?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
There is a global call for action for cervical cancer elimination as a public health problem—WHO Strategy 90-70-90. One of the strategic pillars, alongside vaccination, is cervical cancer screening. This aims to assess the economic consequences and cost-effectiveness of primary DNA-HPV testing for cervical cancer screening in Bulgaria. Combined cost-effectiveness (CEA) and cost-benefit (CBA) analysis was performed to evaluate the net benefit of the HPV genotype testing program among women aged 30–54 in Bulgaria and to compare its cost-effectiveness with the current practice. ICER was presented separately for only direct costs and direct + indirect costs. The CBA analysis for the age group 30-54 shows that the benefit/cost ratio of DNA-HPV primary screening is 1.03 and the net present benefit accounts for 373,023 euro thus making the program beneficial for the healthcare system. CEA shows that HPV screening is cost-effective over the current practice of PAP testing with ICER 1634 euro/LYG when considering only direct costs and—8020 euro/LYG for direct and indirect costs. The ICER in terms of the sensitivity rate of the testing methods is 1316 euros. The LYG earned with the HPV testing account for 4989 for the observed women. Primary hrHPVDNA-based screening is an important scientific and clinical advance since it offers better reassurance of low cancer risk compared to cytology-only screening conducted at the same interval. Primary hrHPV screening can be considered as a dominant alternative to current cytology-based cervical cancer screening approaches including cytology alone and contesting and providing better benefit.
Introduction
Cervical cancer is the 4th most common form of cancer among women worldwide, with 604,127 newly diagnosed cases in 2020 and 341,831 deaths [Citation1]. If diagnosed at early stages and managed effectively, cervical cancer could be a preventable and nearly 100% curable disease. The incidence in most European Union (EU) regions is relatively low, except for Romania and Bulgaria. Rising levels of cervical cancer are also observed in Latvia and Lithuania. In all four above-mentioned countries, population-based screening programs are still not implemented and only testing during routine examinations (opportunistic screening) is available [Citation2, Citation3]. In 2018, the World Health Organization (WHO) called for worldwide efforts for the elimination of cervical cancer using prevention and early testing as highly cost-effective strategies. According to that call, all girls globally should be vaccinated against HPV, as well as that the screening of women over 30 is necessary to prevent the impact of cervical cancer and related diseases [Citation4]. WHO’s global strategy for cervical cancer elimination, endorsed by the World Health Assembly in 2020 aims to achieve a drop in the incidence rate yearly to lower than 4 per 100,000 women. Among the recommended strategies are 90% vaccination rates among girls by the age of 15, a 70% screening rate with high-performance tests by the age of 35 and by the age of 45 with a recommended testing interval of 5 years, and a 90% treatment and management rate of all women with pre-cancer and invasive cancer [Citation5].
Annual expenditures related to HPV-associated diseases increased from $42.6 in 2002 to $180.9 million in 2015 in the USA. Cervical cancer led to the highest cost in 2015 (75.1 million USD) followed by cervical dysplasia and cervical carcinoma in situ [Citation6]. In 2018 HPV-related cancers ranged from $52.700 to $146.100 (2018 US dollars) [Citation7, Citation8]. Over 3 years (2011–2013) the French National Health Insurance spent about €511 million on HPV-potentially related cancers. The highest expenditures are related to hospital care and disability allowance during the first year of diagnostics [Citation9]. The total annual cost of HPV-related cancers is substantial, estimated at €94 million in Sweden. The direct costs accounted for €31.3 million, while premature mortality is estimated at €36 million of indirect costs [Citation10].
Bulgaria is among the leading European countries in terms of incidence and mortality of cervical cancer. Data from the National Cancer Register show that in 2017 the prevalence of cervical cancer among the total population was 431.1/100,000 or 15,691 women. Among them, 908 (24.9/100,000) were newly diagnosed, with the highest number being at the age of 45–49 years (120) and 60–64 years (117). In 2018, the total number of registered malignancies of the cervix was 15,759 (435.8/100,000) with 850 (23.5/100,000) new cases [Citation11]. Data from published studies show an average decline in the incidence rate of 0.97/100,000 (p < 0.001) and an average number of newly diagnosed patients until 2020 of 982 [Citation12].
