Abstract
Bulgarian real-world data on denosumab use for the prevention of skeletal-related events (SREs) associated with bone metastases (BM) from prostate cancer (PC) are lacking. This observational study enrolled 100 adult PC patients with ≥1 BM receiving denosumab (EUPAS26983). Patients’ routine treatment was observed retrospectively for ≤6 months and prospectively for 12 months. Denosumab use and safety, SRE incidence, denosumab persistence (allowing ≤2 missed doses), and pain severity were evaluated using descriptive statistics. Denosumab was initiated at a median (Q1, Q3) of 1.8 (0.6, 9.6) months following BM diagnosis with earlier initiation in hormone-sensitive (HSPC; 1.5 [0.6; 4.2]; n = 74) than castration-resistant PC (CRPC; 7.9 [0.8; 16.4]; p = .082; n = 26) and in younger (<65 years; 1.2 [0.3; 2.8]; n = 19) than older patients (≥65 years; 2.0 [0.7; 9.6]; p = .161; n = 81). Ten SREs were recorded during the retrospective and 10 during the prospective period; none were symptomatic. Denosumab was used for a median of 472 (387, 599) days overall and for 443–464 days without missing more than two doses (persistence). The proportion of patients reporting acceptable pain (score ≤4 of 10) increased from 6% at baseline to 22% at month 12. Twenty-four denosumab-related adverse events were reported, 2 were serious (osteomyelitis, osteitis) and 5 were related to osteonecrosis of the jaw. In Bulgarian clinical practice, denosumab use was associated with the prevention of symptomatic SREs and pain improvement. Denosumab was mostly used persistently. No symptomatic SREs were reported. Safety findings were consistent with previous reports of denosumab use in PC.
Introduction
Prostate cancer (PC) is the most common cancer in men, accounting for 24% of new cancer cases in Bulgarian men in 2020 [Citation1]. It has been shown that breast and PC are especially prone to developing bone metastases due to a favorable interaction of these specific tumor cells with the bone microenvironment [Citation2]. In patients newly diagnosed with PC, the prevalence of bone metastases is low [Citation3]. However, it is estimated that approximately 80–90% will eventually develop metastatic bone disease [Citation4] and survival once bone metastasis is diagnosed is estimated at 3–5 years [Citation5].
Primary prevention of patients with bone metastases from PC before the occurrence of a first skeletal-related event (SRE) has been found to be rare [Citation6]. However, left untreated, half of PC patients with bone metastases experience at least one SRE such as pathological fracture, radiation to bone, spinal cord compression, or bone surgery [Citation7, Citation8]. The risk of subsequent SREs increases with disease progression [Citation9]. To prevent SREs, the European Society for Medical Oncology (ESMO) 2020 guidelines on bone health in cancer recommend the use of bone-targeted agents (BTAs), with denosumab indicated as the preferred BTA from an efficacy, convenience, and renal health perspective [Citation10]. Denosumab, a fully human antibody targeting receptor activator of nuclear factor kappa-B ligand (RANKL), is indicated for the prevention of SREs in adults with advanced malignancies involving bone, and for the treatment of adults and skeletally mature adolescents with giant cell tumor of the bone that is unresectable or where surgical resection is likely to result in severe morbidity [Citation11]. Its superiority versus zoledronic acid in delaying the time to first SRE has been demonstrated in patients with castration-resistant prostate cancer (CRPC), breast cancer and other solid tumors in three large, randomized clinical trials [Citation7, Citation12–14]. In a phase III trial, denosumab showed superior effects on pain and health-related quality of life in patients with metastatic CRPC [Citation15]. However, overall survival times were similar between the denosumab- and placebo-treated groups [Citation16].
In routine clinical practice, long-term drug adherence is critical for therapeutic effectiveness [Citation17]. A recent machine learning-based analysis identified a short duration of denosumab treatment as a risk factor for the development of SREs following denosumab discontinuation [Citation18]. There is very limited knowledge about the adherence to treatment in patients with cancer receiving denosumab. Persistence with denosumab treatment (continuous use of denosumab without exceeding a 35-day gap) has been estimated at 69% to 78% at 24 weeks of treatment in PC patients from Germany, Austria, Bulgaria, the Czech Republic, Hungary, and Slovakia [Citation19–21]. The present study describes treatment patterns of denosumab use for the prevention of SREs in PC patients in Bulgaria.
