department archive of Dicle University Faculty of Medicine between 2012 and 2023. Primary antibodies EBP50 and Merlin were used in the immunohistochemical study. Information regarding patient age, treatment, and clinical follow-up was obtained from Dicle University Department of Obstetrics and Gynecology and Dicle University Department of Medical Oncology. Our study revealed that EBP50 expression was significantly higher in the leiomyosarcoma and STUMP groups, while Merlin expression was significantly higher in only the leiomyosarcoma group. Prognostic parameters were not found to be associated with EBP50 and Merlin expression levels. EBP50 and Merlin expression levels have not been previously studied in uterine smooth muscle tumors. Our study suggests that high expression of EBP50 in STUMP and leiomyosarcoma, as well as Merlin in leiomyosarcoma, may be helpful in the differential diagnosis of uterine smooth muscle tumors.
Keywords:
Introduction
Uterine smooth muscle tumours are neoplasms of smooth muscle. They are classified according to features such as mitosis, necrosis and nuclear atypia, and range from benign leiomyoma to leiomyosarcoma (LMS) [Citation1]. Uterine leiomyomas are the most common cause of solid pelvic masses in women of reproductive age, but uterine LMS is a rare malignancy that accounts for 1%-3% of female malignancies [Citation2]. In some cases a combination of microscopic features including the degree of cytologic atypia, the presence of necrosis and mitotic activity is sufficient to differentiate these tumors. Although there are many subtypes of leiomyomas, mitotically active leiomyomas and leiomyomas with bizarre nuclei (LMB) are sometimes difficult to differentiate with LMS. In addition, when the distinction between leiomyoma variants and LMS cannot be fully made, the definition of uterine smooth muscle tumor of uncertain malignant potential (STUMP) is used. Many immunohistochemical markers such as P16, P21, CD44, Ki67 and aquoporins have been investigated to improve the differential diagnosis of all uterine smooth muscle tumors [Citation3–6].
The NHERF family has four members, NHERF1/EBP50, NHERF2/E3KARP, NHERF3/PDZK1 and NHERF4/IKEPP. Na/H exchanger regulatory factor 1 (NHERF1) protein, first identified as a cofactor required for the regulation of sodium/hydrogen exchanger type 3 activity by cAMP in the kidney, was independently identified in human placental cell lysates in a successful attempt to identify potential ezrin-radixin-moesin (ERM) protein partners and named ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) [Citation7, Citation8]. Since its first discovery, a wide variety of NHERF1/EBP50-interacting proteins have been identified [Citation9]. Many of these molecules are involved in cancer signaling. Thus, NHERF1/EBP50 has been of increasing interest for cancer studies. However, the role of NHERF1/EBP50 as an oncogene or tumor suppressor is still controversial. Some studies have shown that NHERF1/EBP50 has a tumor suppressor function when localized below the plasma membrane, while it acts as a pro-oncogene when its expression is lost or localized in the cytoplasm or nucleus [Citation10]. Furthermore, it interacts with four members of the ERM-NF2 family of cytoskeletal proteins, ezrin, radixin, moesin and Merlin (Moesin-Ezrin-Radixin-Like Protein), through its carboxyl (C)-terminal ERM-binding domain [Citation11, Citation12]. The EBP50 protein regulates the Merlin/NF2 tumor suppressor. Merlin is a member of the ERM family that interacts with the EBP50 ERM binding domain. This interaction prevents the EGFR from being activated at adherens junctions when cells undergo contact inhibition of proliferation [Citation10, Citation11]. Beyond their morphogenetic role, ERM-NF2 is involved in tumorigenesis. Mutations of the tumor suppressor NF2 cause neurofibromatosis type 2, a nervous system cancer predisposition syndrome [Citation13].
The aim of this study was to investigate whether EBP50 and Merlin expression can be used in the differential diagnosis of uterine smooth muscle tumors and to determine their prognostic significance.
Subjects and methods
Ethics statement
Research permission (25-11.10.23) was obtained from Dicle University Medical Faculty Ethics Committee for Noninterventional Studies.
