Abstract
Anise hyssop (Agastache foeniculum (Pursh) Kuntze) is a perennial plant belonging to the Lamiaceae family mainly used in folk medicine to treat various ailments. This study determined the chemical composition of the essential oil and evaluated its antimicrobial and antioxidant activity. The essential oil was obtained from plants cultivated in the experimental field at the Institute of Roses, Essential and Medical Plants, Kazanlak, Bulgaria. The plants were processed by steam distillation, with the essential oil yielding 0.37%, and its main components being methyl chavicol (82.03%) and limonene (9.90%). The most potent antimicrobial action was observed against the gram-positive bacteria Staphylococcus aureus (25.7 mm inhibition zone) and Bacillus cereus (12.3 mm), the yeast Saccharomyces cerevisiae (16.3 mm) and Candida albicans (16.5 mm). The remaining gram-positive bacteria (Staphylococcus epidermidis and Bacillus subtilis), gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, and Salmonella ebony), and fungi (Aspergillus brasiliensis and Fusarium moniliforme) were resistant to the action of the essential oil. The antioxidant activity of the essential oil was ABTS (32.36 µmol TE/mL), DPPH (21.61 µmol TE/mL), CUPRAC (19.94 µmol TE/mL), and FRAP (29.56 µmol TE/mL) in vitro. Overall, the results from this study revealed the biological potential of anise hyssop as a source in pharmaceutical, food, and cosmetic applications.
Introduction
Anise hyssop (Agastache foeniculum) is a perennial herb belonging to the Lamiaceae family, with origins in North America and East Asia [Citation1–3]. This versatile plant has long been utilized in folk medicine for treating various ailments. One of its notable features is its essential oil, which has garnered considerable attention for its medicinal and aromatic properties [Citation4].
Extensive research has been conducted on the chemical composition of anise hyssop essential oil. Studies have consistently identified methyl chavicol as the primary constituent, comprising 88–95% of the oil, contributing to its distinct anise-like smell [Citation5–11]. However, variations in aromatic compounds, such as menthone or pulegone, can alter the scent profile, sometimes imbuing a minty aroma [Citation1,Citation5,Citation12].
While anise hyssop is cultivated primarily as an ornamental plant, its cultivation for essential oil production is also significant [Citation3,Citation6]. The essential oil has demonstrated antibacterial [Citation6,Citation9], antioxidant [Citation6,Citation9–11,Citation13,Citation14], and other biological properties [Citation6,Citation10,Citation12,Citation15], adding to its appeal in various industries.
In Bulgaria, where the plant is commonly used in traditional medicine, research has focused on the composition of its essential oil. Studies have revealed variations in oil composition based on factors, such as plant source and cultivation conditions. For instance, according to Zhekova et al. [Citation5] oils obtained from plants grown in experimental fields in Bulgaria exhibited differing ratios of aromatic compounds, resulting in distinct aromas, ranging from typical anise to sweet menthol.
Anethole, sweet menthol, and menthol were the three types of oil reported by their study [Citation5]. The anethole oil which was composed of around 90% methyl chavicol, had typical anethole aroma with some additional spicy notes. As previously described [Citation6], the other two types of oils were with similar composition, with emerging menthofuran, pulegone, and increasing limonene. The component’s ratio was the reason for the main difference between sweet and typical menthol. The sharper and fresh smell with green, grassy notes was due to the menthol, whereas the sweet menthol created warmer and flavored smell with additional notes [Citation6].
Ivanov et al. [Citation7] investigated the composition of oil obtained from plants purchased from a local drugstore. According to the authors, the main component of the oil is methyl chaviclol (93.45%).
Recent years have witnessed a surge in the demand for novel essential oils and medicinal plants in Bulgaria. Consequently, research efforts have intensified, particularly at the Institute of Roses, Essential and Medical Plants in Kazanlak (Bulgaria).
In this context, the aim of the present study was to determine the chemical composition of anise hyssop essential oil cultivated in Bulgaria, as well as its antimicrobial and antioxidant properties, with potential applications in food and cosmetic formulations.