Subjects and methods
Ethics
The Bulgarian centralized ethics committee for clinical trials provided the ethics approval of the study protocol and the Bulgarian drug agency provided the regulatory approval (registration number: EKKI/CT-0365, approval date: 08 May 2019).
The study was conducted in accordance with country-specific legal and regulatory requirements, as well as with scientific purpose, value, and rigor. It followed the generally accepted research practices described in Good Epidemiological Practice (GEP) guidelines issued by the International Epidemiological Association (IEA). All data were handled in the strictest confidence in conformity with national and European data protection regulations (such as Directive 95/46/EC). Written informed consent was obtained from all individual participants included in the study.
Study design and patient population
This multicenter, prospective observational study included consenting adults (≥18 years) with hormone-sensitive prostate cancer (HSPC) or CRPC and ≥1 imaging-confirmed bone metastasis. Patients must have received ≥1 denosumab dose. Patients receiving bisphosphonates or other BTAs in the 6-month interval before enrolment, those with PC as a second primary malignancy or with brain metastases, and those enrolled simultaneously in a trial with an investigational drug for treatment or prevention of bone metastases and SREs were excluded.
Data collection
Retrospective baseline data (1–6-months from denosumab initiation to study enrolment) were collected from medical files; prospective data (12 months followed by a 1-month safety follow-up) were collected by the investigator during routine visits. To assess the diagnosis and management of pain, the Brief Pain Inventory (BPI) [Citation22] was used. The Beliefs about Medicines Questionnaire (BMQ) [Citation23] and the Perceptions of Adherence Management Questionnaire (PAMQ) [Citation24] were used to assess patient-reported outcomes.
Study objectives
The primary outcome measures were the demographics and disease characteristics of patients with bone metastases from PC receiving denosumab in routine clinical practice, and denosumab treatment patterns and persistence. Secondary outcomes included SREs and symptomatic SREs (SSEs), the time from bone metastasis diagnosis to denosumab initiation, hospitalizations for SREs/SSEs, changes in pain severity over time, and pain medication use.
Statistical considerations
No a priori hypotheses were statistically assessed. All inferential analyses were exploratory. Numeric data were summarized as number of non-missing and missing observations, arithmetic mean, standard deviation (SD), median, first (Q1) and third (Q3) quartiles, minimum and maximum value, and confidence intervals (CI) where applicable. Categorical variables were summarized with counts and percentages, accompanied by Clopper-Pearson 95% two-sided CI, where appropriate. For the Kaplan-Meier survival curves, the CIs were calculated using the SAS-compatible complementary log-log transformation (log cumulative hazard transformation), preventing the CIs from exceeding the [0–1] range. Assessment of proportionality of hazards employed the Grambsch-Therneau χ2 test, assessing the correlation between the covariate-specific scaled Schoenfeld residual and the survival time. The CIs for quantiles of the survival time were calculated using the Brookmeyer and Crowley formula.
Persistence was defined as continuous use of denosumab without exceeding a 60-day gap until discontinuation, study withdrawal, switch to another therapy, or end of study. A descriptive sensitivity analysis was carried out using a 45-day and a 90-day gap. Persistence was calculated using two different scenarios for loss to follow-up: (1) the loss of persistence was set at the last dose before the loss to follow-up + the 60-day gap (Last Observation Carried Forward [LOCF] approach) or (2) patients were considered persistent by the date of loss to follow-up and were subsequently censored (Censoring approach).
All analyses were performed using the validated version of R Statistical Package, version 3.6.3.
Results
Patient disposition
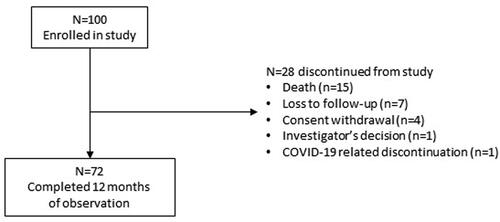
The study enrolled 100 patients (26 with CRPC and 74 with HSPC) from nine study centers. The study period was January 2019 to January 2021. shows the patient disposition and reasons for discontinuation from the study.
