Abstract
Hybridization was performed between the rice materials carrying the rice blast resistance genes Pi1, Pi2, and Pi-ta (R283) as the maternal parent and the broad compatibility restorer line R110 as the paternal parent. Molecular marker-assisted breeding techniques were used to detect the target genes in segregating generations. Through field selection and resistance identification, three stable lines with multiple resistance, strong restorer ability, and excellent agronomic traits were bred. Their agronomic and economic traits were analyzed along with their resistance to different physiological races of rice blast. These three stable lines were then crossed with seven different sterile lines (Chunjiang 23A, FA1, FA3, FA4, 81, 57, and 212A) to analyze the agronomic traits and resistance to rice blast pathogens in the hybrid combinations. JR11, JR12, and JR13 were stable, homozygous polymerized lines carrying Pi1, Pi2, and Pi-ta resistance genes and exhibited resistance frequencies ranging from 90.19 to 96.08% against different physiological races of rice blast disease. This indicates the excellent resistance of these three stable lines to rice blast disease. The seed-set frequencies in the hybrid combinations of the seven sterile lines with the three stable lines ranged from 66.4 to 84.1%, showing strong recovery ability. The yield of the selected combination of 23 A/JR11, 81 A/JR11, and 212 A/JR13 was 2.2–2.3% higher than that of the control Yongyou 1540. The research results showed that the three stable aggregated rice blast resistance genes Pi1, Pi2, and Pi-ta rice restorer lines created have good practical value in production.
Introduction
Rice blast disease, caused by the fungal pathogen Magnaporthe oryzae (Anamorph Pyricularia grisea Sacc), is a major threat to rice production [Citation1]. Globally, rice yields suffer a reduction of 10–30% annually due to the impact of rice blast disease [Citation2]. In affected areas, yield losses can reach 10–50%, with severe cases leading to complete crop failure [Citation3,Citation4]. Presently, high doses of pesticides are commonly used to control rice blast disease, imposing significant environmental and production cost pressure. Long-term practices have shown that developing rice varieties resistant to rice blast disease is the safest and most economical method for disease management [Citation5,Citation6]. However, continuous variation in physiological races of the rice blast pathogen has made rice varieties carrying only a single resistance gene susceptible to losing their resistance. In practical farming, it has been observed that aggregating multiple resistance genes broadens the spectrum of resistance against rice blast disease, enhancing the ability to resist the disease, thus proving to be the most scientific, environmentally friendly, and effective measure in disease prevention and control.
Currently, through molecular marker technology, at least 69 resistance loci and over 100 major rice blast disease resistance genes have been identified, with 36 of these resistance genes cloned [Citation7]. The majority of these resistance genes are located on the 6th and 11th chromosomes, with a smaller number situated on the 1st, 9th, and 12th chromosomes. Additionally, individual resistance genes have been found on the 2nd, 4th, and 8th chromosomes [Citation8]. Different types of genes exhibit varying degrees of effectiveness in combating rice blast disease. Genes, such as Pi1, Pi2, and Pi-ta demonstrate broad-spectrum, high resistance capabilities against rice blast disease, holding significant scientific value for breeding rice varieties resistant to the disease [Citation8,Citation9]. The Pi1 gene originates from the African japonica rice variety LAC23; the gene is located at the terminal end of the long arm of chromosome 11, between the RFLP markers G181 and RZ536 [Citation10]. Derived from a highly resistant indica rice variety, 5173, the Pi2 gene is located near the centromere of the short arm of chromosome 6. Research has shown that the Pi2 allelic series, such as C101A51, exhibits resistance to 455 physiological races in various regions of the Philippines and over 92% of races in 792 physiological races from central and southern China [Citation11]. The Pi-ta gene originates from the Philippine indica rice variety Tadukan, located on rice chromosome 12. This gene’s coding region comprises 2 exons and 1 intron, which encodes a protein consisting of 928 amino acids. It exhibits resistance against the majority of rice blast disease physiological races in China [Citation11,Citation12]. In recent years, many breeders have utilized molecular marker-assisted technology based on Pi1, Pi2, and Pi-ta resistance genes to develop a series of rice materials resistant to rice blast disease [Citation8,Citation12,Citation13], although research on the aggregation of these three resistance genes simultaneously remains limited.