Tissue collection and examination
Our study included patients diagnosed with uterine smooth muscle tumor between 2012 and 2023 at the Department of Pathology, Faculty of Medicine, Dicle University. Patients’ paraffin-embedded tissues were retrieved from the archive for re-evaluation. Eighty patients with adequate paraffin-embedded tissue and clinical data were included in the study. The specimens were re-evaluated by two experienced pathologists and divided into five groups according to diagnosis: LMB (n = 15), STUMP (n = 15), LMS (n = 20), mitotically active leiomyoma (n = 15), and leiomyoma (n = 15). Information regarding patient age, treatment and clinical follow-up was obtained from Dicle University’s Department of Obstetrics and Gynecology and Department of Medical Oncology. All uterine smooth muscle tumors were restaged according to the 2009 revised Federation of Gynecology and Obstetrics (FIGO) staging system [Citation14].
Tumor type and histopathologic grade were assessed according to the 2020 World Health Organization Classification of Tumors of the Breast and Female Genital Organs. The diagnostic criteria for LMB were as follows: (1) specific smooth muscle cell type; (2) presence of pleomorphic mononucleated or multinucleated bizarre tumor cells (comprising at least 5% of the tumor); and (3) mitotic index <5 per 10 high-power field (HPF). Mitotically active leiomyoma was diagnosed in cases with 6-14/HPF mitosis without necrosis and atypia. In the presence of any of the following criteria: (1) tumors with focal/multifocal or diffuse nuclear atypia with 5-9 mitoses/10 HPF, (2) tumors with tumor cell necrosis but no other worrisome features, (3) tumors without cytologic atypia and tumor cell necrosis but with ≥ 15 mitoses/10 HPF were diagnosed as STUMP. The diagnosis of LMS was confirmed when the tumor had at least two of the following characteristics: mitotic index of 10 per 10 HPFs, moderate to severe cytologic atypia, and tumor cell necrosis [Citation15]. Immunohistochemically ki67 stained preparations were retrieved from the archive and re-evaluated by two expert pathologists.
Immunohistochemical staining
For immunohistochemical markers of EBP50 and Merlin, 4 μm thick sections were taken for each case on adhesive slides (Isotherm, Germany) using a microtome device (Shandon, USA) from the blocks where routine tissue follow-up procedures were performed. EBP50 (Affinity, USA, polyclonal rabbit mAb, catolog number: DF8271, dilution: 1/100, incubation time: 64 min) and Merlin (Genetex, USA, polyclonal rabbit mAb, catolog number: GTX50579, dilution: 1/100, incubation time: 64 min) primary antibodies were used. The immunohistochemical study was performed automatically on a Ventana BenchMark Ultra device (Roche Diagnostics, USA).
The immunohistochemical staining evaluation involved determining and scoring the degree of intensity and percentage for each case. The distribution of EBP50 and Merlin immunoreactivity was scored semi-quantitatively using a 0-4 scale based on the percentage of stained cells. A score of 0 indicates no staining, 1 indicates staining in 1% to 25% of cells, 2 indicates staining in 26% to 50% of cells, 3 indicates staining in 51% to 75% of cells, and 4 indicates staining in 76% to 100% of cells. Immunohistochemical staining intensity was graded from 0 to 3, where 0 indicating no staining, 1 indicating weak staining, 2 indicating moderate staining, and 3 indicating strong staining. The scores were combined by multiplying the percentage and intensity scores. The final scores were then graded as follows: negative = 0, weak = 1-4, moderate = 5-8, and strong = 9-12 [Citation16].
Statistical method
IBM SPSS 21.0 for Windows (Statistical Package for Social Sciences) was used for the statistical evaluation of our research data. The frequency of the variables, the means and the standard deviations of the numerical data were obtained. Measurement variables were presented as mean ± standard deviation (SD), and categorical variables were presented as number and percentage (%). The conformity of continuous variables to the normal distribution was measured by the Kolmogorov-Smirnov test. In statistical evaluations, an independent t-test was used to evaluate the means of normally distributed continuous variables between two groups, and those that did not comply with normal distribution were analyzed with Kruskal Wallis and Mann Whitney U test. The Chi-Square test was used for the statistical evaluation of categorical variables. The effects of EBP50 and Merlin expressions, clinical and pathological characteristics on general survival time of the patients were evaluated by the Kaplan–Meier method in univariate analysis and by the Cox regression method in multivariate analysis. Spearman’s test was used to evaluate the correlation of continuous variables; the hypotheses were bidirectional, and differences were considered statistically significant at the level of p < 0.05.
Results
In our study, a retrospective evaluation was conducted on 80 cases diagnosed as leiomyoma, mitotically active leiomyoma, LMB, STUMP and LMS at the Department of Pathology, Faculty of Medicine, Dicle University between 2012 and 2023. displays the clinical, histopathologic and prognostic features of the patients. Immunohistochemical EBP50 and Merlin expression results are given in .
Table 1. The clinical, histopathologic, and prognostic features of the patients.
Table 2. Results from immunohistochemical analysis of EBP50 and Merlin expression.
Significantly higher EBP50 expression was observed in the STUMP and LMS groups compared to the other groups (). Although EBP50 usually showed cytoplasmic staining, nuclear staining was detected in 5 LMS, 3 STUMP and 2 mitotically active leiomyoma cases (). There was a significant relation between EBP50 expression and tumor type (p = 0.00). Significantly higher Merlin expression was observed in LMS group compared to the other groups (). There was a significant relation between Merlin expression and tumor type (p = 0.00). Pairwise group comparisons of EBP50 and Merlin expressions are given in .
Figure 1. EBP50 staining images in uterine smooth muscle tumour types of some patients. A. Leiomyoma showed no immunoreactivity with EBP50 (immunoperoxidase, 200×). B. Mitotically active leiomyoma showed weak immunoreactivity with EBP50 (immunoperoxidase, 200×). C. STUMP showed moderate immunoreactivity with EBP50 (immunoperoxidase, 200×). D. LMS showed strong immunoreactivity with EBP50 (immunoperoxidase, 200×). Scale bar = 500 µm.
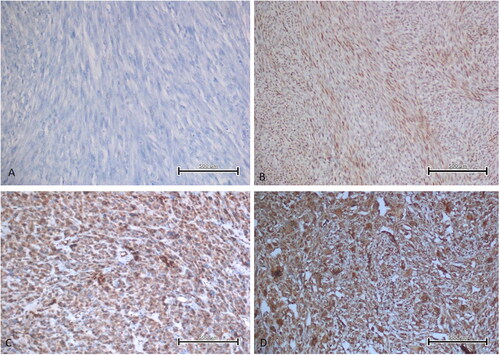
Figure 2. Strong nuclear EBP50 expression seen in some cases of LMS. A, B. Diffuse, nuclear EBP50 expression in two LMS cases (immunoperoxidase, 100×). Scale bar = 500 µm.
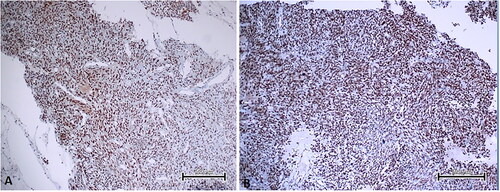
Figure 3. Merlin staining images in uterine smooth muscle tumour types of some patients. A. Leiomyoma showed moderate immunoreactivity with Merlin (immunoperoxidase, 200×). B. Mitotically active leiomyoma showed moderate immunoreactivity with Merlin (immunoperoxidase, 200×). C. STUMP showed moderate immunoreactivity with Merlin (immunoperoxidase, 200×). D. LMS showed strong immunoreactivity with Merlin (immunoperoxidase, 200×). Scale bar = 500 µm.
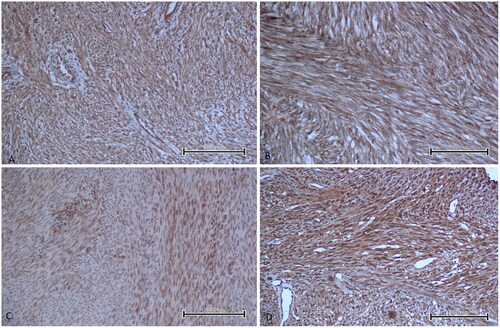
Table 3. Pairwise comparison of uterine smooth muscle tumor groups in terms of EBP50 and Merlin expression.