Patient population
At enrolment, the mean (SD) age was 72 (8.3) years; 19% were aged <65 years and 81% were aged ≥65 years. The median (Q1, Q3) Gleason score was 8.0 (7.0, 9.0) and the Gleason grade group was 4.0 (2.0, 5.0). Most patients had high (35%) or very high-risk cancers (22%). shows baseline tumor characteristics for all patients and by cancer type.
Table 1. Tumor diagnosis and tumor characteristics at baseline.
At baseline, most patients (63%) had Eastern Cooperative Oncology Group (ECOG) performance status 1 and ECOG status generally remained stable over time (Supplemental Table S1). Calcium supplementation was recorded in 82% of patients and vitamin D supplementation in 67%. The most frequent comorbidities (>10% of 110 comorbidity reports) at baseline were cardiovascular disorders (67 reports, 60.9% of reports) and diabetes mellitus/metabolic disorders (16 reports, 14.5% of reports).
Ninety-seven patients were receiving anticancer therapy during the baseline period: 90% (n = 87/97) hormone therapy, 29% (n = 29/97) chemotherapy, 14% (n = 14/97) radiation therapy, 8% (n = 8/97) targeted therapy, and 4% (n = 4/97) surgery (multiple therapy counts were possible).
Denosumab use
The median (Q1, Q3) time between the diagnosis of bone metastases and initiation of denosumab was 1.8 (0.6, 9.6) months and was shorter in patients with HSPC (1.5 [0.6, 4.2] months) than in those with CRPC (7.9 [0.8, 16.4] months; hazard ratio [HR] = 0.65; p = .082), and in adults (<65 years) compared to elderly (≥65 years) patients (1.2 [0.3, 2.8] months versus 2.0 [0.7, 9.6] months [HR = 0.68, p = .161], respectively). Overall, the median (Q1, Q3) duration of denosumab treatment from first dose to end of study was 15.5 months (12.7, 19.6) and comprised 5.0 (2.0, 9.0) administrations.
Thirty patients temporarily or permanently discontinued denosumab during the prospective study period. The most frequent reasons were death (37%, n = 11), investigator’s decision (27%, n = 8), loss to follow-up (23%, n = 7), and (serious) adverse events related to denosumab (10%, n = 3).
Bone metastases at baseline and during study
Per inclusion criteria, all patients had at least one bone metastasis at enrolment. In the 6-month retrospective period, a total of 305 bone metastases were recorded. Of confirmed metastases, 84.6% (n = 258) were asymptomatic and detected by imaging, while 14.8% (n = 45) were symptomatic; for 0.7% (n = 2) the method of detection was unknown. During months 1–6 of prospective observation, 13 new metastases were found. Of these, 92.3% (n = 12) were asymptomatic and 7.7% (n = 1) were symptomatic. During months 7–12, 18 new metastases were reported, all of which were asymptomatic. Supplemental Table S2 shows metastases by location at baseline, months 1–6, and 7–12.
SREs at baseline and during study
At baseline, 10 SREs in 7 patients and no SSEs had been recorded. During the study period, 10 new SREs in 7 patients and no SSEs were identified. The newly occurring SREs were radiation to bone (n = 9) and pathological fracture (n = 1). There were five patients with SREs recorded only at baseline, with no further events; two patients had SREs at both baseline and during the 12 months of prospective observation; five patients with SREs only during prospective observation; two patients had recurrent events during prospective observation. shows the occurrence of SREs and SSEs over time. In the prospective study period, the duration of hospitalization due to SREs ranged from 3–19 days.
Table 2. Skeletal-related events and symptomatic skeletal-related events over time.