Materials and methods
Plant material
Anise hyssop (A. foeniculum (Pursh) Kuntze) was used as seed material of German origin, without mentioning the variety of the seed package. It was grown in a cultivation facility under controlled conditions in containers. Planting was done at the beginning of July 2022 under conditions of drip irrigation in the experimental field of the Institute of Roses, Essential and Medical Plants, Kazanlak, Bulgaria (42.61°94′418″ N 25.39°29′58″ E, altitude 407 m) (). The sample material will be planted at the experimental field of the Rose Institute in the city of Kazanlak during the next year. Air-dried material in the amount of 100 g was also preserved.
Figure 1. Anise hyssop cultivated in the Institute of Roses, Essential and Medical Plants, Agricultural Academy, Kazanlak, Bulgaria (authors’ images).

The soil characteristics of the Institute’s experimental field play a crucial role in comparing the chemical composition of essential oils. Therefore, understanding the soil-climatic conditions is vital during the plants’ growth stages. The soils in the area are leached cinnamon-forest, develop on old diluvial deposits, structureless with good aeration and water permeability, with acidity pH 5.6 and poorly stocked with nitrogen 20.5 mg/1000 g, phosphorus 4.25 mg/100 g, but well stocked with potassium 21.75 mg/100 g, humus content 1.8% [Citation8].
The following planting scheme was implemented in this study: inter-row distance was 86 cm, and intra-row distance was between 23 cm and 5 plants/m2.
Essential oils extraction
The raw material was collected during the mass flowering phase (end of August 2022).
The moisture content of the raw materials (aerial mass that includes leaves and flowers) was determined by drying to constant weight [Citation16], and the results from the essential oil yield were given further on a dry weight (DW) basis.
The essential oil was extracted through steam distillation using a 5-L capacity laboratory copper distillation apparatus (). The process lasted for 2 h, mimicking production conditions where steam distillation is commonly employed. Each of the three apparatuses contained 500 g of raw material, previously cut into 2–5 cm pieces. Steam, with a temperature of 100 °C, was supplied into the distillation apparatus from a separate generator. Only the primary essential oil was collected in a receiver during distillation, while the resulting distillation waters were not further processed for secondary oil extraction.
The obtained oil was then dried using anhydrous sulfate and stored in tightly sealed dark vials at 4 °C until further analysis. The samples were transported in secure and protective packaging with temperature-controlled shipping options (0–4 °C).
Chemical composition of essential oils
The chemical composition of the essential oil was determined by gas chromatography (GC) analysis using an Agilent 7890A gas chromatograph (Santa Clara, CA, USA), with a DB-5 ms column (30 m × 0.32 mm × 0.25 µm). The temperature program used for the analysis was: from 65 °C, 1 °C/min to 230 °C, temperature of injector and detector 250 °C; temperature of flame ionization detector (FID): 250 °C; helium as a carrier at a constant rate of 0.8 mL/min; split 100:1. The GC/MS analysis was performed on a mass spectrometer (Agilent 5975C) with a carrier gas helium. The column and the temperature program were the same as those described for the GC analysis. The identification of the chemical compounds was made by comparing their retention time and library data [Citation17].
Antimicrobial activity of essential oils
The antimicrobial activity of the essential oil was tested against the following test microorganisms: gram-positive bacteria Staphylococcus aureus ATCC 6538, Staphylococcus epidermidis ATCC 12228, Bacillus subtilis ATCC 6633, and Bacillus cereus ATCC 10876; Gram-negative bacteria: Escherichia coli ATCC 8739, Pseudomonas aeruginosa АТСС 9027, and Salmonella abony NCTC 6017; the yeasts: Candida albicans ATCC 10231 and Saccharomyces cerevisiae ATCC 9763 and the fungi Aspergillus brasiliensis ATCC 16404 and Fusarium moniliforme (clinical isolate). All test microorganisms strains were supplied by the National Bank for Industrial Microorganisms and Cell Cultures, Sofia, Bulgaria.