Materials and methods
Experimental materials
Donor parents
Rice materials R283 (carrying rice blast disease resistance genes Pi1, Pi2, and Pi-ta) were provided by the Jiaxing Academy of Agricultural Sciences.
Recipient parents
The wide compatibility restorer line R110, possessing excellent agronomic traits and yield, was also provided by the Jiaxing Academy of Agricultural Sciences.
Sterile lines
Seven different types of sterile lines, A1-A7, namely: Chunjiang 23 A (Variety rights number: CNA20140460.3), FA1 (Breeding combination: Xiushui110A//Changjing1B/Jiahe212), FA3 (Breeding combination: Xiushui110A//Xiushui134/Jinjing818), FA4 (Breeding combination: Xiushui110A//Xiushui134/Jinjing212), 81 A (Rice approval number: 2021019), 57 A (Rice approval number: 2011013), and 212 A (Variety rights number: CNA20180822.2), were provided by Jiaxing Academy of Agricultural Sciences. These lines were utilized for evaluating the restoration ability of stable strains, identifying hybrid vigor in crossbreeds, selecting superior hybrid combinations with comprehensive traits, and assessing resistance to rice blast disease.
Identification of pathogenic strains
As shown in , the strains of rice blast inoculation and resistance evaluation were provided by the Jiaxing Academy of Agricultural Sciences. The samples were collected from 18 county-level cities in Zhejiang Province and Jiangsu Province. In this study, the physiological races of 51 isolates of Magnaporthe grisea were identified by using seven Chinese cultivars (Tetepu, Zhenlong 13, Sifeng 43, Dongnong 363, Guandong 51, Hejiang 51, and Lijiang xintuan Heigu). According to the resistance performance of physiological races of M. grisea in each identified cultivar, the population structure of physiological races of M. grisea in Zhejiang and Jiangsu provinces was determined. The populations included ZA (frequency of occurrence: 3.92% in japonica rice), ZB (frequency of occurrence: 74.51% in japonica rice), ZC (frequency of occurrence: 11.76% in japonica rice), and ZE (frequency of occurrence: 9.80% in indica-japonica rice). These strains exhibited relatively strong pathogenicity.
Table 1. Information on rice blast inoculum strains.
Experimental methods
Process of multi-gene material aggregation
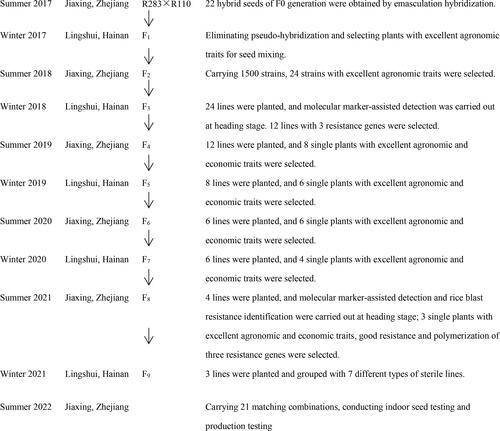
In July 2017, at the experimental fields of the Jiaxing Academy of Agricultural Sciences, hybridization was performed between the material R283 carrying rice blast disease resistance genes Pi1, Pi2, and Pi-ta as the maternal parent, and the wide compatibility restorer line R110 as the paternal parent. In December 2017, F1 hybrids were obtained from these crosses in Lingshui, Hainan, China. Subsequently, multiple generations of self-pollination and field-based selection for agronomic traits were carried out. Stable strains designated as JR10, JR11, JR12, and JR13, used for rice blast disease molecular marker detection, were developed. Specific breeding methods are illustrated in .