Persistence with denosumab treatment
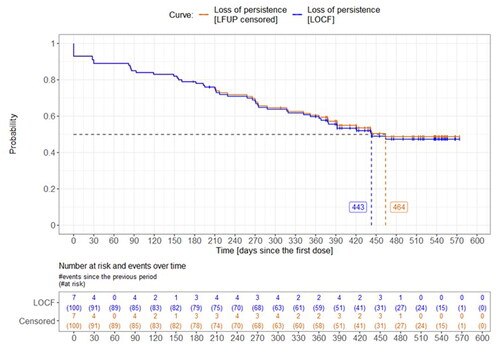
When considering the entire study period, that is, 6 months of retrospective plus 12 months of prospective observation, the median time from first known denosumab dose to loss of persistence was 443–464 days, depending on the approach to the loss to follow-up (). At the end of observation (day 540), 47–49% were still persistent to denosumab. When considering only the 12-month prospective observation period, the median time to loss of persistence was 300–365 days. At the end of the prospective observation period (day 360), 51–52% were persistent. In adult patients aged <65 years, the median time to non-persistence was not reached; in elderly patients aged ≥65 years, it was 464 days. Persistence was lower when the allowed time window between two denosumab administrations was shorter (45 days; HR = 1.81) than the standard 60-day window and higher when it was longer (90 days; HR = 0.74). The reasons for non-persistence at each allowed time window are summarized in Supplemental Table S3.
Figure 2. Time from first denosumab dose to non-persistence with denosumab. LFUP, loss to follow-up; LOCP, last observation carried forward. Calculated from the first dose within the 6-month retrospective period. Persistence was assessed for the 60-day gap. LOCF approach: the loss of persistence was at the last dose before the loss to follow-up + the 60-day gap). LFUP censored approach: patients were considered persistent by the date of loss to follow-up and then they were censored.
Diagnosis and management of pain
Patients completed the BPI questionnaire at baseline and in 3-monthly intervals during the observation period. For nearly all questions, patients’ responses suggested an improvement in pain over time (Supplemental Table S4).
The proportion of patients reporting pain scores ≤4 (acceptably low level of pain on a scale of 1–10) increased over time (Supplemental Table S5). In patients with a pain score <4 at baseline, 2–6 patients reported a clinically relevant increase in pain (≥2 points) from any 3-month period to the next, while 2–3 patients reported an increase in pain score >4. Pain medication was prescribed to 38 patients, mostly metamizole sodium (34%, n = 13/38).
Physicians’ perceptions of persistence
All 9 investigators completed the PAMQ at baseline and at the end of observation (Supplemental Table S6). Physicians had strong confidence in their knowledge of adherence in general and in their ability to influence their patients’ adherence-related behavior. Most physicians reported similar responses at baseline and at the end-of-study responses.
Patients’ beliefs about medicines
The BMQ was completed by 98 patients at baseline and at the end of observation. Patients’ responses initially showed some level of hesitation and concern about side effects and potential discomfort caused by medicines in general (Supplemental Figure S1). However, there was generally a good understanding of the reasons for having to take the medicines prescribed to them and there appeared to be fewer concerns than in the “medicines in general”-part of the questionnaire (Supplemental Figure S2).
Adverse events
Overall, 93 adverse events – irrespective of denosumab causality – were reported (Supplemental Table S7). These adverse events occurred in 38 patients, 20 patients had serious adverse events and 26 had non-serious adverse events. Twenty-four adverse events were related to denosumab (). Of these, two were serious (osteomyelitis, osteitis) and of severe intensity. Osteonecrosis of the jaw or related events occurred in five patients (loose tooth, n = 1; pain in jaw, n = 2; osteonecrosis, n = 1; osteonecrosis of the jaw, n = 1).
Table 3. Summary of denosumab-related adverse drug reactions (by MedDRA classification).
Discussion
For HSPC and CRPC as well as older and younger patients participating in the study, this group of Bulgarian PC patients receiving denosumab for the prevention of SREs was representative of the general population of PC patients with bone metastases. Most patients had high (35%) and very high (22%) risk tumors.
The present study shows trends in denosumab treatment patterns in Bulgaria. Denosumab was initiated earlier in adults <65 years than elderly patients ≥65 years (not statistically significant, HR = 0.68; p = .161). Although the group of adult patients was small (n = 19) and results need to be interpreted with caution, this numerical difference could be related to the need for elderly patients to schedule and receive their treatment while managing comorbidities and logistical support requirements.
Patients with HSPC were also initiated on denosumab earlier than those with CRPC (not statistically significant, HR = 0.65; p = .082). A recent study from Canada also found that patients with CRPC rarely received primary prevention before the first occurrence of an SRE [Citation6]. This may be due to the challenges and practical complexities of establishing the ‘hormone resistant’ tumor state (i.e. failure of at least one hormonal therapy, indicated by a rising prostate-specific antigen concentration) [Citation25].