The antimicrobial activity was determined by the agar well diffusion method. The growth media were Tryptic soy agar (Merck) for the tested bacteria and Sabouraud-Dextrose-Agar (Merck KGaA, Darmstadt, Germany) for yeast and fungi. The media were inoculated with 24 h suspension of the bacterial species and 48 h of the yeast and fungi with turbidity—0.5 McFarland standard. Melted media that had cooled to 50 ± 2 °C were inoculated with 1% of the prepared suspensions of the test microorganisms. Twenty milliliters of inoculated media were poured into sterile petri dishes (∅ = 90 mm). The agar plates were allowed to solidify. A cork borer was used to punch holes (∅ = 8 mm) in the agar. A solution of essential oil in dimethyl sulfoxide (1:10, v/v) was prepared and 50 μL were added dropwise to each well. Then, the petri dishes were placed in thermostatic chambers and incubated at 37 or 28 °C for 24 and 48 h according to the microbial species. After cultivation, the zone of growth inhibition around the wells was measured using a digital caliper. The diameter of zones, including the diameter of the well, was recorded in mm. The tests were performed in parallel with solvent controls (50 μL) [Citation18].
Antioxidant activity of essential oils
Chemicals and reagents
Chemicals and reagents were purchased from Sigma-Aldrich.
ABTS assay
The Trolox Equivalent Antioxidant Capacity (TEAC) was determined by using the colorimetric method reported by Re et al. [Citation19] with slight modifications [Citation20]. For this assay, cation radical (ABTS•+) solution was prepared by dissolving 7 mmol/L of ABTS in 2.45 mmol/L K2S2O8. This mixture was shaken for 12–16 h at ambient temperature (25 ± 1 °C) in the dark until obtaining a stable oxidative state. For the study of the essential oils, the ABTS•+ stock solution was diluted with methanol until absorbance became 0.70 ± 0.02 at 734 nm. Sample analysis was performed as follows: 3 mL of ABTS solution and 30 μL of sample or standard were mixed. The absorbance of samples was measured at 734 nm with a spectrophotometer (Camspec M508, UK) after the samples were incubated at 25 ± 1 °C for 7 min. The calibration curve was plotted by using Trolox as a standard. The results were expressed as µmol equivalents per milliliter of essential oil (µmol TE/mL).
DPPH assay
Antioxidant activity was measured according to the procedure of Brand-Williams et al. [Citation21]. In the test tubes, 150 µL of essential oil or Trolox and 2.85 mL of 0.12 mmol/L DPPH reagent, prepared with 4.8 mg of DPPH dissolved in 100 mL of methanol), were mixed. The mixtures were shaken and then incubated for 30 min at room temperature (25 ± 1 °C). The absorbance was recorded at 517 nm with a spectrophotometer (Camspec M508, UK). To quantify the antioxidant activity, a standard Trolox curve was used with concentrations from 0.045 to 1.5 mmol Trolox. The results were expressed as µmol TE/mL. IC50 (mg/mL) is defined as the concentration of essential oil that causes a 50% loss of colour. The mean IC50 was calculated based on three repetitions and by means of interpolation of graphical dependence of concentration and degree of inhibition of the ABTS and DPPH radical.
Cupric ion reducing antioxidant capacity (CUPRAC) assay
The essential oil was investigated by the cupric ion-reducing antioxidant capacity (CUPRAC) method described by Apak et al. [Citation22]. In test tubes were mixed 1 mL CuCl2 solution (10 mmol/L), 1 mL neocuproine alcoholic solution (7.5 mmol/L) and 1 mol/L ammonium acetate buffer solution (pH = 7), 0.2 mL tested essential oil or Trolox, and 0.9 mL distilled water. Absorbance against a blank sample was measured at 450 nm with a spectrophotometer (Camspec M508, UK) after incubation for 30 min in darkness at room temperature (25 ± 1 °C). The calibration curve was plotted using Trolox as a standard ethanolic solution at concentration ranges between 0.045 and 1.5 mmol/L. The Trolox equivalent antioxidant capacity was plotted as µmol Trolox equivalents per milliliter essential oil (µmol TE/mL).