According to the Europe Beating Cancer Plan [Citation13] and the newly adopted National Anticancer Plan for Bulgaria [Citation14] screening for cervical cancer is among the top priorities together with ensuring advanced diagnostic capabilities for patients and equal access to diagnostic methods, centers, and leading specialists. The European Beating Cancer Plan recommends the primary testing to be performed with HPV genotype test, approved for HPV primary cervical cancer screening. Among the recommended strategies for cervical diseases in the Bulgarian Anticancer plan are recommended HPV genotype testing, Papanicolaou smears (PAP), and colposcopy as follow-up confirmation of cervical abnormalities. Those discrepancies provoke our interest in comparing both testing approaches within the national context in Bulgaria.
The present study aims to assess the economic consequences and cost-effectiveness of DNA-HPV genotype testing, as a primary test for cervical cancer screening in Bulgaria.
Materials and methods
The study applies two pharmacoeconomic methods cost-effectiveness and cost-benefit. The cost-effectiveness method compares two strategies for testing: HPV genotype testing vs current PAP testing.
The cost-benefit (CBA) analysis evaluates the economic consequences of HPV genotype testing among women aged 30–54 in Bulgaria for 5 years (2024–2028).
We assumed that women who visited the gynecologists for regular examination would be tested with either HPV genotype test or PAP test.
Testing approaches for cervical cancer screening
The compared alternative approaches for testing are, as follows:
The HPV testing approach as primary screening will be offered to women aged 30–54 years who attend for routine screening: HPV genotype testing at least once in 5 years is performed with subsequent colposcopy and dual-stain cytology test if positively evaluated.
The HPV genotype testing approach is based on the recommendations published by Wentzensen et al. [Citation15] ().
PAP cytology testing, which is the currently applied strategy, includes women aged 30–54 who attend the routine prophylactic examination and are offered PAP testing with subsequent colposcopy if needed once every year.
Table 1. Screening algorithm for HPV adapted from Wentzensen et al. [Citation15].
According to the updated World Health Organization (WHO) cervical cancer prevention guideline, HPV-DNA detection is recommended to be performed as the primary testing method for the general population of women starting at the age of 30 years with regular testing every 5–10 years [Citation16]. We chose the age group 30–54 as the cohort at the highest risk in Bulgaria [Citation17]. This choice is supported by the local epidemiological data for this age group as it is associated with a nearly 30% risk of HPV infection compared to other age groups and is associated with the highest risk of progression to cervical cancer [Citation18].
Costs included in the calculations
For both methods were calculated direct medical costs and indirect costs related to premature mortality.
The direct medical costs for the first approach: HPV screening, included costs for primary DNA-HPV genotype testing, colposcopy for HPV16/18, dual stained cytology test (p16/Ki67) for triage-risk stratification of identified other 12 hrHPV genotypes, and physician visits which are calculated in proportions described in .
The direct costs for the second approach of current practice included costs for PAP cytology testing, colposcopy, and physician visits.
For both approaches, the micro-costing method was applied to calculate the total costs using the following equations:
and
The indirect costs for both approaches included life year lost (LYL) due to cervical cancer-related mortality and were calculated using the human capital approach and the following formula:
The future costs were calculated in euro (fixed rate 1 euro = 1.95583 BGN) and discounted with a discounting coefficient of 3.5% [Citation19].
For CEA and CBA, the total average annual cost for each approach was derived by summarizing the yearly cost from 2024 to 2028 and dividing it by the number of years.
The input cost data for the pharmacoeconomic methods are summarized in .
Table 2. Cost data for the pharmacoeconomic methods.
Result measures
The long-term results were measured as life years gained (LYGs) based on the difference in cancer detection rate of the two testing procedures. Average LYGs were calculated for the two examined approaches based on epidemiology data for Bulgaria which with the current practice of PAP testing, shows 503 mortality cases from cervical cancer yearly and at the average age of 59 years [Citation18, Citation21]. The number of additional LYG by using the HPV genotype test was calculated based on an expected decrease in mortality from cervical cancer with 34.2%, which will lead to a 90% increase in 10-year survival [Citation22].
