Abstract
Repurposing of drugs is a strategy for discovering new uses for already authorized or tested medicines outside their original indications. Bevacizumab is humanized antibody, observed to have anti-VEGF (Vascular Endothelial Growth Factor) effect, which is indicated for colorectal, lung and other types of carcinomas. We conducted research on the historical development and current regulatory status of bevacizumab. In addition, analysis of the data on completed clinical trials with bevacizumab showing potential for repurposing was performed. The analysis for the period 01.01.2019-31.12.2022 found 131 trials, 42 (32%) of which have potential for repurposing. The development and influence of bevacizumab-biosimilar medicines was studied in the whole clinical trials database with biosimilars included in 23 trials (15 in Phase I and 8 in Phase III). The performed research and analysis show that bevacizumab with its pharmacological mechanism of action can affect crucial point in the pathogenesis of different diseases. In addition to this, the process of repurposing can discover new pathways of treatment for patients, shorten the time for reaching the market, lower public healthcare expenses and companies’ budgets. With all the results pointed out, this biotechnological product has potential for repurposing in broad spectrum of new indications.
Introduction
Bevacizumab, a monoclonal antibody, is the first medicine which successfully uses the molecular signal path of vascular endothelial growth factor (VEGF), and its receptor (VEGF-R), as a pharmacological target. This biotechnological product exercises its effect by blocking the interaction between the ligand (especially type A) and the receptor (types 1 and 2) [Citation1, Citation2]. It was first approved as medicine by FDA in 2004 with primary indication ‘metastatic colorectal carcinoma’. In the following years bevacizumab was authorized for use in several additional indications – for example ‘metastatic breast carcinoma’ in combination with paclitaxel, ‘unresectable advanced metastatic or relapsing non-small cell lung cancer’ and others [Citation3–5].
In help of extending the knowledge for adverse drug reactions and long-term efficacy many Phase IV clinical trials and non-interventional studies are performed. Their prolonged duration and vast number of patients enrolled determine the larger volume of data on drug use, which is gathered and processed. Additional estimate of key parameters such as long-term safety, improved efficacy or proof of new indication can be performed by aggregation and combination with data from clinical trials Phase I-III [Citation6]. There are different regulatory and marketing strategies to extend medicines product life cycles. Repurposing for example is one effective strategy in identifying new pharmacological or therapeutic indications for developed and established drug molecules. When a drug interacts with a common signal pathway in the human body which is part of different pathological mechanisms, this can lead to discovery of new therapeutic alternatives for existing conditions [Citation7]. With its anti-angiogenic effect bevacizumab shows potential for repurposing in various areas: oncology, ophthalmology, angiology and others [Citation8–10]. The wide use of this anti-VEGF drug also contributes for the successful manufacturing and authorization for use of biosimilar medicinal products from the drug regulators in USA and Europe [Citation11]. The scientific idea behind our research and analysis is to investigate the repurposing process for a well-established drug product in order to define specific characteristics in the conducted clinical trials. In addition, the investigation of the clinical trials including a drug product can show absence or presence of further repurposing potential. The aim of this paper is to describe the development and modern state-of-art of bevacizumab, to analyze the potential for repurposing in different indications and to study the development and influence of biosimilars of bevacizumab.
Materials and methods
In this paper we performed literature search and discussed the published data of historical development and current state-of-art of repurposing of bevacizumab as a methodology for conducting the scientific research. Two of the most significant and crucial articles in the development of bevacizumab were summarized.
Detailed analyses of clinical trials database [Citation12] with public access were carried out for the original as well as the biosimilars with INN bevacizumab. The database was chosen due to its amount of registered clinical trials (together with description, related article information, protocol, etc.), dominant use in science literature, diverse advance search options and easy accessibility for operation. All clinical trials subjected to the analysis were extracted, analyzed and included in the study. From a methodological and scientific point of view, this is the main limitation of the study. In addition, the period, selected areas and indications, studies grouping, transparency of clinical trials funding, etc. could be discussed as potential limitations with lower impact.