Ferric reducing antioxidant power (FRAP) assay
The essential oil was investigated by the ferric-reducing antioxidant power (FRAP) method described by Benzie and Strain [Citation23]. The FRAP reagent was freshly prepared before being analyzed by mixing 0.3 mol/L acetate buffer (pH 3.6), 10 mmol/L TPTZ in 40 mmol/L HCl and 20 mmol/L FeCl3.6H2O in distilled water in a ratio of 10:1:1. In test tubes were mixed 0.15 mL tested oil or standard Trolox and 2.85 mL FRAP reagent. Absorbance against a blank sample was measured at 593 nm with a spectrophotometer (Camspec M508, UK) after 15 min incubation in darkness at room temperature (25 ± 1 °C). The calibration curve was obtained using Trolox as a standard ethanolic solution at concentration ranges between 0.045 and 1.5 mmol/L. The Trolox equivalent antioxidant capacity was plotted as µmol Trolox equivalents per milliliter essential oil (µmol TE/mL).
Statistical analysis
All analyses were performed in triplicate. The data were expressed as mean values with standard deviation (±SD). Differences were considered statistically significant at the level of p < 0.05. Statistical program SPSS 19.0 software was used for data analysis by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test to evaluate differences between mean values of activities (SPSS Inc., Chicago, IL, USA).
Results
Chemical composition of essential oil
The essential oil is a mobile light yellow liquid with a specific anethole smell. The essential oil yield was 0.37%.
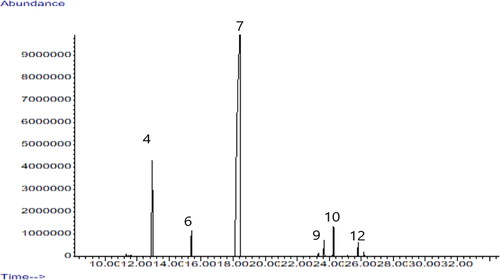
The chemical composition of the essential oil is presented in and . Thirteen compounds were identified, representing 99.61% of the identified content. Seven had a concentration below 1%, and the remaining six were over 1%. The main compounds (concentration above 3%) were methyl chavicol (82.03%) and limonene (9.90%).
Table 1. Chemical composition of anise hyssop essential oil, %.
The distribution of the main groups of compounds (% of the composition) in the oil is presented in . The data showed that phenyl propanoids predominate in the oil, followed by monoterpene hydrocarbons.
Antimicrobial activity
The diameters of the zones of inhibition are presented in . The data showed that the oil is only active against the gram-positive bacteria S. aureus and B. cereus, and yeasts C. albicans and S. cerevisiae, while the other test cultures were found to be resistant to the action of the studied essential oil sample.
Table 2. Antimicrobial activity of anise hyssop essential oil.
Antioxidant activity
The results of the antioxidant activity of the essential oil are presented in . The values of antioxidant activity calculated as Trolox equivalents ranged from 19.94 to 32.36 µmol TE/mL; for IC50, they were between 83.33 and 99.93 mg/mL.
Table 3. Antioxidant activity of anise hyssop essential oil.
Discussion
The essential oil yield differed from the data in the literature: 1.87% [Citation2], 0.2% [Citation7], 0.07–2.45% [Citation9], and 0.1–0.3% [Citation10]. This could be explained by the different geographical and climatic conditions of growth, the method of processing of the raw material, as well as the processed plant part, also found in other plants [Citation24].
In terms of the content of the main component methyl chavicol, the essential oil studied by us did not differ from the data in the literature, but its amounts were different as compared to previous reports, for example, 87.5% [Citation2], 97.66–98.42% [Citation3], 90% [Citation5], 93.45% [Citation7], 0.10–3.00% [Citation10], 83.1% [Citation11]. This difference in quantities could be explained by the conditions described above. According to some authors, however, the main components of essential oil were oxygenated monoterpenes: pulegone and menthone [Citation1,Citation5,Citation14].
The comparative analysis showed differences in the composition of the oil studied by us and that of other Bulgarian authors. According to Zhekova et al. [Citation5], there were differences in the composition of the oil, although the raw material was from the same field. The variation in results can be attributed to the impact of soil and climatic conditions on plant development, especially considering the time lapse of over 12 years since the previous study. Furthermore, methodological differences exist, as the previous authors utilized hydrodistillation in a modified Clevenger apparatus, whereas we employed steam distillation in our study. Unlike hydrodistillation, where both primary and secondary oils are collected, steam distillation only yields primary oil. Additionally, the origin of the plants examined in the previous study by Zhekova et al. [Citation5] was not specified, adding another variable to consider.