For the CBA, the LYG was transformed into monetary units using the human capital approach (LYG × GDP per capita) for the additional years of life provided with HPV genotype testing.
The average life expectancy for women in the Bulgarian population is 74.5 years and the GDP based on the latest published statistical data is 10.330 euro [Citation23].
The increased sensitivity of the DNA-HPV test compared with PAP cervical cytology favors DNA-HPV testing as a primary screening test [Citation24].
For the CEA we performed additional analysis based on the surrogate endpoint related to the sensitivity of each testing approach in terms of detecting HPV infection—97% on average for HPV genotype testing vs 43.1% for PAP testing according to published literature [Citation25, Citation26].
Cost-effectiveness and net benefits analyses
For cost-effectiveness analysis, the incremental cost-effectiveness ratio (ICER) was calculated. ICER was presented separately for only direct costs and direct + indirect costs using the following formula:
For the cost-benefit analysis calculated net present value and benefit/cost ratio for HPV screening alternative using the following formulas:
Sensitivity analysis
The robustness of the results of both methods was examined through deterministic sensitivity analysis. Data that were considered uncertain were the number of screened individuals, cost of HPV genotype test, dual-stained cytology test p16/Ki67 for risk stratification of hrHPV genotypes, PAP cytology testing, colposcopy, and physician visits. They were varied within ±30% interval and a Tornado diagram was built to evaluate how ICER and net benefit would change.
Results
Distribution of patients and results per approaches
Approach 1 and Approach 2 are equal in terms of several screened populations because both approaches include the same birth cohort and women who go through routine prophylaxis and are eligible for testing. The difference between the approaches is in the difference of testing methods: only PAP cytology testing is performed for the current practice, compared to HPV genotype testing as a primary test, followed by dual-stained cytology test p16/Ki67 in the first approach, which further influence the number of patients who would be eligible for the triage test and immediate risk stratification of identified hrHPV genotypes.
The increased sensitivity of the DNA-HPV test compared with PAP cervical cytology favors DNA-HPV testing as a primary screening test [Citation23].
If HPV genotype testing is performed, most of the patients at risk will be discovered in the earlier stages of cervical abnormalities resulting in the detection of cancer at an early stage, decreased mortality, and increased 10-year survival rate [Citation27]. Expected live years gained (LYG) when HPV testing is applied accounted for 4989. It is also expected for HPV genotype testing to decrease the false-negative results of the current PAP testing by 43.1% ().
Table 3. Distribution of patients and results per approach.
Cost of the screening approaches
The main cost driver in the cost analysis was the HPV genotype testing (). Nevertheless, the higher total cost of HVP testing compared to current PAP testing is associated with a reduced risk of false negative results and underdiagnosis.
Table 4. Cost of the testing approaches (euro).
Cost-effectiveness analysis
CEA shows that HPV testing is cost-effective over the current practice of PAP testing with ICER 1634 euro/LYG when considering only direct costs and dominant with −8020 euro/LYG for direct and indirect costs. The suggested willingness to pay (WTP) threshold for Bulgaria based on the WHO approach is 1×–3× GDP/capita (10,330–30,990 euro/capita). The life years that would be saved due to reduced YLL earned through the HPV testing account for 4988 for the observed cohort of women ().
Table 5. Cost-effectiveness analysis of HPV genotype test approach.
The incremental-cost effectiveness ratio in terms of sensitivity rate of the testing method of the two testing approaches measured as several women tested positive for HPV is 1.316 euros per woman tested positive for HPV ().
Table 6. Cost-effectiveness analysis of HPV genotype test screening approach in terms of surrogate endpoint related to sensitivity of testing.
Cost benefit-analysis
The CBA analysis shows that the benefit/cost ratio of HPV testing is 3.16 and the net present benefit accounts for 35,205,139 from the payer perspective and 1.18 and 7,948,245 euro when including indirect costs also, thus making the approach beneficial for the healthcare system in Bulgaria ().