In the case of original bevacizumab using the ‘Advance search option’ the following criteria were applied:
Other terms – ‘bevacizumab’
Study type – ‘Interventional studies (Clinical Trials)’
Status – ‘Recruitment – Completed’
Primary completion – From 01.01.2019 to 31.12.2022
After reviewing the obtained results, the following criteria were applied:
Inclusion criteria – bevacizumab part of control or experimental arm.
Exclusion criteria – condition/s in the clinical trial part of authorized therapeutic indications and/or bevacizumab not included as intervention and/or bevacizumab only as part of previous therapy and/or primary purpose is ‘diagnostic’ and ‘basic science’.
This search and selection strategy was implemented to limit the results to only ongoing and completed clinical trials, which include bevacizumab and therapeutic indication outside of the already authorized ones. No criteria were applied to ‘Age’ and ‘Study Phase’ since they represent major variables in the repurposing process.
The analysis concerning bevacizumab biosimilars was performed including all available data until 05 February 2023. Using the ‘Advance search option’, the following criteria were applied:
Other terms – ‘bevacizumab biosimilar’ and ‘Avastin biosimilar’
Study type – ‘Interventional studies (Clinical Trials)’
This different approach for the biosimilars was undertaken because of the limited number of trials including bevacizumab-biosimilars, which eliminated the need of implementation of advanced search options and imposed in-depth analysis of all available trials. After reviewing the obtained results, the results were combined in three subgroups:
Clinical trials comparing bevacizumab-biosimilars against Avastin© (originally developed bevacizumab)
Clinical trials with bevacizumab-biosimilars as part of experimental or control arm
Clinical trials not including bevacizumab-biosimilars arm.
The first group included only trials which contain in section ‘Interventions’ both Avastin© and bevacizumab biosimilar (or code name of it clarified as bevacizumab biosimilar in the available trial description or related article). The remaining results were distributed into group 2 and 3. Trials, which included only ‘bevacizumab biosimilar’ and/or related drug code in their Study title and/or Interventions, were included in group 2. For trials, which did not meet this criterion, trial record was opened and reviewed for additional information on whether biosimilar drug was part of either arm. If the record was not definitive enough on that question, the article related to the trial outcomes was searched for ‘biosimilar’ and trade or code names of authorized bevacizumab biosimilars. Clinical trials showing lack of information for biosimilar both in the trial record and in the related article were combined in Group 3.
Results and discussion
Our literature search resulted in defining two articles who are the most cited and marked the main stages of the drug development process for bevaicuzmab. These include the article of Judah Folkman [Citation13] in which he describes VEGF and its significant role in tumor growth and the article of Ferrara et al. [Citation2], which describes in depth the discovery and development of bevacizumab.
Тhe analysis of Judah Folkman’s paper [Citation13] supported the significance of the neovascularization for tumors. The author stated that growth of solid tumors is dependent on the formation of new blood vessels and if this does not occur the tumor will reach size up to 2–3 mm and enter dormant yet viable state. If the growth of tumor outweighs the growth of new vessels, drop in the speed of tumor increase is observed. The author also hypothesized that there is a factor, which contributes to better blood flow to the tumor and suggests that if this factor is blocked, the malignant growth will be interrupted [Citation13].
Ferrara et al. [Citation2] further broaden the knowledge for VEGF by isolating the factor and investigating it. The main effects described in the work are:
promotion of the growth of vascular endothelial cells derived from arteries, veins and lymphatics
increase in vascular permeability
prevention of endothelial-cell apoptosis induced by serum starvation
induction of the expression of the anti-apoptotic proteins BCL2 and A1
Furthermore, a big part of the study was focused on the receptors for VEGF and found three types in general. However, type receptor 3 is largely restricted to lymphatic endothelial cells and was not researched extensively in this article. The most important for the mitogenic, angiogenic and permeability enhancing effects of VEGF is type 2 receptor. However, the authors concluded that without blocking type 1 receptor as well type 2 the effect would not be sufficient in some tumors. There is also analysis of VEGF’s role in tumors and it is described that many human tumors express the factor. There are many differences in expression between not only various kinds of tumors but also cases of the same kind of tumors. The described development of bevacizumab started with humanization of mouse anti-VEGF monoclonal antibody in 1997 which neutralizes only isoform A of VEGF and does not interact with other members of the factor’s family (). In preclinical phase of development, it was found that it inhibits the growth of human tumor cell lines in nude mice and the magnitude of the inhibition is inversely related to the content of stromal-derived mouse VEGF. Bevacizumab safety was demonstrated in Macaca fascicularis (species showing complete identity with the human’s VEGF). Clinical trials in Phase I were initiated in April 1997 and concluded that the drug was relatively non-toxic and did not lead to increased toxicity when used in combination with chemotherapy. The article includes the data of five Phase II and one Phase III (in colorectal carcinoma) trials which show encouraging results [Citation2].