DNA extraction and PCR amplification detection
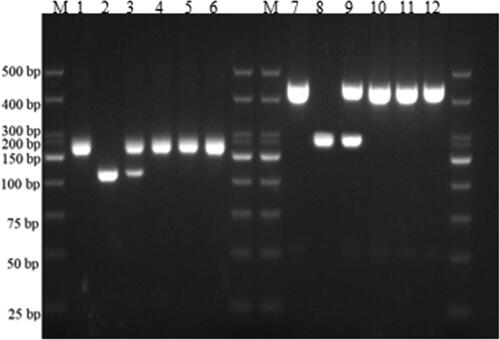
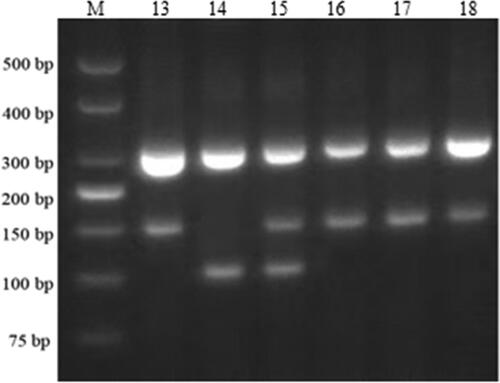
DNA Extraction and PCR Amplification Detection: PCR-specific primers for rice blast disease resistance genes Pi1, Pi2, and Pi-ta, along with their respective chromosome locations, annealing temperatures, and amplified fragment details, are detailed in . The PCR system for the rice blast resistance genes Pi1 and Pi2 (20 μL) was as follows: 2 × PCR mix 10 μL, sterile water 8 μL, primer forward (10 μmol/L) and reverse (10 μmol/L) each 0.5 μL, template 1 μL. The PCR amplification conditions were as follows: pre-denaturation at 94 °C for 4 min; 94 °C denaturation 30 s, 55 °C annealing 30 s, 72 °C extension 30 s, 35 cycles; 72 °C for final extension for 5 min. The reaction products were detected by 4% agarose gel electrophoresis, 200 V electrophoresis 60 min. The 237 bp band of PCR product contained Pi1 resistance gene, and the band without Pi1 resistance gene was <237 bp. The 200 bp band of PCR product contained Pi2 resistance gene, and the band without Pi2 resistance gene was <200 bp.
Table 2. Molecular marker information closely linked to rice blast resistance genes.
The PCR reaction system for the rice blast resistance gene Pi-ta (20 μL) was as follows: 2 × PCR mix 10 μL, sterile water 7.5 μL, 4 primers (10 μmol/L) each 0.5 μL, template 0.5 μL. The PCR amplification conditions were: pre-denaturation at 94 °C for 4 min; Denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s, extension at 72 °C for 35 s, 35 cycles; and final extension at 72 °C for 5 min. The reaction products were detected by 4% agarose gel electrophoresis. 200 V electrophoresis for 60 min showed that two bands of 309 and 202 bp could be amplified for those containing Pi-ta gene, two bands of 309 and 156 bp could be amplified for those without Pi-ta gene, and three bands of 309, 202 and 156 bp could be amplified for those containing heterozygous Pi-ta gene. The primers () and the 500 bp DNA Marker were produced by Sangon Biotech (Shanghai) Co., Ltd. (cat. no. B600303; https://store.sangon.com/).
Rice blast resistance inoculation identification
The test strains were provided by the Plant Protection Group of Jiaxing Academy of Agricultural Sciences. Inoculation methods followed the guidelines outlined in the Technical Regulations for Rice Blast Disease Resistance Identification of Rice Varieties published by the Ministry of Agriculture [Citation12–15]. The experimental materials were sown in the resistance identification nursery. When the rice seedlings reached 15 days of age, they were sprayed with a concentration of ∼2 × 10^5 spores/mL of rice blast disease pathogen. The inoculated plants were kept under controlled conditions at a temperature of 25 °C, humidity of 80%, and shade in a dark environment for 24 h. Disease symptoms were assessed and recorded 5–7 days after inoculation, and disease severity was graded based on the percentage of leaf area covered by lesions. The resistance reactions were categorized into nine levels: 0–3 indicated resistant (R) reactions, while 4–9 indicated susceptible (S) reactions, following criteria referenced from previous studies [Citation16].