Non-persistence with denosumab was defined as not receiving any denosumab administration on the scheduled date nor during a 60-day window thereafter. Since denosumab is administered on a once-monthly schedule in the oncology setting, a 60-day gap represents two missed doses. With the first known denosumab dose within the retrospective period as the index date, the median time to non-persistence was 443–464 days, depending on the approach to handling loss to follow-up. When starting calculation from the enrolment date, the median time to non-persistence was 365 days, suggesting median persistence spanning the full 12 months of prospective observation. Irrespective of the duration under consideration (retrospective plus prospective observation or prospective observation alone) approximately half of patients remained persistent with denosumab. In previously published studies of persistence with denosumab in Germany, Austria, and the CEE region [Citation19–21], which used a 35-day window corresponding to one missed dose, it was observed that persistence varied among tumor types: patients with prostate and breast cancer generally showed higher persistence than those with lung and other cancers. Persistence also differed by country with Bulgaria showing the second lowest persistence among the participating countries. Although definitions were different, real-world evidence from the United States and from Germany indicated better compliance in patients using denosumab than those using zoledronic acid [Citation26–28].
The violation of the allowed gap between administrations was the most frequent reason for non-persistence in the present study as well as the persistence study from the CEE region [Citation19]. Importantly, denosumab safety was not an important reason. The reported adverse events were consistent with previous treatment experience with denosumab in PC or other solid tumors [Citation7, Citation12–14].
It is important to understand the risk of extending denosumab administration intervals, for example, to align denosumab dosing with common chemotherapy schedules in clinical practice. For instance, data from Canada showed administration intervals up to once every 12 weeks are common [Citation29]. A retrospective study of different denosumab dosing intervals (<5, 5–11, or ≥12 weeks) found no significant differences in time to first SRE and median overall survival with extended dosing schedules [Citation30]. However, there appears to be contradicting evidence from another study which found a higher SRE incidence when denosumab was administered at intervals of 31–56 days versus 27–30 days [Citation31]. In the present study, very few SREs and no SSEs were recorded during observation, precluding meaningful analysis of differences in SRE rate among patients with different administration windows.
The reported pain levels in the present study improved over the treatment period. This is in line with the previous persistence studies from Austria, Germany and the CEE region, where only very few patients shifted from no or weak opioid analgesics (Analgesic Quantification Algorithm [AQA] score ≤ 2) at baseline to an AQA category >2 (i.e. strong opioids at increasing doses) at later timepoints [Citation19, Citation20].
Questionnaires on perception of persistence and beliefs about medicine were used to understand the factors contributing to patients’ non-adherence to their prescribed medicines. An understanding of these contributors could inform future physician education efforts and patient support programs. It was found that physicians were aware of the importance of persistence with treatment and believed to understand the factors that contributed to non-persistence. They also felt confident to educate their patients accordingly. From the patients’ perspective, there appeared to be a gap between their beliefs about medicines in general and medicines prescribed specifically for them. Patients generally understood why they had to take their medicines and felt confident about their safety, even when they did not fully trust medicines in general. It has previously been found [Citation32] that patients typically conduct some kind of benefit-risk assessment when deciding to take and/or adhere to a medicine and will be more likely to adhere to treatment when they have a positive decisional balance, i.e. the perceived benefit is considered higher than the risk [Citation32]. The decisional balance is strongly impacted by the tumor stage, cancer treatment context (curative versus palliative), fear of recurrence, etc. Adequate patient education on expected treatment benefit and side effects is therefore critical to avoid any misunderstanding of the benefits and risks of treatment [Citation32]. A respectful and trusting physician-patient relationship was shown to be important and even sway patients’ treatment decisions despite previously held negative beliefs about medicines [Citation32].
Potential limitations of the study relate to its observational design. Data source limitation was expected to be low due to the mainly prospective design. In the case of unexpected or incorrect data, clarifications were sought based on medical records. Any inconsistencies were reviewed and resolved during frequent data extractions. Adverse drug reactions, such as osteonecrosis of the jaw, were not independently adjudicated by an expert panel. There were two major areas of potential selection bias for the study. Firstly, recruited patients were required to have metastatic stage of the disease and to have received at least one dose of denosumab prior to enrolment. Secondly, there might have been a small group of patients who ceased treatment after one dose, usually because of death or disease progression. The survival bias may therefore have been slightly inflated and have impacted other collected data. However, due to the prospective design of the study, patients were alive at enrolment. The nature of an observational study involves some difficulties in obtaining data, therefore bias due to missing data was anticipated, and the number of missing data values was explicitly shown for each statistic.
Conclusions
The present study showed that in Bulgaria denosumab was initiated sooner after bone metastasis diagnosis in HSPC than CRPC patients and in adult patients <65 years compared to ≥65 years with PC. Rates of new SREs were very low and no symptomatic SREs occurred during the study. Median persistence with denosumab was approximately 15 months counting from the first known dose when allowing two missed doses to define persistence. The denosumab safety profile was consistent with previous evidence.
Authors’ contributions
V.I. and K.B. contributed substantially to the study design and concept. A.T. and M.P. were involved in data acquisition. A.O. conducted the data analyses. All authors assisted with interpretation of the data. All authors were involved in drafting of the manuscript, provided critical revisions for important intellectual content, approved the final version submitted for publication, and agreed to be accountable for all aspects of the work.
Ethics
The Bulgarian centralized ethics committee for clinical trials provided the ethics approval of the study protocol and the Bulgarian drug agency provided the regulatory approval (registration number: EKKI/CT-0365, approval date: 08 May 2019).
The study was conducted in accordance with country-specific legal and regulatory requirements, as well as with scientific purpose, value, and rigor. It followed the generally accepted research practices described in GEP guidelines issued by the International Epidemiological Association (IEA). All data were handled in the strictest confidence in conformity with national and European data protection regulations (such as Directive 95/46/EC). Written informed consent was obtained from all individual participants included in the study.
Supplemental Material
Download PDF (1.2 MB)Acknowledgements
The authors would like to thank Margit Hemetsberger, PhD, of Hemetsberger Medical Services, Vienna, Austria, for medical writing support, funded by Amgen Bulgaria, EOOD. The authors would like to thank all the investigators and patients.
Disclosure statement
A.T. and M.P. were principal investigators of this study. V.I., K.B. are employees of Amgen and hold Amgen stocks. A.O. declares no conflict of interest.
Data availability statement
Qualified researchers may request data from Amgen clinical studies. Complete data are available at the following: https://wwwext.amgen.com/about/how-we-operate/policies-practices-and-disclosures/ethical-research/clinical-data-transparency-practices/clinical-trial-data-sharing-request.
Additional information
Funding
References
- International Agency for Research on Cancer. Cancer Fact Sheets: Bulgaria 2020. Available from: https://gco.iarc.fr/today/data/factsheets/populations/100-bulgaria-fact-sheets.pdf.
- Lu J, Hu D, Zhang Y, et al. Current comprehensive understanding of denosumab (the RANKL neutralizing antibody) in the treatment of bone metastasis of malignant tumors, including pharmacological mechanism and clinical trials. Front Oncol. 2023;13:1. doi: 10.3389/fonc.2023.1133828.
- Ottosson F, Baco E, Lauritzen PM, et al. The prevalence and locations of bone metastases using whole-body MRI in treatment-naïve intermediate- and high-risk prostate cancer. Eur Radiol. 2021;31(5):2747–10. doi: 10.1007/s00330-020-07363-x.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755.
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591.
- Phillips WJ, Saad F, Leigh J, et al. Real-world evaluation of primary versus secondary prevention of skeletal-related events in metastatic castration-resistant prostate cancer. Oncologist. 2024:oyae036 (Online ahead of print). doi: 10.1093/oncolo/oyae036.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6.
- Nørgaard M, Jensen AØ, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol. 2010;184(1):162–167. doi: 10.1016/j.juro.2010.03.034.
- Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879–882. doi: 10.1093/jnci/djh141.
- Coleman R, Hadji P, Body J-J, et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2020;31(12):1650–1663. doi: 10.1016/j.annonc.2020.07.019.
- European Medicines Agency. Xgeva(R) (denosumab) – Summary of product characteristics: European Medicines Agency [Internet]. 2022 [updated 2022 July25]. Available from https://www.ema.europa.eu/en/documents/product-information/xgeva-epar-product-information_en.pdf.
- Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082–3092. doi: 10.1016/j.ejca.2012.08.002.
- Stopeck AT, Lipton A, Body J-J, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101.
- Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679–687. doi: 10.1007/s00520-013-2022-1.
- Hegemann M, Bedke J, Stenzl A, et al. Denosumab treatment in the management of patients with advanced prostate cancer: clinical evidence and experience. Ther Adv Urol. 2017;9(3-4):81–88. doi: 10.1177/1756287216686018.
- Scher HI, Morris MJ, Basch E, et al. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol. 2011;29(27):3695–3704. doi: 10.1200/JCO.2011.35.8648.
- Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x.
- Jacobson D, Cadieux B, Higano CS, et al. Risk factors associated with skeletal-related events following discontinuation of denosumab treatment among patients with bone metastases from solid tumors: a real-world machine learning approach. J Bone Oncol. 2022;34:100423. doi: 10.1016/j.jbo.2022.100423.
- Haslbauer F, Petzer A, Safanda M, et al. Prospective observational study to evaluate the persistence of treatment with denosumab in patients with bone metastases from solid tumors in routine clinical practice: final analysis. Support Care Cancer. 2020;28(4):1855–1865. doi: 10.1007/s00520-019-04988-7.
- Diel IJ, Greil R, Janssen J, et al. Medication adherence with denosumab in patients with bone metastases from solid tumors treated in routine clinical settings: a retrospective study. Support Care Cancer. 2022;30(11):9267–9278. doi: 10.1007/s00520-022-07333-7.
- Porubská M, Němcová A. Persistence of denosumab in Slovak patients with bone metastases – a prospective observational study. Klin Onkol. 2023;36(1):54–64. doi: 10.48095/ccko202354.
- MD Anderson Cancer Center. The Brief Pain Inventory. 1991. Available from: https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html.
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. doi: 10.1080/08870449908407311.
- Verbrugghe M, Timmers L, Boons CC, et al. Adherence to oral anticancer agents: healthcare providers’ perceptions, beliefs and shared decision making in Belgium and the Netherlands. Acta Oncol. 2016;55(4):437–443. doi: 10.3109/0284186X.2015.1119307.
- Morote J, Aguilar A, Planas J, et al. Definition of castrate resistant prostate cancer: new insights. Biomedicines. 2022;10(3):689. doi: 10.3390/biomedicines10030689.
- Hernandez RK, Quigley J, Pirolli M, et al. Patients with bone metastases from solid tumors initiating treatment with a bone-targeted agent in 2011: a descriptive analysis using oncology clinic data in the US. Support Care Cancer. 2014;22(10):2697–2705. doi: 10.1007/s00520-014-2251-y.
- Qian Y, Bhowmik D, Kachru N, et al. Longitudinal patterns of bone-targeted agent use among patients with solid tumors and bone metastases in the United States. Support Care Cancer. 2017;25(6):1845–1851. doi: 10.1007/s00520-017-3583-1.
- Diel I, Ansorge S, Hohmann D, et al. Real-world use of denosumab and bisphosphonates in patients with solid tumours and bone metastases in Germany. Support Care Cancer. 2020;28(11):5223–5233. doi: 10.1007/s00520-020-05357-5.
- AlZahrani M, Clemons M, Vandermeer L, et al. Real-world practice patterns and attitudes towards de-escalation of bone-modifying agents in patients with bone metastases from breast and prostate cancer: a physician survey. J Bone Oncol. 2021;26:100339. doi: 10.1016/j.jbo.2020.100339.
- Abousaud AI, Barbee MS, Davis CC, et al. Safety and efficacy of extended dosing intervals of denosumab in patients with solid cancers and bone metastases: a retrospective study. Ther Adv Med Oncol. 2020;12:1758835920982859. doi: 10.1177/1758835920982859.
- Kettle JK, Patel PB. Feasibility of extended dosing intervals of denosumab. J Oncol Pharm Pract. 2017;24(5):343–347. doi: 10.1177/1078155217703791.
- Marshall VK, Given BA. Factors associated with medication beliefs in patients with cancer: an integrative review. Oncol Nurs Forum. 2018;45(4):508–526. doi: 10.1188/18.Onf.508-526.