Figure 1. Mechanism of action of bevacizumab [Citation11].
![Figure 1. Mechanism of action of bevacizumab [Citation11].](/cms/asset/082cd7ef-aec1-4885-b602-cd4b1827e209/tbeq_a_2357191_f0001_c.jpg)
Based on the data from the Phase III trial and the clinical benefit of bevacizumab measured by survival, progression-free survival and objective response seen in all pre-specified subject subgroups and other clinical trials, FDA grants approval in 2004 followed by EMA in 2005 [Citation3, Citation14].
During the conducted analysis for the original bevacizumab we found 131 results. From them, 42 (32%) show the molecule’s potential for repurposing in indications that are not part of SmPC (Supplemental data, Appendix 1). Results with repurposing potential were categorized by Therapeutic area, Phase, Masking, Intervention model, Primary purpose and Funder type and the distribution is shown and discussed below.
The most important aspect of the analysis of repurposing potential is the Therapeutic area distribution presented in . It shows that 19 (45%) of the trials with potential for repurposing belong to Ophthalmology as the most covered area. This can be explained by the importance of angiogenesis and vessel permeability in the pathology of these indications and the blocking mechanism of bevacizumab. In addition, bevacizumab is widely used off-label in Ophthalmology which adds to the repurposing potential in these conditions [Citation15]. Second with 18 (43%) is Oncology area with repurposing potential for glioma, glioblastoma, collecting duct carcinoma, etc. Ever since Judah Folkman’s article angiogenesis is one of the main targets when fighting tumors and anti-VEGF drugs are candidates for treatment of different tumor types especially ones strongly dependent on angiogenesis like gliomas [Citation16]. Three (7%) and two (5%) of the trials stand for Angiology and Infectious diseases (COVID-19) respectively. With four different therapeutic areas, in which bevacizumab can help treating variety of conditions, the molecule shows huge potential for repurposing.
Table 1. Original bevacizumab clinical trials distribution on the basis of therapeutic area.
It is interesting to point out the distribution by phases and the big share of clinical trials Phase II and III which together constitute 64% of all results and Phase I trials (including Early Phase I) are only 4 (10%) ().
Table 2. Original bevacizumab clinical trials distribution on the basis of phase.
This corresponds well with the concept of using a well-researched drug based on safety data from previous experience and can also eliminate the need of strict Phase I studies of safety and tolerability. In our analysis, 3 of the 4 trials in Phase I are an exception of this model, as the targeted ophthalmology indications represent new therapeutic area with limited data and require more research for tolerability and dosage. Repurposing allows for more saved funds and resources and shortens the time for access to the market as shown by Pushpakom et al. [Citation17] and Hernandez et al. [Citation18] In their 2020 report for drug repurposing ‘Research and Markets’ describe that the cost of developing and marketing a repurposed drug has been estimated to be USD 300 million, compared to the USD 2–3 billion for a novel one [Citation19].
Part of this cost reduction can derive from the use of in silico repurposing approaches, which use highly sophisticated systems to recognize a connection between drug and disease. There are two main ways to perform this according to Cha et al. [Citation20] – one is using computerized molecular approaches, ‘OMIC data’ and predicting interaction between chemical structure and drug targets like the one established by Lee et al. [Citation21]. Other is Real World Data (RWD) approaches, which use data collection methods focused on identification of unknown and/or unexpected relationship between trends in prescribing and patient outcomes that can support regulatory decisions [Citation22]. On one hand, these can reduce the cost of preclinical development and eliminate the need of animals testing. On the other, savings can come from avoided expenses due to unacceptable toxicity or inability to prove clinical benefit in the course of later phases [Citation23].