Evaluation of multi-gene aggregate restoration lines and their hybrid agronomic traits
In April 2023, at the Hainan breeding base of Jiaxing Academy of Agricultural Sciences in Lingshui, the multi-gene aggregated rice blast disease resistance restorer lines containing Pi1, Pi2, and Pi-ta genes were crossed with the afore-mentioned 8 sterile lines for hybrid seed production. In June 2023, three restored lines and the hybrid seeds were planted in the experimental fields of the Jiaxing Academy of Agricultural Sciences. Each material was planted in one plot, consisting of six rows with six plants per row, arranged in a randomized sequence with three replicates. Standard field management practices for water, fertilizer, pest, and disease control were applied. During the whole growth period, test materials were fertilized at sowing with 96 kg N·ha−1, 45 kg P2O5 ha−1, and 135 kg K2O ha−1, respectively. The rice was fertilized at the tillering stage and panicle initiation stage with 72 kg N·ha−1, respectively. Water management was done in accordance with the local rice irrigation practice of early-stage irrigation, mid-stage sunshine, and late-stage alternating wet and dry conditions. Yongyou 1540 was set as the control of the test cross combination, which was the control variety of the regional test of indica-japonica hybrid rice in China and Zhejiang Province. In November 2023, investigations were conducted on various agronomic traits of rice, including growth stages, plant height, panicle length, grains per panicle, unfilled grain number, grain drop rate, seed setting rate, single panicle weight, length-to-width ratio of grains, and yield.
Statistical analyses
The data were analyzed by one-way analysis of variance (ANOVA) with SPSS 20.0 statistical software, compared with Duncan’s new complex error, and plotted and tabulated with Microsoft Office Excel 2010 software. Differences were considered statistically significant at the level of p < 0.05.
Results
PCR detection of rice blast disease resistance genes Pi1, Pi2, and Pi-ta
Using specific primers for the rice blast disease resistance genes markers Pi1, Pi2, and Pi-ta, the stable strains JR10, JR11, JR12, and JR13, resulting from the hybridization of the multi-gene aggregated resistant materials R283 and the wide compatibility restorer line R110, were individually tested at the single-plant level. In the PCR results, the presence or absence of specific bands indicates the presence of resistance genes. For Pi1, the PCR product of 237 bp indicates the presence of the resistance allele, while a slightly smaller band than 237 bp indicates its absence. Regarding Pi2, a PCR product of ∼200 bp was observed; the band indicating the presence of the resistance allele was smaller than the band without the resistance allele. For the Pi-ta, amplification resulted in bands of 309 and 202 bp for the presence of the allele, and bands of 309 and 156 bp for the absence of the allele. Moreover, the presence of a heterozygous Pi-ta allele displayed bands of 309, 202, and 156 bp. The results shown in and indicated successful amplification of the 237, 200, 309, and 202 bp bands in the parental strain R283 and stable strains JR11, JR12, and JR13, respectively, confirming the presence of Pi1, Pi2, and Pi-ta disease-resistant alleles. However, no amplification bands were observed for the wide compatibility restorer line R110, while JR10 displayed a heterozygous band. This confirms the successful aggregation of the Pi1, Pi2, and Pi-ta resistant alleles into the stable strains.
Rice blast disease resistance performance of multi-gene aggregated restoration lines
The three improved restoration lines, JR11, JR12, and JR13, along with the wide compatibility restorer line R110, displayed varying levels of resistance to 51 different strains of rice blast disease pathogens in Jiangsu and Zhejiang provinces, as shown in . Specifically, JR11 exhibited susceptibility to ZA9 and ZA41 in Xiuzhou and ZE3 in Haiyan, while displaying resistance to the other 38 strains, resulting in a resistance frequency of 94.12%. JR12 showed susceptibility to ZA9 in Xiuzhou and ZE3 in Dafeng, yet displayed resistance to the other 39 strains, with a resistance frequency of 96.08%. JR13 exhibited susceptibility to ZA9 in Xiuzhou, ZB9 in Xinchang and Yuyao, and ZE3 in Haiyan and Dafeng, while demonstrating resistance to the other 36 strains, resulting in a resistance frequency of 90.19%. On the other hand, with a resistance frequency of 33.33%, the control wide compatibility restorer line R110 demonstrated resistance to 17 strains. The resistance frequencies of JR11, JR12, and JR13 to rice blast were 2.82 times, 2.88 times, and 2.71 times higher than those of the control R110, respectively. This indicates that JR11 shows a significant improvement in rice blast resistance. Research has shown that it is feasible to enhance the restoration line by aggregating three resistance genes.