Ivanov et al. [Citation7] studied raw materials purchased from the commercial market without indicating its origin. It was processed by water distillation in a glass laboratory apparatus.
Many essential oils contain aromatic substances that could be potential allergens for the skin or hair when included in various cosmetic products’ compositions. Therefore, their presence should be indicated on the labels of these preparations. Of those specified in the EU Cosmetics Regulation [Citation25], the essential oil we studied contained monoterpene hydrocarbon limonene [Citation26,Citation27], which was present in an amount of 9.90%, as well as oxygenated monoterpene linalool [Citation28]. Still, it was in low concentrations of 0.08%. The main component in the essential oil, methyl chavicol, did not appear on that list, but its isomer anethole [Citation29–31] was considered a potential allergen.
When developing cosmetic preparations with this essential oil, the concentration of allergens should be declared on the label or in an accompanying information leaflet if it is higher than 0.01% for skin or hair wash-off products or if it is higher than 0.001% for preparations that remain on the skin or hair [Citation32]. Additionally, it is crucial to adhere to regulatory guidelines and safety standards when incorporating this essential oil into cosmetic formulations. The concentration of allergens must be clearly declared on the product label or in accompanying informational materials, particularly if it exceeds certain thresholds. Transparency regarding allergen content ensures consumer awareness and enables individuals to make informed decisions, especially those with known sensitivities or allergies. By following these regulations and providing comprehensive product information, cosmetic manufacturers can uphold product safety and consumer trust while harnessing the potential benefits of this valuable natural extract.
In the literature, there were no restrictions on essential oil use in food products; even its use as a preservative is recommended [Citation11]. However, it is essential to conduct thorough safety assessments and comply with food regulations to ensure its suitability and efficacy as a preservative. Additionally, further research may be needed to evaluate its impact on food flavor, stability, and sensory characteristics. By integrating this essential oil responsibly into food formulations, manufacturers can potentially enhance product shelf-life and safety while tapping into its natural preservative properties. Continued exploration of its applications in food science holds promise for addressing current challenges in food preservation and meeting consumer demand for clean label and minimally processed products.
Ivanov et al. [Citation7] found that anise hyssop essential oil inhibited the gram-positive and gram-negative bacteria they studied, except for Enterococcus faecalis. According to Hashemi et al. [Citation11], essential oil exhibited antibacterial and antifungal activity, the gram-positive bacterium B. subtilis being the most resistant strain against the essential oil, and the gram-negative bacterium E. coli being the most sensitive. The difference between these data and ours could be explained by the lower content of the main component, the geographical and growth conditions described above, and the different analysis methods.
Methyl chavicol is known to have pronounced antimicrobial, antioxidant, and biological activity [Citation32–34]. It was found that, isolated from the essential oil of the species Agastache rugosa (Fisch. & C.A.Mey.) Kuntze, it exhibited activity against various molds and yeasts, and its minimum inhibitory concentration was several times higher than the oil [Citation35].
Our data from the analysis of the antioxidant activity of the essential oil differed from those in the literature. For example, according to Ivanov et al. [Citation7], the anise hyssop essential oil showed a strong radical scavenging ability IC50 = 6.54 μL/mL. On the other hand, Hashemi et al. [Citation11]) reported that 92.1% of DPPH radical was inhibited at a concentration of 10 mg/mL. These differences could be explained by the different geographical and growth conditions described above and the different analysis methods. In considering future prospects, it is evident that the chemical composition of the studied plant holds promise for various applications, particularly in cosmetics and food products due to its notable antimicrobial and antioxidant activity. However, further research should optimize its efficacy for these purposes. Future studies could focus on enhancing the content of the main active ingredients through various methods. Moreover, exploring synergistic combinations with other natural compounds could potentially amplify its beneficial effects. Ultimately, continued investigation into this extract’s properties and its potential applications will pave the way for innovative developments in both the cosmetic and food industries, offering consumers safer and more sustainable alternatives.
Conclusions
This study analyzed the chemical composition, as well as the antimicrobial and antioxidant properties of an essential oil extracted via steam distillation of the flowering aerial parts of cultivated anise hyssop (A. foeniculum (Pursh) Kuntze). The plants were cultivated at the Institute of Roses, Essential and Medical Plants in Kazanlak, Bulgaria, under drip irrigation conditions. The primary constituents of the essential oil were identified as methyl chavicol and limonene. While the essential oil displayed weak antimicrobial activity overall, it showed a notable effect against the gram-positive bacterium S. aureus. Additionally, the oil exhibited a low antioxidant effect. The findings suggest potential applications of the essential oil as a flavoring agent in cosmetic and food products, warranting further research in this area.
Authors contributions
Conceptualisation, S.M. S.S., D.B., S.M., I.K., S.D., H.F., and A.S.; data analysis and validation, M.S.S., D.B., S.M., I.K., S.D., H.F., A.S., S.E., and A.A.; writing—original draft preparation, S.M. S.S., D.B., H.F., S.M., R.U., A.B., and A.S.; writing—review and editing, M.S.S., D.B., S.M., I.K., S.D., H.F., A.S., S.E., R.U., A.A., and A.B.; visualisation, S.D., S.E, A.A., R.U., and A.B.; collection of biological material, S.D.; writing—review and editing, S.M. S.S., D.B., S.M., I.K., S.D., H.F., and A.S.; supervision, A.S.; funding acquisition, R.U. and A.B. All authors have read and agreed to the published version of the manuscript.
Acknowledgment
Authors wish to thank Researchers Supporting Project Number(RSP2024R346) at King Saud University Riyadh Saudi Arabia for financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author (H.F.) upon reasonable request.
Additional information
Funding
References
- Chumakova V, Popova O. Lofant anisovy (Agastache foeniculum L.). A perspective source of obtaining drugs. Pharm Pharmacol. 2013;1:1–9.
- Omidbaigi R, Sefidkon F. Essential oil composition of Agastache foeniculum cultivated in Iran. J Essent Oil Res. 2003;15(1):520–531.
- Moghaddam M, Estaji A, Farhadi N. Effect of organic and inorganic fertilizers on morphological and physiological characteristics, essential oil content and constituents of agastache (Agastache foeniculum). J Essent Oil Bear Plants. 2015;18(6):1372–1381.
- Marcel D, Vârban D, Muntean S, et al. Use of species Agastache foeniculum (Pursh) Kuntze. Hop Med Plant. 2013;2:41–42.
- Zhekova G, Dzhurmanski A, Dobreva A. Gas-chromatography and organoleptic analysis of the essential oil of Agastache foeniculum (Pursh.) Kuntze. Agric Sci Technol. 2010;2(2):102–104.
- Lim TK. Agastache foeniculum. In: Edible medicinal and non medicinal plants (volume 8, flowers). Dordrecht: Springer; 2014. p. 151–155. doi: 10.1007/978-94-017-8748-2_7.
- Ivanov I, Vrancheva R, Petkova N, et al. Phytochemical compounds of anise hyssop (Agastache foeniculum) and antibacterial, antioxidant, and acetylcholinesterase inhibitory properties of its essential oil. J Appl Pharm Sci. 2019;9(2):72–78.
- Mollova S, Stanev S. Specific features of an immortal (Helichrisum italicum L.) grown on the territory of Bulgaria. Bulg J Crop Sci. 2019;56(5):60–65.
- Lashkari A, Najafi F, Kavoosi G, et al. Evaluating the in vitro anti-cancer potential of estragole from the essential oil of Agastache foeniculum [Pursh.] Kuntze. Biocatal Agric Biotechnol. 2020;27:101727. doi: 10.1016/j.bcab.2020.101727.
- Bălănescu F, Botezatu A, Marques F, et al. Bridging the chemical profile and biological activities of a new variety of Agastache foeniculum (Pursh) Kuntze extracts and essential oil. Int J Mol Sci. 2023;24(1):828.
- Hashemi M, Ehsani A, Hassani A, et al. Phytochemical, antibacterial, antifungal and antioxidant properties of Agastache foeniculum essential oil. J Chem Health Risks. 2017;7(2):95–104.
- Najafi F, Kavoosi G, Siahbalaei R, et al. Anti-oxidative and anti-hyperglycemic properties of Agastache foeniculum essential oil and oily fraction in hyperglycemia-stimulated and lipopolysaccharide-stimulated macrophage cells: in vitro and in silico studies. J Ethnopharmacol. 2022;284:114814. doi: 10.1016/j.jep.2021.114814.
- Najar B, Marchioni I, Ruffoni B, et al. Volatilomic analysis of four edible flowers from Agastache genus. Molecules. 2019;24(24):4480. doi: 10.3390/molecules24244480.
- Kovalenko N, Supichenko G, Leontiev V, et al. Composition of essential oil of plants some species of the genus Agastache L. introduced in Belarus. Proc Nat Acad Sci Belarus Biol Ser. 2019;64(2):147–155.
- Ebadollahi A, Khosravi R, Jalali Sendi J, et al. Toxicity and physiological effects of essential oil from Agastache foeniculum (Pursh) Kuntze against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) larvae. Ann Rev Res Biol. 2013;3:649–658.
- Association of Official Analytical Chemists (AOAC). Official methods of analysis. 20th ed. Geithersburg (MD): AOAC International; 2016.
- Adams R. Identification of Essential Oil Components By Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007.
- Zaika L. Spices and herbs: their antimicrobial activity and its determination. J Food Saf. 1988;9:97–118.
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237.
- Bojilov D, Manolov S, Ivanov I, et al. Investigation of antioxidant activity of different extracts of Helichrysum italicum from Bulgaria. J Int Sci Publ Mater Methods Technol. 2019;13:241–249.
- Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30.
- Apak R, Güçlü K, Ozyürek M, et al. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nutr. 2006;57(5–6):292–304.
- Benzie I, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292.
- Başer HCK, Buchbauer G. Handbook of essential oils: science, technology, and applications. 1st ed. Boca Raton, FL: Taylor and Francis Group, LLC CRC Press; 2010:33487–2742.
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off J Eur Union. 2009;L342/59. [cited 2017 Oct 20]. Available from: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF
- Pesonen M, Suomela S, Kuuliala O, et al. Occupational contact dermatitis caused by D-limonene. Contact Dermat. 2014;71(5):273–279. doi: 10.1111/cod.12287.
- Deza G, García-Bravo B, Silvestre J, et al. Contact sensitization to limonene and linalool hydroperoxides in Spain: a GEIDAC prospective study. Contact Dermat. 2017;76:74–80.
- Bennike N, Zachariae C, Johansen J. Non-mix fragrances are top sensitizers in consecutive dermatitis patients – a cross-sectional study of the 26 EU-labelled fragrance allergens. Contact Dermat. 2017;77:270–279.
- Poon T, Freeman S. Cheilitis caused by contact allergy to anethole in spearmint flavoured toothpaste. Australas J Dermatol. 2006;47(4):300–301. doi: 10.1111/j.1440-0960.2006.00300.x.
- Aschenbeck K, Hylwa S. Brushing your way to allergic contact dermatitis: anethole allergy. Dermatitis. 2017;28(3):219–220. doi: 10.1097/DER.0000000000000276.
- Horst N, Leysen J, Mellaerts T, et al. Allergic contact cheilitis from anethole-containing toothpastes: a practical solution. J Eur Acad Dermatol Venereol. 2017;31(8):e374–e375. doi: 10.1111/jdv.14174.
- Costa L, Pinto J, Bertolucci S, et al. In vitro antifungal activity of Ocimum selloi essential oil and methyl chavicol against phytopathogenic fungi. Cienc Agron. 2015;2:428–435.
- Zielińska S, Matkowski A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (lamiaceae). Phytochem Rev. 2014;13(2):391–416. doi: 10.1007/s11101-014-9349-1.
- Santos B, Pires A, Yamamoto C, et al. Methyl chavicol and its synthetic analogue as possible antioxidant and antilipase agents based on the in vitro and in silico assays. Oxid Med Cell Longev. 2018;2018:2189348.
- Ebadollahi A. Chemical constituents and toxicity of essential oil from Agastache foeniculum (Pursh) Kuntze against two stored-product insect pests. Chil J Agric Res. 2011;71:212–217.