Table 7. Cost-benefit analysis.
Sensitivity analysis
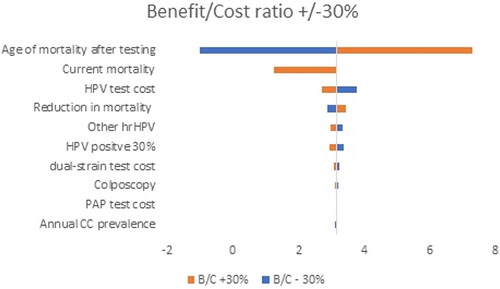
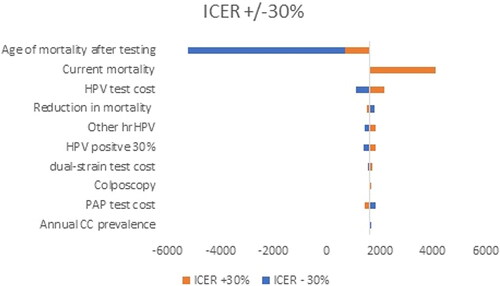
To test the uncertainty in our analysis, we performed deterministic sensitivity analysis and varied uncertain parameters within the +/−30% range. The results are presented via a Tornado diagram for both analyses separately. The variables tested in the analysis are annual cervical cancer (CC) prevalence, reduction in mortality, current mortality, age of mortality after screening, colposcopy, HPV positive 30%, CINTec+, other hrHPV, PAP test cost, dual-stain test cost, HPV test cost.
The results reveal that the age of mortality screening, and levels of current and HPV test costs are among the parameters that affected the most significantly the benefit/cost ratio () and ICER ().
Discussion
In this study, we evaluated the economic consequences of changing the current practice for HPV testing in Bulgaria from PAP cytology to HPV genotype testing for women with regular prophylactic exams. The input data in the model is the difference in the cancer detection rate of the two screening procedures. Our results show that HPV genotype testing is both cost-effective and cost-beneficial for the Bulgarian healthcare system. Thus, we confirm that the WHO recommendations for introducing HPV testing should be the primary approach for HPV diagnosis in Bulgaria. Based on these results, we can consider that the Bulgarian cancer plan should include such a recommendation.
The EU set its first cancer screening guidelines in 1993, which were further updated in 2008 and 2015 [Citation31, Citation32]. Currently, the EU recommends population-based screening programs with HPV genotype testing starting at the age of 30 at 5-year intervals [Citation33]. However, these guidelines are not obligatory, and each EU country has its specific policy in terms of screening and prophylaxis of oncology diseases [Citation34]. A systematic review of the HPV screening practices in European and non-European countries shows that differences between the screening guidelines and practices within EU countries still exist. Overall, 6 out of 11 countries have national screening registries (USA, France, Germany, Sweden, UK and Australia). All European countries have implemented HPV genotype testing every 5 years above the age of 30 [Citation12]. To close the gaps between the EU countries, the European Beating Cancer Plan was established with the main focus on the detection of cancers at an early stage, increase in the number and adherence to screenings, covering more target groups, and respectively more cancers are identified at an early stage [Citation35].
The Netherlands was one of the first countries to implement nationwide HPV-genotype testing in 2017, with the cytology-based screening starting at the age of 30. The HPV-positive women are triaged with cytology and repeat cytology after 6 months, while the women with positive cytology are referred to colposcopy. For women with a negative previous HPV test, the interval for the next examination is 5 years. The new HPV-genotype program is expected to result in increasing costs during the first five years, but lowering is expected as compared to cytology-based testing five years after implementation of the new program [Citation14, Citation36].
Cost-effectiveness calculations for low-income countries show that co-testing via HPV-genotype testing and cytology is an economically dominant screening strategy compared with primary HPV testing calculated for 3- and 5-year periods. It resulted in a slight decrease in referral colposcopies per woman compared with primary DNA-HPV testing, a greater number of QALYs per woman, and a lower rate of false positive colposcopies [Citation37]. The switch from cytology to primary DNA-HPV testing in the cervical cancer screening program of Brazil is less costly and cost-effective than the currently used cytology program [Citation38].