Table 3. Evaluation of resistance of rice blast resistance improved restorer lines to different types of rice blast fungus.
Performance of major agronomic traits in multi-gene aggregated restoration lines
According to , when compared to the control wide compatibility restorer line R110, the stable strain JR11 exhibited a significant increase of 10.2 and 20.4% in panicle length and yield, respectively. Additionally, there was a significant reduction of 76.7% in unfilled grains per panicle. The stable strain JR12 displayed significant increases of 13.0% in panicle length, 32.0% in effective panicles per plant, and 80% in grain length-to-width ratio. However, it experienced significant decreases of 7.6% in 1000-grain weight, 8.1% in grains per panicle, and 14.3% in single panicle weight. As for stable strain JR13, there were significant increases of 6.5% in panicle length, 42% in effective panicles per plant, 139.5% in unfilled grains per panicle, and 49.9% in yield. The three selected restoration lines showed no significant differences in other agronomic traits compared to the control.
Table 4. Performance of major agronomic traits in improved blast disease-resistant restorer lines.
Performance of major agronomic traits in hybrid combinations of multi-gene aggregated restoration lines
The experimental results () revealed that in the hybrid combinations of improved restoration lines, three combinations—namely, Chunjiang 23 A/JR11, 81 A/JR11, and 212 A/JR13—exhibited increased yield compared to the control, with yield increments of 3.8, 11.0, and 5.2%, respectively. Moreover, the Chunjiang 23 A/JR11 combination showed a 20% increase in effective panicles per plant, a 0.4% increase in grains per panicle, and a 0.9% increase in seed-setting rate compared to the control. In the case of the 81 A/JR11 combination, there were increases of 3.0% in effective panicles per plant, 15.4% in 1000-grain weight, 1.8% in unfilled grains per panicle, 11.6% in seed-setting rate, and 11.6% in single panicle weight compared to the control. Additionally, the 212 A/JR13 combination exhibited increments of 14.0% in plant height, 14.0% in panicle length, 17.0% in effective panicles per plant, and 7.5% in 1000-grain weight compared to the control. By aggregating the Pi1, Pi2, and Pi-ta resistance genes, Chunjiang 23 A/JR11, 81 A/JR11, and 212 A/JR13 demonstrated resistance rates of 72.54, 73.50, and 64.71%, respectively, against different strains of rice blast disease, all surpassing the control Yongyou 1540 (58.82%). These combinations displayed a higher level of resistance.
Table 5. Performance of agronomic traits in combinations of improved blast disease-resistant restorer lines.
Discussion
Utilizing molecular marker-assisted selection techniques for breeding rice materials that aggregate multiple resistant genes has become a crucial method in molecular breeding for rice. Ma et al. [Citation17] aggregated the Pi-ta and Pib genes to breed the rice variety ‘Tiejing 16’, which exhibited broad resistance spectrum and strong resistance to blast disease [Citation17]. The aggregation of Pi5 and Pi-ta genes resulted in rice lines with robust resistance to blast disease [Citation18]. Liu et al. [Citation19] utilized molecular marker-assisted selection techniques to aggregate the Pi-ta, Pib, and Pi9 genes, breeding a new strain named ‘Yandao 1626’ [Citation19]. Bhat Muzaffar et al. [Citation20] hybridized the rice variety Jhelum with a donor parent possessing three blast disease resistance genes (Pi54, Pi1, and Pi-ta) to breed a new strain with robust resistance [Citation20]. However, studies have shown that some resistant R genes have narrow resistance spectra, which makes it challenging to address the diversity and mutation rate of field physiological races. Moreover, there are instances where other R genes are linked with unfavorable economic traits, leading to a compromise between disease resistance and economic yield [Citation21–23].