During clinical trials, savings could be generated by omitting some of the development phases during gathering of information needed for regulatory authorization. Martin et al. [Citation24] described that median costs are as follows – Phase I with 3.4 million, Phase II − 8.6 million and Phase III − 21.4 million. These costs depend mainly on number of subjects randomized, countries and sites involved, but type of molecule researched was not significant factor for the costs. Having this in mind, bigger savings might occur when a molecule proven to be safe and efficacious is repurposed in close to its previous authorized indications and Phase III trials are conducted only.
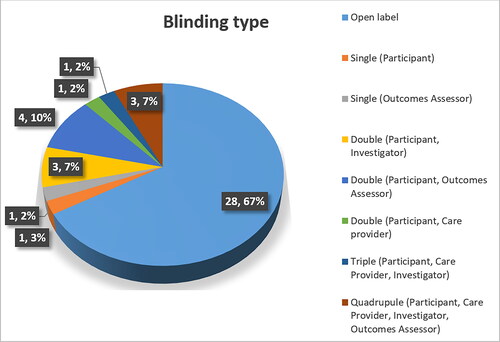
In regards to ‘Blinding’ the results from our research with original bevacizumab show 28 (67%) trials were conducted as open-label (). The masked trials (regardless of the type) included conditions in ophthalmology and hematology, and none in oncology. This results from the need of robust data in new therapeutic areas which is generally gathered in masked trial, as found by Karanicolas et al. [Citation25].
For ‘Intervention model’ our research found that 22 (52%) of the repurposing potential trials used parallel assignment and 18 (43%), single group assignment. Only 2 (5%) were designed with sequential assignment – this number can be explained with the tendency of not reporting results with this kind of assignment transparently and interpretably enough, as stated by Bigirumurame et al. [Citation26]. In addition, this type of design may involve many sub-parts and variables within the same trial which can pose greater challenges to regulatory authorities in terms of trial authorization and to both investigators and pharmaceutical companies – for implementation of the assignment, as shown by Kidwell et al. [Citation27].
Primary purpose analysis shows 40 (95%) trials for treatment, 1 (2.5%) for supportive care and 1 (2.5%) for prevention. The ‘supportive care’ trial studied the pain and sub conjunctival hemorrhage control of cooled anesthetic eye drops and antiseptics following intravitreal application of bevacizumab in different ophthalmologic conditions. The prevention-oriented trial studied intravitreal bevacizumab against review of cases with no additional treatment in recurrent retinal detachment. These results also confirm the importance of repurposing bevacizumab and specifically in Ophthalmology.
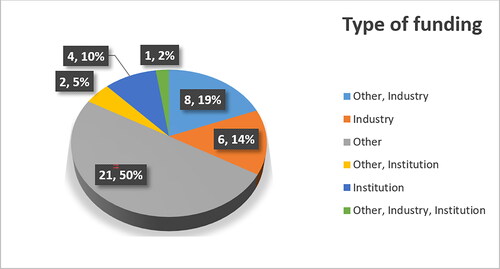
The type of funding is another important aspect included in the research which underscores important relation (). Trials funded with the partnership of industry were relatively less numerous (with total of 15 or 35.7%). These include 6 (14%) funded by industry alone, 8 (19%) funded by industry and other sides and one is funded by other, industry and institution.
The term ‘Other’ corresponds to sponsors/collaborators such as hospitals, medical and research centers, institutes, universities, etc. some of which are public and some are financed by foundations, non-profit organization, etc. This once again consolidates the importance of repurposing because in most cases funding is offered by public institutions rather than private ones. As discussed by Cha et al. academics are interested in collaborating in these types of trials in order to achieve science breakthrough and attract talents, government funding and other partnerships [Citation20]. Moreover, most of the cases of repurposing before the digitalization era have been triggered by case reports descriptions by doctors and retrospective analyses by scientists. Therefore, the real and primary source of repurposing are indeed hospitals and universities. Typical example is the repurposing of the key drug for the development of strict drug regulations – thalidomide. Initially marketed as morning sickness pill in pregnant women it was found to have teratogen potential and to cause phocomelia in newborn babies. RJ D’Amato with his theory that drug’s antiangiogenic effect can be used in oncology initiated the process of repurposing thalidomide for multiple myeloma [Citation28]. One of the clinical trials also had institutional funding and was conducted by scientists at the University of Arkansas. Both of the repurposing methods for thalidomide show comparable initiatives and funding as the major part of repurposing trials for bevacizumab.