In the current practice of PAP testing in Bulgaria, almost 40% of the women remain underdiagnosed [Citation17]. In countries, where PAP cervical screening is not well standardized, the testing process could be interfered with by many factors, such as low cytology sensitivity, requiring frequent repetition to achieve longitudinal sensitivity, low coverage of the target population at regular intervals, and poor-quality control of cytology services [Citation39, Citation40]. These factors could contribute to implementing alternative strategies for testing, such as HPV molecular testing [Citation41].
Progression to cervical cancer could be impacted by different biological, behavioral, healthcare, and socio-economic factors. To achieve effective prevention, management and improved access to screening and treatment strategies, we must consider and understand them [Citation42].
Countries should consider implementing evidence-based policies in their national health strategies for primary prophylaxis, screening programs, and improved access to innovative testing and treatment protocols. These steps could improve vaccination coverage and decision-making in terms of healthcare and financial resource allocation [Citation43].
HPV is known to cause at least 99% of cervical cancer worldwide. Moreover, it is proven that the duration of HPV persistence after primary conization could be considered as a risk factor for CIN2+ recurrence thus increasing the risk of developing HPV-related diseases such as cervical dysplasia and cervical cancer [Citation44].
In this respect, early detection is important for women at a higher risk of developing cervical cancer, as HPV infections are more likely to lead to cervical cancer if left untreated over time [Citation45]. The use of a dual-stained cytology test for triage of positive HPV test enables efficient patient management by sending only women to colposcopy who can benefit most from it. Dual-stain cytology test p16/Ki67 advances cytology and detects cells undergoing oncogenic transformation. On the other hand, triage stratification helps to find other hrHPV genotypes immediately for optimal HPV risk stratification: women who will benefit from immediate intervention when transforming HPV infections are present. This will help to minimize the risk of losing women to follow-up.
Several randomized clinical trials and prospective studies have shown the superiority of DNA-HPV testing in detecting early cervical cancer when compared to cervical cytology [Citation25, Citation46–49].
WHO guideline from 2021 for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention shows that DNA-HPV primary screening by HPV alone detects more CIN2 + at screening than PAP cytology [Citation16]. The 6.5-year risk of cervical cancer was 40% lower in the HPV-based screening group. Cervical cancer mortality was 41% lower in the HPV-based testing group than in the cytological screening group after a cumulative follow-up of 8 years.
A meta-analysis study of four European clinical trials analyzing the screening with the DNA-HPV test showed that women aged 30 years and older would benefit from testing in five-year intervals [Citation41, Citation50].
The ATHENA study evaluated the DNA-HPV as a primary screening option for cervical cancer, and the findings support the use of a combination of HPV-16/18 genotyping and reflex cytology only for the group of other hr-HPV positive, non-HPV-16/18 women redirected for colposcopy triage [Citation8].
The DNA-HPV test has a negative predictive value of approximately 100% and allows a screening interval of at least five years, longer than the 3-year interval for cytology. These would reduce the total number of tests performed, affecting costs.
The study reveals that primary HPV genotype testing using the mentioned DNA-HPV assay, followed by colposcopy evaluation of women with HPV16/18 positive results was applied, whereas the women positive for other 12 hrHPV genotypes are referred to cytology or colposcopy (if necessary). The women with negative results in primary screening are tested every 3 years [Citation46].
Our results are similar to the model showing that screening by use of HPV genotyping test as a primary screening test combined with dual stain cytology as the triage option of HPV-positive women is cost-effective compared with conventional PAP cytology. The covered population is 30–65 years old women. The incremental cost-effective ratio (ICER) per QALY gained $1395 [Citation51].