The resistance frequencies of the three stable restorer lines (JR11, JR12, and JR13) selected in this study to different types of physiological races were 90.19–96.08%, and the resistance level was higher than that of the control wide compatibility restorer line R110, indicating that the donor gene in this study conferred broad-spectrum resistance and strong resistance to rice blast. In addition, the three stable restorer lines selected in this study had excellent economic traits, and the plot yield was higher than that of the wide compatibility restorer line R110. Compared with the control, the plot yield of JR13 line was significantly increased by 49.9%. The results showed that it was effective and feasible to use Pi1, Pi2, and Pi-ta genes for comprehensive breeding that could consider both yield and resistance.
Many previous studies have introduced rice blast resistance genes Pi9, Pigm, and Pi-kh into susceptible sterile lines or restorer lines through molecular assisted selection technology, and selected sterile lines or restorer lines with strong resistance to rice blast. The yield traits of hybrid rice combinations formulated with restorer lines containing rice blast resistance genes were good [Citation24–26]. The current experiment revealed that the restoration rates of the improved restoration lines containing three aggregated blast disease-resistant genes ranged from 66.4 to 84.7%, indicating a strong recovery ability of the developed improved restorer lines. Although the yield of restorer lines was not significantly higher than that of the control Yongyou 1540, the yield of three combinations of Chunjiang 23 A/JR11, 81A/JR11, and 212 A/JR13 increased by 3.8, 11.0, and 5.2%, respectively, compared with the control.
Limitations
Due to the limitation of rice blast inoculation conditions, this study did not carry out the rice blast inoculation test for the selected restorer lines and combinations in the whole growth period of rice in the field environment. Therefore, certain data regarding blast disease resistance testing remains incomplete and requires further refinement in future studies. Future studies could attempt to molecularly mark Pi1, Pi2, and Pi-ta according to different primers to find a simpler and faster method.
Conclusions
In this study, we successfully created three broad affinity restorer lines containing three Pi1, Pi2, and Pi-ta rice blast resistance genes through molecular marker-assisted breeding and three hybrid combinations. They not only improved rice blast resistance and yield and economic traits, but also gained rich and diverse genetic materials, providing good parental materials for selecting and breeding disease-resistant and high-quality hybrid rice combinations, and laying a good foundation for realizing the breeding of breakthrough rice varieties.
Author contributions
Conceptualization, KC and JC; methodology, HY and LW; formal analysis, BL; resources, YS, JL, and JY; data curation, ZT; writing-original draft preparation, HY, LW, and JC; writing-review and editing, KC and JC; supervision, JC; funding acquisition, HY and JC. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data that support the findings reported in this study are available from the corresponding authors [KC and JC] upon reasonable request.
Additional information
Funding
References
- Woan-Fei LJ, Hooi-Leng S, Khan TM, et al. The potential of streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front Microbiol. 2017;8:3. doi: 10.3389/fmicb.2017.00003.
- Fernandez J, Wilson RA. Cells in cells: morphogenetic and metabolic strategies conditioning rice infection by the blast fungus Magnaporthe oryzae. Protoplasma. 2014;251(1):37–47. doi: 10.1007/s00709-013-0541-8.
- Li B, Li YC, Liu ZS, et al. Development and application of Pi2 specific molecular markers for rice blast resistance gene. Mol Plant Breed. 2021;19(8):6.
- Yi NA, Li W, Dai LY. Cloning of rice blast resistant genes and research progress in molecular breeding. Mol Plant Breed. 2015;(7):1653–1659.
- Yang HL, Wang L, Li B, et al. Research progress on rice blast disease in Zhejiang Province. Chinese Rice. 2023;29(3):46–50.
- Yang DW, He NQ, Huang FH. Using molecular marker assisted selection to aggregate rice disease resistance genes Pigm-1 and Xa23. J Northw A&F Univ. 2023;51(11):37–45.