Furthermore, 7 (17%) of all identified repurposing trials had institutional backing and 4 (10%) of them were entirely funded by institutions. NIH and its different Institutes grants all 4 funding for diverse indications in the areas of Oncology and Ophthalmology. The most important sector for repurposing in NIH is the National Center for Advancing Translational Sciences (NCATS), which has dedicated page with information on this topic. The center’s purpose is to offer support in searching new home for approved drugs in early- or late-stage. In early-stage, repurposing NCATS can help with approved drugs libraries, which researchers can screen for potential candidates. There scientists conduct high-throughput screening (HTS), computerized modeling and various other analyses so that they can help the repurposing process. For the late-stage, repurposing NCATS aid investigators, which have, found a promising molecule by collecting data for authorization of the clinical trial and provide funding on a competitive basis to academic researchers who carry out repurposing projects. All this confirms the importance of repurposing bevacizumab with its wide government back up and funding [Citation29].
Bevacizumab-biosimilars trials analysis show the following distribution in the pre-defined groups − 16 (23%) results in Group 1 (Supplemental data, Appendix 2), 19 (28%) in Group 2 (Appendix 3) and 33 (49%) in Group 3 (Appendix 4) ().
Table 3. Distribution of clinical trials included in bevacizumab-biosimilars analysis.
In Group 1 we found ten clinical trials Phase I and six – Phase III. This is due to the regulatory requirement of conducting phases I and III in order to prove biosimilarity. Phase I are usually conducted as pharmacokinetic study, preferably in healthy volunteers and if necessary pharmacodynamic studies can be added. Comparative Phase III trials are performed in vaster population groups (for bevacizumab – patients with non-small cell lung cancer) and using endpoint both of which are sensitive enough to identify any meaningful clinical differences between biological and biosimilar product [Citation30]. Based on the results from such trials EMA has authorized 8 bevacizumab-biosimilar products and 2 others were withdrawn by marketing authorization holder. These numbers make bevacizumab equal in number of authorized biosimilars with pegfilgrastim. Both are outnumbered by adalimumab which has 10 confirmed by the results found in EMA’s database for biosimilar medicinal products [Citation31]. This confirms the significance of the molecule in clinical practice as it holds second place by authorized biosimilars in EMA. The analysis focuses on results from EMA as it is the world’s leader in biosimilar regulation and approval with first guidelines created in 2005 (followed by an update in 2015). Meanwhile FDA adopted its first regulation on the subject of biosimilars in 2010 (The Biologics Price Competition and Innovation Act) and began applying it in 2015 [Citation32, Citation33]. Other major difference between the two agencies is the FDA requirement of transition studies in which patients treated with the biological are switched to the biosimilar in order to evaluate safety and efficacy. Despite these differences, both agencies have created initiatives to facilitate biosimilars’ authorization and gathering enough evidence for biosimilars quality, safety and efficacy.
Group 2 results show that total of 7 (37%) out of 19 trials with bevacizumab-biosimilar as intervention used indication hepatocellular carcinoma and 5 (26%) ovarian carcinoma. The results from this group reconfirm the clinical importance of bevacizumab and its mechanism of action. Moreover, results from trials in the first indication, which is not included in the EMA approved SmPC [Citation34], are in support of the thesis that bevacizumab has potential for repurposing. Having in mind the aforementioned data, biosimilars have enormous impact on clinical practice – widens patient access to new and innovative therapies, saves resources for different types of healthcare payers, poses challenges to healthcare providers in the choice of appropriate medicine, etc. Repurposing can play a significant role in the development of biosimilars and by adding new indications can broaden the sphere of influence of biosimilars. This will facilitate the discovery of more sensitive population for conducting comparative clinical trials.