To our knowledge, our study is the first in Bulgaria to evaluate the economic consequences of HPV genotype testing based on the WHO Strategy and European Anticancer Plan and the first to use the methodology of cost-benefit analysis at the national level. Most of the published studies analyze the cost-effectiveness of the screening program in terms of life years gained. The cost-effectiveness analysis proves that the HVP genotype testing program for 30–54-year-old women in Bulgaria is cost-effective with an estimated ICER of 1634 euros. When considering the societal perspective, the strategy is even dominant with ICER accounting for −8020 euro/LYG. The cost-benefit analysis shows that from the payer perspective, HPV screening has a benefit-cost ratio of 3.16 and 1.18 from the point of view of society, thus making the approach beneficial. The strategy with the HPV genotype test is also expected to reduce the mortality from cervical cancer and reduce productivity losses by almost 3.6 times.
Limitations
Our study has some limitations. First, it covers a population between the ages of 30 and 54; however, different simulations show that this is the cohort group with the highest benefit from the screening [Citation52, Citation53]. The calculations of C/E and C/B outputs did not incorporate primary-order and secondary-order sources of uncertainty. Some of the limitations, however, were tested within the Sensitivity analysis, which showed that age of testing, levels of current and HPV test cost are among the parameters that affected most significantly the benefit/cost ratio and the incremental cost-effectiveness ratio.
Conclusions
Primary hrHPV DNA genotype testing, followed by cytology triage of hrHPV genotypes provides important scientific and clinical advances in cervical cancer testing since it offers better reassurance of low cancer risk compared to cytology-only testing conducted at the same interval. Primary HPV testing can be considered as a dominant alternative to current cytology-based cervical cancer approaches including cytology alone and providing better benefit for the healthcare system and the society in Bulgaria.
Author contributions
MD and ZM performed the research. MD and ZM drafted the manuscript and prepared calculations. MP analyzed and interpreted the final data. MP and GP critically reviewed the paper and prepared the final manuscript. All authors have provided valuable contributions to the manuscript, and read, and approved the final manuscript.
Acknowledgments
The results presented in the manuscript have been reported as a poster abstract at ISPOR Europe 2022, 6–9 November; Available from: https://www.ispor.org/conferences-education/conferences/past-conferences/ispor-europe-2022/program/program/session/euro2022-3564/121563
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The raw data supporting the conclusions of this article is available upon request from the corresponding author.
Additional information
Funding
References
- GLOBOCAN. 2020. Available from: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf.
- Poljak M, Rogovskaya SI, Kesić V, et al. Trends in cervical cancer incidence and mortality in the Baltic countries, Bulgaria and Romania. Int J Cancer. 2011;31: h80–h82. doi: 10.1016/j.vaccine.2013.04.038.
- Arbyn M, Antoine J, Mägi M, et al. Trends in cervical cancer incidence and mortality in the Baltic countries, Bulgaria and Romania. Int J Cancer. 2011;128:1899–1907.
- Cervical Cancer: an NCD we can overcome. Geneva: Intercontinental Hotel; 2018 [cited 2023 Mar 1]. Available from: https://www.who.int/reproductivehealth/DG_Call-to-Action.pdf.
- WHO. Cervical cancer elimination initiative. Available from: https://www.who.int/initiatives/cervical-cancer-elimination-initiative.
- Ki M, Choi HY, Han M, et al. The economic burden of human papillomavirus infection-associated diseases in the Republic of Korea, 2002-2015. Vaccine. 2018;36(31):4633–4640. doi: 10.1016/j.vaccine.2018.06.046.
- Gan BK, Macalino GE, Nsouli-Maktabi H, et al. Human papilloma-virus seroprevalence among men entering military service and seroincidence after ten years of service. MSMR. 2013;20(2):21–24.
- Hesson HW, Ekwueme DU, Saraiya M, et al. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30(42):6016–6019. doi: 10.1016/j.vaccine.2012.07.056.
- Abramowitz L, Lacau Saint Guily J, Moyal-Barracco M, et al. Epidemiological and economic burden of potentially HPV-related cancers in France. PLoS One. 2018;13(9):e0202564. doi: 10.1371/journal.pone.0202564.
- Östensson E, Silfverschiöld M, Greiff L, et al. The economic burden of human papillomavirus-related precancers and cancers in Sweden. PLoS One. 2017;12(6):e0179520. doi: 10.1371/journal.pone.0179520.
- Karcheva M, Iordanov A, Tzvetkova S. Current burden of cervical cancer in Bulgaria - epidemiological study. Eur J Public Health. 2020;30(Supplement_5):ckaa166.1091. doi: 10.1093/eurpub/ckaa166.1091.
- Yordanov A, Vasileva-Slaveva M, Galai N, et al. Cancer of the cervix in Bulgaria: epidemiology of a crisis. Healthcare (Basel). 2023;11(3):318. doi: 10.3390/healthcare11030318.
- European Commission. Europe beating cancer plan; [cited 2023 Mar]. Available from: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/promoting-our-european-way-life/european-health-union/cancer-plan-europe_en.
- National Anticancer Plan. 2030; [cited 2023 Mar]. Available from: https://www.mh.government.bg/media/filer_public/2022/07/08/bg_national_cancer_plan_2030_-_site.pdf.
- Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol. 2016;76 Suppl 1(Suppl 1):S49–S55. doi: 10.1016/j.jcv.2015.11.015.
- WHO recommends DNA testing as a first-choice screening method for cervical cancer prevention; [cited 2023 Mar]. Available from: https://www.who.int/europe/news/item/11-09-2021-who-recommends-dna-testing-as-a-first-choice-screening-method-for-cervical-cancer-prevention#:∼:text=For%20the%20general%20population%20of%20women%2C%20HPV%2DDNA%20detection%20is,screening%20every%203%E2%80%935%20years.
- National Cancer Register. 2017; [cited May 2023]. Available from: https://www.sbaloncology.bg/index.php/bg.html.
- Kovachev S, Slavov V. Prevalence of human papillomavirus infection in women in Bulgaria: a 2017 update. J Med Virol. 2018;90(6):1142–1149. doi: 10.1002/jmv.25050.
- Ordinance on terms, rules and procedure for regulation and registration of prices for medicinal products. Adopted in 19 April 2013; last amended and supplemented SG N. 28 of 6 April 2021. https://ncpr.bg/en/regulations/bulgarian-legislation/regulations.html.
- National Health Insurance Fund: National Frame Contract 2020–2022; [cited 2023 Feb]. Available from: https://nhif.bg/bg/nrd/2020-2022/medical.
- Human Papillomavirus and Related Diseases Report Bulgaria 2023; [cited 2023 Mar]. Available from: https://hpvcentre.net/statistics/reports/BGR.pdf?t=1679479185980.
- Canfell K, Kim JJ, Brisson M, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):591–603. doi: 10.1016/S0140-6736(20)30157-4.
- National Statistics Institute; [cited 2019 Feb]. Available from: www.nsi.bg.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197. doi: 10.1016/j.ygyno.2014.11.076.
- Whitlock EP, Vesco KK, Eder M, et al. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive services task force. Ann Intern Med. 2011;155(10):687–697. doi: 10.7326/0003-4819-155-10-201111150-00376.
- Wright TC, Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144(1):51–56. doi: 10.1016/j.ygyno.2016.10.031.
- Basoya S, Anjankar A. Cervical cancer: early detection and prevention in reproductive age group. Cureus. 2022;14(11):e31312. doi: 10.7759/cureus.31312.
- Doorbar J, Egawa N, Griffin H, et al. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25(Suppl 1):2–23. doi: 10.1002/rmv.1822.
- Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6.
- Liu G, Markowitz LE, Hariri S, et al. Seroprevalence of 9 human papillomavirus types in the United States, 2005-2006. J Infect Dis. 2016;213(2):191–198. doi: 10.1093/infdis/jiv403.
- Coleman D, Day N, Douglas G, et al. European guidelines for quality assurance in cervical cancer screening. Europe against cancer programme. Eur J Cancer. 1993;29A(Suppl 4):S1–S38.
- Jordan J, Arbyn M, Martin-Hirsch P, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology. 2008;19(6):342–354. doi: 10.1111/j.1365-2303.2008.00623.x.
- von Karsa L, Arbyn M, De Vuyst H, et al. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res. 2015;1:22–31. doi: 10.1016/j.pvr.2015.06.006.
- Wang W, Arcà E, Sinha A, et al. Cervical cancer screening guidelines and screening practices in 11 countries: a systematic literature review. Prev Med Rep. 2022;28:101813. doi: 10.1016/j.pmedr.2022.101813.
- European Commission: A cancer plan for Europe; [cited 2023 May]. Available from: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/promoting-our-european-way-life/european-health-union/cancer-plan-europe_en
- Polman NJ, Snijders PJF, Kenter GG, et al. HPV-based cervical screening: rationale, expectations and future perspectives of the new Dutch screening programme. Prev Med. 2019;119:108–117. Feb doi: 10.1016/j.ypmed.2018.12.021.
- Jeffrey DM, Vilalta A, Troeger KA. Revisiting the cost-effectiveness of HPV co-testing versus primary HPV testing for cervical cancer screening. J Women’s Health Dev. 2021;4:151–162.
- Vale DB, Silva MT, Discacciati MG, et al. Is the HPV-test more cost-effective than cytology in cervical cancer screening? An economic analysis from a middle-income country. PLoS One. 2021;16(5):e0251688. doi: 10.1371/journal.pone.0251688.
- Denny L. Cytological screening for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2012;26(2):189–196. doi: 10.1016/j.bpobgyn.2011.08.001.
- Wright TC, Jr, Kuhn L. Alternative approaches to cervical cancer screening for developing countries. Best Pract Res Clin Obstet Gynaecol. 2012;26(2):197–208. doi: 10.1016/j.bpobgyn.2011.11.004.
- Teixeira JC, Vale DB, Bragança JF, et al. Cervical cancer screening program based on primary DNA-HPV testing in a Brazilian city: a cost-effectiveness study protocol. BMC Public Health. 2020;20(1):576. doi: 10.1186/s12889-020-08688-4.
- Asare M, Elizondo A, Dwumfour-Poku M, et al. Intervention to increase cervical cancer screening behavior among medically underserved women: effectiveness of 3R communication model. Healthcare (Basel). 2023;11(9):1323. doi: 10.3390/healthcare11091323.
- Wilailak S, Kengsakul M, Kehoe S. Worldwide initiatives to eliminate cervical cancer. Int J Gynaecol Obstet. 2021;155(Suppl 1):102–106. doi: 10.1002/ijgo.13879.
- Bogani G, Sopracordevole F, Ciavattini A, et al. Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV); the investigators of the Italian HPV study group (iHPV study group). Duration of human papillomavirus persistence and its relationship with recurrent cervical dysplasia. Eur J Cancer Prev. 2023;32(6):525–532. doi: 10.1097/CEJ.0000000000000822.
- Onuki M, Matsumoto K, Tenjimbayashi Y, et al. Human papillomavirus genotype and prognosis of cervical cancer: favorable survival of patients with HPV16-positive tumors. Papillomavirus Res. 2018;6:41–45. doi: 10.1016/j.pvr.2018.10.005.
- Schiffman M, Herrero R, Hildesheim A, et al. HPV DNA testing in cervical cancer screening: results from women in a high-risk province of Costa Rica. JAMA. 2000;283(1):87–93. doi: 10.1001/jama.283.1.87.
- Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst. 2003;95(1):46–52. doi: 10.1093/jnci/95.1.46.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol. 2010;11:249–257.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370(9601):1764–1772. doi: 10.1016/S0140-6736(07)61450-0.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials: international HPV screening working group. Lancet. 2014;383(9916):524–532.
- Termrungruanglert W, Khemapech N, Tantitamit T, et al. Cost effectiveness analysis of HPV primary screening and dual stain cytology triage compared with cervical cytology. J Gyneco lOncol. 2019;30(2):e17. doi: 10.3802/jgo.2019.30.e17.
- Burger EA, Kim JJ, Sy S, et al. Age of acquiring causal human papillomavirus (HPV) infections: leveraging simulation models to explore the natural history of HPV-induced cervical cancer. Clin Infect Dis. 2017;65(6):893–899. doi: 10.1093/cid/cix475.
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol. 2019;154:118–123. doi: 10.1016/j.ygyno.2019.04.680.