- Cao N, Chen Y, Ji ZJ, et al. Research progress on the molecular mechanism of rice blast resistance. Chinese Rice Sci. 2019;33(6):489–498.
- Li JB, Xia MY, Wei HX. Acquisition and resistance evaluation of rice blast resistance genes Pi1 and Pi2 polymer lines. Anhui Agric Sci. 2015;43(5):12–14.
- Xu X, Jiang WG, Liang HB, et al. Cloning of rice blast resistance gene Pi-ta2 and analysis of defense related gene expression mediated by it. J Central South Univ Nat. 2021;40(1):26–31.
- Yu Z H, Mackill DJ, Boman JM, et al. Tagging genes for blast resistance in rice via linkage to RFLP markers. Theor Appl Genet. 1991;81:471–476.
- Andersen JR, Lübberstedt T. Functional markers in plants. Trends Plant Sci. 2003;8(11):554–560. doi: 10.1016/j.tplants.2003.09.010.
- Cai HY, Zhou L, Jiao CM, et al. Development and application of Pi-ta specific molecular markers for rice blast resistance gene. Mol Plant Breed. 2017;15(2):589–593.
- Yu J, Lv DA, Guo CL, et al. Molecular marker assisted breeding of a new rice restoration line with resistance to rice blast Pi2 gene. Hubei Agric Sci. 2017;56(1):13–17 + 25.
- Pan QH, Zhang YL, Liang ZJ, et al. A comprehensive and precise method for identifying, mining, and cloning alleles of the Pi-ta resistance gene family in rice blast disease. CN202111116143.5[P]. CN113981122B[2023-11-26]; 2021.
- Luo CP, Ni L, Chen ZY, et al. Inoculation techniques for rice blast disease and resistance identification of 2009 regional trial varieties in Jiangsu Province. Jiangsu Agric Sci. 2009;6:178–179.
- Yang D, Li S, Xiao Y, et al. Transcriptome analysis of rice response to blast fungus identified core genes involved in immunity. Plant Cell Environ. 2021;44(9):3103–3121. doi: 10.1111/pce.14098.
- Ma ZB, Zhao JM, Cui YF, et al. Molecular marker assisted breeding of a new rice variety ‘Tiejing 16’ resistant to rice blast. Mol Plant Breed. 2021;19(2):512–517.
- Ma ZB, Quan DX, Shi Y, et al. Molecular marker assisted breeding of new rice lines with aggregated resistance genes Pi5 and Pi-ta to rice blast. Mol Plant Breed. 2021;19(1):173–179.
- Liu K, Wan BJ, Zhao SL, et al. Using molecular marker assisted selection to aggregate rice Pi-ta, Pi-b, and Pi-9 genes. Southw Agric J. 2021;34(5):926–931.
- Bhat Muzaffar A, Khan Gazala H, Saba M, et al. Marker-assisted selection for pyramiding of three blast resistance genes in rice variety Jhelum. SKUAST J Res. 2021;23(2):127–134.
- Wang J, Zhou L, Shi H, et al. A single transcription factor promotes both yield and immunity in rice. Science. 2018;361(6406):1026–1028. doi: 10.1126/science.aat7675.
- Rebecca N, Tyr W, Randall W, et al. Navigating complexity to breed disease-resistant crops. Nat Rev Genet. 2018;19(1):21–33.
- He HY, Chai YR, Qiu HP, et al. Distribution and resistance evaluation of five blast resistant genes in rice varieties in Zhejiang Province. Zhejiang Agric J. 2019;31(6):922–929.
- Huang YL, Yan Z, Wang H, et al. Molecular marker-assisted selection for directional improvement of rice blast resistance in sterile line Q211S. China Agric Bull. 2018;34(24):135–140.
- Xiang C, Ren XM, Lei DY, et al. Molecular marker-assisted selection to improve rice blast resistance of C815S. J Hunan Agric Univ. 2018;44(1):62–65.
- Qiu Y, Li CF, Chen LL, et al. Using Pi-kh gene to improve rice blast resistance of restorer lines. Mol Plant Breed. 2019;17(1):119–124.