Drug repositioning is a well-known strategy for establishing new uses for authorized or investigational medicines that are beyond the original initially approved indication. Bevacizumab as an anti-VEGF humanized antibody is indicated in colorectal, lung and other types of carcinomas. The study conducted on the historical development and current status of bevacizumab repositioning is very indicative of the treatment potential of the molecule and confirms data from other similar studies. In addition, an analysis of data from completed clinical trials with original bevacizumab and potential for repositioning and study of the development and impact of entry of biosimilar medicinal products containing bevacizumab was performed. The analysis of completed bevacizumab CTs and the results obtained support the hypothesis of future potential for repositioning. In the context of biosimilarity, there are 23 clinical trials investigating bevacizumab-biosimilars, mostly in early phases, so regulatory approval of new medicines and new therapeutic indications can be expected in the future.
Conclusions
The performed analysis shows that the mechanism of action of bevacizumab can affect an important step in the pathogenesis not only of tumors but in different diseases as well, namely neoangiogenesis. For this reason, the molecule has potential for repurposing in wide range of indications. This process opens up new possibilities for treatment of patients, shortens the time for medicinal products to reach marketing approval and lowers healthcare systems expenses. Other instrument for expanding access to biological therapies is development and authorization of biosimilar drugs. The significant number of clinical trials including bevacizumab-biosimilars comes in support of this statement. Based on the results of such trials EMA and FDA have authorized several bevacizumab biosimilar therapies in recent years. In conclusion, bevacizumab is identified as a promising molecule, which is keeping its potential and represents a subject of interest for both repurposing and development of biosimilars.
Authors’ contributions statement
Conceptualization: IG, VG-K; Supervision EG, IG; Methodology: AI, VG-K; Visualization: AI, VB; Writing—original draft preparation AI, VG-K; Writing—review and editing VG-K, EG, VB, IG; Funding acquisition: IG, VG-K. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download PDF (556.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study is available within the article.
Additional information
Funding
References
- CRISP_ Special Edition August 2021.pdf. Available from: https://tinyurl.com/yc5c5v9e
- Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi: 10.1038/nrd1381.
- Drug Approval Package: Avastin (Bevacizum) NDA #125085. [Internet]. [cited 2024 Mar 25]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/STN-125085_Avastin.cfm
- Avastin EPAR Scientific Discussion - 08/08/2007. [Internet]. [cited 2022 May 3]. Available from: https://www.ema.europa.eu/en/documents/scientific-discussion-variation/avastin-h-c-582-ii-0008-epar-scientific-discussion-variation_en.pdf
- Avastin EPAR Scientific Discussion - 12/10/2007. [Internet]. [cited 2022 May 3]. Available from: https://www.ema.europa.eu/en/documents/scientific-discussion-variation/avastin-h-c-582-ii-0009-epar-scientific-discussion-variation_en.pdf
- Phase IV of Drug Development - PMC [Internet]. [cited 2023 Jul 18]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3148611/
- Sahoo BM, Ravi Kumar BVV, Sruti J, et al. Drug repurposing strategy (DRS): emerging approach to identify potential therapeutics for treatment of novel coronavirus infection. Front Mol Biosci. 2021;8:628144. doi: 10.3389/fmolb.2021.628144.
- Fatemi N, Karimpour M, Bahrami H, et al. Current trends and future prospects of drug repositioning in gastrointestinal oncology. Front Pharmacol. 2023;14:1329244. [cited 2024 Mar 25]. Available from: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2023.1329244
- El Bairi K, Trapani D, Petrillo A, et al. Repurposing anticancer drugs for the management of COVID-19. Eur J Cancer. 2020;141:40–61.
- Jose V, Radhakrishna S, Pipalava P, et al. Bevacizumab for eye diseases – legal, regulatory, and ethical overview. Indian J Pharmacol. 2019;51(6):377–383. doi: 10.4103/ijp.IJP_413_19.
- Bachu RD, Abou-Dahech M, Balaji S, et al. Oncology biosimilars: new developments and future directions. Cancer Rep (Hoboken). 2022;5(11):e1720. Epub 2022 Oct 4. PMID: 36195576; PMCID: PMC9675387. doi: 10.1002/cnr2.1720.
- Home | Beta ClinicalTrials.gov [Internet]. [cited 2023 Jul 18]. Available from: https://clinicaltrials.gov/
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108.