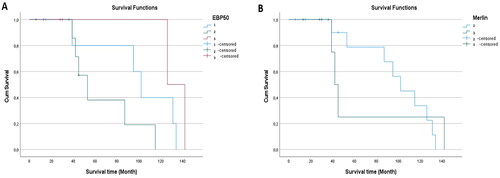
The youngest of the cases was 30 years old, and the oldest was 66 years old, and the mean age was 47.90 ± 10.38. There was a significant relation between age and increased EBP50 expression (p = 0.032) (). There was no significant relationship between age and immunohistochemical Merlin expression (p = 0.079). Our study showed that the mean survival time was 66.15 ± 45.27 months, with the shortest survival time being 6 months and the longest, 142 months. We did not find a significant relation between the survival time and EBP50 and Merlin expression levels (p = 0.052, p = 0.906) ().
Figure 4. The distribution of age across all groups of uterine smooth muscle tumors is presented based on the expression of EBP50 (A) and Merlin (B).
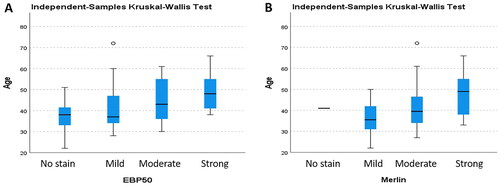
Twelve of the LMS cases were grade 3; five were grade 2 and three were grade 1, and no association was observed between grade and EBP50 and Merlin expression levels (p = 0.133, p = 0.186). Stage 4 was diagnosed in 9 cases due to distant metastasis. There was no significant relationship between EBP50 and Merlin expressions and the stage (p = 0.223, p = 0.054). Significantly decreased survival time was found in Stage 4 LMSs and a significant correlation was found between stage and survival time (p = 0.000). However, no significant relationship was observed between grade and survival in LMSs (p = 0.056). The LMS group exhibited a higher ki67 proliferation index compared to the other groups. However, no significant relation was found between ki67 proliferation index and EBP50 and Merlin expression levels (p = 0.099, p = 0.295). Although the mitotic index was significantly increased in the LMS group, no significant relation was found between mitotic index and EBP50 and Merlin expression levels (p = 0.053, p = 0.670).
Discussion
Uterine LMS is a rare malignancy that originates from the smooth muscle of the uterine wall. Clinical symptoms are not present in most patients with LMS. The diagnosis of LMS is usually made through pathological examination of hysterectomy or mass excision samples. Uterine LMS can be easily diagnosed in most cases by the presence of histopathological features like severe nuclear atypia, a high mitotic rate (exceeding 10 mitotic figures per 10 HPF), necrosis and atypical mitotic figures. However, differential diagnosis can be challenging due to atypical histological features and unusual growth patterns that mimic malignancy in STUMP and some leiomyoma variants [Citation17, Citation18]. Our study analyzed 20 cases of LMS, 15 cases of BLM, 15 cases of mitotically active leiomyoma and 15 cases of STUMP, which may pose challenges for differential diagnosis. In addition, 15 cases of leiomyoma were examined as a control group. As expected, the LMS group had a higher patient age, mitotic count and ki67 index compared to the other groups.
The NHERF1, also known as EBP50, emerged in the late 1990s as a co-regulator of the sodium-hydrogen antiporter 3 in rabbit kidney epithelia. EBP50 has been shown to play a critical role in the regulation of signal transduction pathways by acting as a scaffolding protein and has been shown to be overexpressed in certain human tumors such as breast cancer, schwannoma and hepatocellular carcinoma [Citation19]. Studies have shown that EBP50 has direct biological effects on gynecologic tumors. The overexpression of EBP50 appears to play a crucial role in directing the early stages of cancer initiation in various tumor types of ovarian and cervical origin [Citation19–22]. Although EBP50 expression has been investigated in some gynecologic tumors, it has not been previously studied in uterine smooth muscle tumors. In our study, we observed significant EBP50 overexpression in STUMP and LMS cases. Although there was higher expression in LMSs compared to STUMPs, it was not statistically significant. We can say that EBP50 expression is a useful marker in the differential diagnosis of leiomyoma variants that may resemble their malignant counterparts and STUMP and LMS. However, studies with larger case series are needed to fully elucidate the role of EBP50 in the differential diagnosis of STUMP and LMS.
Wang et al. [Citation23] showed that EBP50 acts as a protein that suppresses tumor metastasis in breast cancer and cervical carcinoma cells. Matsumoto et al. [Citation22] demonstrated that EBP50 is an unfavorable prognostic indicator in clear cell ovarian carcinomas. Another study [Citation21] reported that EBP50 increases cisplatin sensitivity in cervical cancer cells. Hoque at al. [Citation24] demonstrated that EBP50 increases the expression and function of multidrug resistance protein 4 and has an effect on treatment and prognosis. Although EBP50 overexpression was observed in LMS patients in our study, the relationship between this expression and grade, stage, metastasis and prognosis could not be demonstrated. The small number of LMS cases is a limitation of our study and this issue needs to be clarified with larger series.
EBP50 localization is altered during cell transformation, resulting in a shift from the membrane to the cytoplasm and nucleus. This phenomenon has been observed in various types of cancer including breast, liver, brain, colon, kidney and prostate cancer [Citation8, Citation25]. Lema et al. [Citation8] showed nuclear and membranous EBP50 expression in renal cell carcinoma cases. Shibata et al. [Citation26] observed that nuclear EBP50 expression may be present in hepatocellular carcinoma. Lin et al. [Citation27] described aberrant nuclear EBP50 expression in colorectal carcinoma. In a study of colorectal carcinogenesis, nuclear expression of EBP50 was associated with a more aggressive phenotype; a significant association was also found between NHERF1 levels, disease progression and the appearance of liver metastases [Citation28]. In breast cancer patients, long-term follow-up of a clinical cohort demonstrated the prognostic value of nuclear expression of EBP50. Patients with expression of nuclear EBP50 showed greater disease-free survival compared to patients with no nuclear expression [Citation29]. In our study, nuclear EBP50 expression was observed in 5 LMS cases, but no relation was observed between nuclear expression and prognosis. In addition, nuclear EBP50 expression was detected in 3 STUMP and 2 mitotically active leiomyoma cases.
The Neurofibromin 2 gene is located on chromosome 22 and consists of 17 exons. It encodes a 70-kDa protein called NF2, or Merlin. Mutations in NF2 cause neurofibromatosis type 2. This disease is autosomal dominant and is mainly associated with benign tumours of the nervous system such as bilateral vestibular schwannomas, meningiomas and ependymomas [Citation30]. Merlin deficiency has been identified in various malignant tumors, such as mesothelioma, prostate cancer, melanoma, glioma and breast cancer, in addition to its initial investigation in Neurofibromatosis type 2 disorder [Citation30–33]. Quan et al. [Citation34] demonstrated that Merlin inhibited tumor growth and metastasis in vivo in pancreatic ductal adenocarcinoma. This suggests that Merlin is an important tumor suppressor for this type of cancer. While various studies have shown the role of Merlin in some tumors, to our knowledge, no study has examined Merlin expression in uterine smooth muscle tumors. In our study, we observed higher Merlin expression in the LMS group compared to the other groups. The results of our study indicate that the overexpression of Merlin, in conjunction with morphological features such as mitosis, necrosis and nuclear atypia, may serve as a means of differentiating LMS from other uterine smooth muscle tumors. Although Merlin overexpression was observed in LMS patients in our study, the relationship between Merlin expression and grade, stage, metastasis and prognosis could not be demonstrated. Although these results may be significant, as noted previously for EBP50, the small number of LMS cases is a limitation of our study and this issue needs to be clarified with larger series.
Conclusions
EBP50 and Merlin overexpression, previously shown in some tumor types, were demonstrated for the first time in uterine smooth muscle tumors in this study. Some previous studies on EBP50 and Merlin have shown that some malignant tumor types as well as a benign tumor such as schwannoma can express these markers. In our study, EBP50 and Merlin expression was observed in a small number of cases in normal myometrium and in some leiomyomas and benign variants, which casts doubt on the usefulness of these markers in the differential diagnosis, but supports the idea that they may be useful in differentiating these tumors because they were expressed at a much higher rate in LMS. Furthermore, the absence of strong EBP50 and Merlin expression in any of the leiomyomas and variants provided a strong indication that strong expression of these markers can be seen in LMS. In order to make definitive comments on this subject, we believe that studies with large series including more cases should be performed. Moreover, further studies with large series will provide support, positive or negative, for the idea that EBP50 and Merlin do not have a prognostic significance as shown in this study.
Author contributions
All authors contributed significantly to the work and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. UA and ANK made substantial contributions to the conception and design of the work. UA, ANK, RG, IY and ZU were involved in project management, literature search, data acquisition, interpretation, drafting, and reviewing of the manuscript. UA and ANK were involved in visualization. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [UA], upon reasonable request.
Additional information
Funding
References
- Horn LC, Hiller GGR, Mayr D, et al. Practical diagnostic aspects of uterine leiomyosarcoma in the context of the 2020 WHO classification. Pathologe. 2022;43(3):196–201. doi: 10.1007/s00292-022-01064-6.
- Tsukada H, Muramatsu T, Miyazawa M, et al. Long term prognostic implications of expression of glucose transporter- 1 and hexokinase II in patients with stage I uterine leiomyosarcoma. Acta Histochem Cytochem. 2012;45(2):147–154. doi: 10.1267/ahc.11063.
- Gökaslan H, Türkeri L, Kavak ZN, et al. Differential diagnosis of smooth muscle tumors utilizing p53, pTEN and Ki-67 expression with estrogen and progesterone receptors. Gynecol Obstet Invest. 2005;59(1):36–40. doi: 10.1159/000080933.
- Poncelet C, Walker F, Madelenat P, et al. Expression of CD44 standard and isoforms V3 and V6 in uterine smooth muscle tumors: a possible diagnostic tool for the diagnosis of leiomyosarcoma. Hum Pathol. 2001;32(11):1190–1196. doi: 10.1053/hupa.2001.28935.
- Ünver NU, Acikalin MF, Öner Ü, et al. Differential expression of P16 and P21 in benign and malignant uterine smooth muscle tumors. Arch Gynecol Obstet. 2011;284(2):483–490. doi: 10.1007/s00404-010-1690-z.
- Alabalık U, Türkcü G, Keleş AN, et al. Can aquaporins be used as diagnostic and prognostic markers for uterine smooth muscle tumours? 2017; Biotechnology & Biotechnological Equipment. 2017;31(1):148–155. doi: 10.1080/13102818.2016.1240018.
- Weinman EJ, Steplock D, Wang Y, et al. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane na(+)-H + exchanger. J Clin Invest. 1995;95(5):2143–2149. doi: 10.1172/JCI117903.
- Lema BE, Patricio GM, Kreimann EL. Nuclear expression of NHERF1/EBP50 in clear cell renal cell carcinoma. Acta Histochem. 2021;123(5):151717. doi: 10.1016/j.acthis.2021.151717.
- Centonze M, Saponaro C, Mangia A. NHERF1 Between promises and hopes: overview on cancer and prospective openings. Transl Oncol. 2018;11(2):374–390. doi: 10.1016/j.tranon.2018.01.006.
- Vaquero J, Nguyen Ho-Bouldoires TH, Clapéron A, et al. Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: from signaling regulation to clinical relevance. Oncogene. 2017;36(22):3067–3079. doi: 10.1038/onc.2016.462.
- Nguyen R, Reczek D, Bretscher A. Hierarchy of merlin and ezrin N- and C-terminal domain interactions in homo- and heterotypic associations and their relationship to binding of scaffolding proteins EBP50 and E3KARP. J Biol Chem. 2001;276(10):7621–7629. doi: 10.1074/jbc.M006708200.
- Georgescu MM, Mobley BC, Orr BA, et al. NHERF1/EBP50 and NF2 as diagnostic markers for choroid plexus tumors. Acta Neuropathol Commun. 2016;4(1):55. doi: 10.1186/s40478-016-0329-0.
- Curto M, Cole BK, Lallemand D, et al. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177(5):893–903. doi: 10.1083/jcb.200703010.
- Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104(3):177–178. doi: 10.1016/j.ijgo.2008.12.008.
- Matias-Guiu X, Oliva E, McCluggage WG, et al. WHO classification of tumours of female genital tumours: tumours of the uterine corpus. 5th ed. Lyon (France): International Agency for Research on Cancer; 2020.
- Fuchs I, Vorsteher N, Bühler H, et al. The prognostic significance of human epidermal growth factor receptor correlations in squamous cell cervical carcinoma. Anticancer Res. 2007;27:959–963.
- Kaur K, Kaur P, Kaur A, et al. Uterine leiomyosarcoma: a case report. J Midlife Health. 2014;5(4):202–204. doi: 10.4103/0976-7800.145175.
- Sait HK, Anfinan NM, El Sayed ME, et al. Uterine sarcoma. Clinico-pathological characteristics and outcome. Saudi Med J. 2014;35:1215–1222.
- Sonnessa M, Sergio S, Saponaro C, et al. The biological relevance of NHERF1 protein in gynecological tumors. Front Oncol. 2022;12:836630. doi: 10.3389/fonc.2022.836630.
- Oh YS, Heo K, Kim EK, et al. Dynamic relocalization of NHERF1 mediates chemotactic migration of ovarian cancer cells toward lysophosphatidic acid stimulation. Exp Mol Med. 2017;49(7):e351. doi: 10.1038/emm.2017.88.
- Tao T, Yang X, Qin Q, et al. NHERF1 enhance cisplatin sensitivity in human cervical cancer cells. Int J Mol Sci. 2017;18(1):5. doi: 10.3390/ijms18010005.
- Matsumoto T, Yoki A, Konno R, et al. Cytoplasmic EBP50 and elevated PARP1 are unfavorable prognostic factors in ovarian clear cell carcinoma. Carcinogenesis. 2021;42(9):1162–1170. doi: 10.1093/carcin/bgab070.
- Wang L, Qi Y, Xiong Y, et al. Ezrin-Radixin-moesin binding phosphoprotein 50 (EBP50) suppresses the metastasis of breast cancer and HeLa cells by ınhibiting matrix metalloproteinase-2 activity. Anticancer Res. 2017;37(8):4353–4360. doi: 10.21873/anticanres.11829.
- Hoque MT, Cole SP. Down-regulation of na+/H + exchanger regulatory factor 1 ıncreases expression and function of multidrug resistance protein 4. Cancer Res. 2008;68(12):4802–4809. doi: 10.1158/0008-5472.CAN-07-6778.
- Georgescu MM, Morales FC, Molina JR, et al. Roles of NHERF1/EBP50 in cancer. Curr. Mol. Med. 2008;8:459–468. doi: 10.2174/156652408785748031.
- Shibata T, Chuma M, Kokubu A, et al. EBP50, a beta-catenin-associating protein, enhances WNT signaling and is over-expressed in hepatocellular carcinoma. Hepatology. 2003;38(1):178–186. doi: 10.1053/jhep.2003.50270.
- Lin YY, Hsu YH, Huang HY, et al. Aberrant nuclear localization of EBP50 promotes colorectal carcinogenesis in xenotransplanted mice by modulating TCF-1 and β-catenin interactions. J Clin Invest. 2012;122(5):1881–1894. doi: 10.1172/JCI45661.
- Mangia A, Saponaro C, Malfettone A, et al. Involvement of nuclear NHERF1 in colorectal cancer progression. Oncol Rep. 2012;28(3):889–894. doi: https://doi.org/10.3892/or.2012.1895.
- Paradiso A, Scarpi E, Malfettone A, et al. Nuclear NHERF1 expression as a prognostic marker in breast cancer. Cell Death Dis. 2013;4(11):e904. doi: 10.1038/cddis.2013.439.
- Mota M, Shevde LA. Merlin regulates signaling events at the nexus of development and cancer. Cell Commun Signal. 2020;18(1):63. doi: 10.1186/s12964-020-00544-7.
- Petrilli AM, Fernández-Valle C. Role of merlin/NF2 inactivation in tumor biology. Oncogene. 2016;35(5):537–548. doi: 10.1038/onc.2015.125.
- Sato T, Sekido Y. NF2/Merlin ınactivation and potential therapeutic targets in mesothelioma. Int J Mol Sci. 2018;19(4):988. doi: 10.3390/ijms19040988.
- Sekido Y, Sato T. NF2 alteration in mesothelioma. Front Toxicol. 2023;5:1161995. doi: 10.3389/ftox.2023.1161995.
- Quan M, Cui J, Xia T, et al. Merlin/NF2 suppresses pancreatic tumor growth and metastasis by attenuating the FOXM1-mediated wnt/β-Catenin signaling. Cancer Res. 2015;75(22):4778–4789. doi: 10.1158/0008-5472.CAN-14-1952.