- Avastin: EPAR - Scientific Discussion. First published 24/01/2006 [Internet]. [cited 2024 Mar 25]. Available from: https://www.ema.europa.eu/en/documents/scientific-discussion/avastin-epar-scientific-discussion_en.pdf
- Yorston D. Anti-VEGF drugs in the prevention of blindness. Community Eye Health. 2014;27(87):44–46. PMID: 25918459; PMCID: PMC4322736.
- Würdinger T, Tannous BA. Glioma angiogenesis: towards novel RNA therapeutics. Cell Adh Migr. 2009;3(2):230–235. Epub 2009 Apr 22. PMID: 19262177; PMCID: PMC2679892. doi: 10.4161/cam.3.2.7910.
- Pushpakom S, Iorio F, Eyers P, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58. doi: 10.1038/nrd.2018.168.
- Hernandez JJ, Pryszlak M, Smith L, et al. Giving drugs a second chance: overcoming regulatory and financial hurdles in repurposing approved drugs as cancer therapeutics. Front Oncol. 2017;7:273. [cited 2023 Jan 8]. Available from: doi: 10.3389/fonc.2017.00273.
- Research and Markets. Drug repurposing service providers market, 2020–2030. Roots Analysis Private Ltd; 2020 Jul; ID: 5141824 [Internet]. [cited 2023 Jan 10]. Available from: https://www.researchandmarkets.com/reports/5141824/drug-repurposing-service-providers-market-2020
- Cha Y, Erez T, Reynolds IJ, et al. Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol. 2018;175(2):168–180. Jan
- Lee HS, Bae T, Lee J-H, et al. Rational drug repositioning guided by an integrated pharmacological network of protein, disease and drug. BMC Syst Biol. 2012;6(1):80. doi: 10.1186/1752-0509-6-80.
- Commissioner O of the FDA. Real-world evidence; FDA; 2023. [cited 2023 Jul 19]. Available from: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence
- Schad F, Thronicke A. Real-world evidence—current developments and perspectives. Int J Environ Res Public Health. 2022;19(16):10159. doi: 10.3390/ijerph191610159.
- Martin L, Hutchens M, Hawkins C, et al. How much do clinical trials cost? Nat Rev Drug Discov. 2017;16(6):381–382. doi: 10.1038/nrd.2017.70.
- Karanicolas PJ, Farrokhyar F, Bhandari M. Blinding: who, what, when, why, how? Can J Surg. 2010;53(5):345–348.
- Bigirumurame T, Uwimpuhwe G, Wason J. Sequential multiple assignment randomized trial studies should report all key components: a systematic review. J Clin Epidemiol. 2022;142:152–160. doi: 10.1016/j.jclinepi.2021.11.007.
- Kidwell KM, Postow MA, Panageas KS. Sequential, multiple assignment, randomized trial designs in immuno-oncology research. Clin Cancer Res. 2018;24(4):730–736. doi: 10.1158/1078-0432.CCR-17-1355.
- D'Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91(9):4082–4085. doi: 10.1073/pnas.91.9.4082.
- Repurposing Drugs. | National Center for Advancing Translational Sciences. [Internet]. [cited 2023 Jan 15]. Available from: https://ncats.nih.gov/preclinical/repurpose
- Hurwitz HI, Yi J, Ince W, et al. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14(1):22–28. doi: 10.1634/theoncologist.2008-0213.
- EMA (European Medicines Agency). Medicines. [cited 2023 Feb 6]. Available from: https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_field/Human/ema_group_types/ema_medicine/field_ema_med_status/authorised-36/ema_medicine_types/field_ema_med_biosimilar/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar
- Koyfman H. Biosimilarity and interchangeability in the biologics price competition and innovation act of 2009 and FDA’s 2012 draft guidance for industry. Biotechnol Law Rep. 2013;32(4):238–251. doi: 10.1089/blr.2013.9884.
- Schiestl M, Zabransky M, Sörgel F. Ten years of biosimilars in Europe: development and evolution of the regulatory pathways. Drug Des Devel Ther. 2017;11:1509–1515.
- avastin-epar-product-information_en.pdf. [Internet]. [cited 2023 Jul 19]. Available from: https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf