Abstract
Biocontrol agents against rice blast fungus Magnaporthe oryzae have attracted more attention recently. In this study, the antifungal activity of AMEP412 protein against M. oryzae was assessed. AMEP412 induced a visible inhibition zone on M. oryzae coated agar plates, which preliminarily verified its antifungal activity. On complete medium agar plates, the aerial hyphae growth was significantly slowed down by AMEP412 (0.0125–0.05 mg/mL), with inhibition rates ranging from 21.25 to 54.40%. The half maximal effective concentration (EC50 value) was 0.041 mg/mL. On oats medium agar plates, the spore development was also inhibited both in quantity and in pathogenicity. The treatment with AMEP412 (0.0125, 0.025 and 0.05 mg/mL) was associated with a decrease in the spore number by 78.13%, 88.98% and 95.72%, and the lesion area on rice leaves by 38.2%, 58.0% and 63.0%, respectively. Under microscopic observation, the aerial hyphae showed irregular external shape and the spores showed abnormal transverse septum. Fluorescence labeling revealed that AMEP412 localized on the surface of aerial hyphae and the septum of spores, indicating that the membrane integrity and permeability were probably disrupted. The predicted structure model of AMEP412 displayed obvious positive net charge and hydrophobic face, which supported the interaction between AMEP412 and M. oryzae. Finally, the pathogenicity test on rice seedlings showed that AMEP412 (0.05 mg/mL) reduced the lesion area by 90.8%. Our results revealed that AMEP412 had antifungal activity against M. oryzae in vivo and in vitro, and could be developed as an antifungal agent in the control of rice blast.
Introduction
China is the world’s largest rice producer and more than 60% of the Chinese population consumes rice as a staple food [Citation1, Citation2]. However, rice production is challenged by numerous viral, bacterial and fungal diseases. Among these, rice blast disease is considered as the most serious threat [Citation3]. Rice blast is mainly caused by Magnaporthe oryzae, which infects the aerial parts of the rice plant at different growth stages and affects both quality and yield. Rice blast leads to 10–30% losses in the rice grain yield annually and complete loss (100%) in the years of outbreaks [Citation4, Citation5]. In China, it affects more than 3.8 million hectares of rice field and reduces the yield by 1 billion kg every year, causing huge economic losses and threatening national food security [Citation6]. Thus, the Ministry of Agriculture and Rural Affairs of China classified rice blast as a Class I crop pest [Citation7]. To manage this disease, many strategies have been employed, such as breeding resistant cultivars and the use of numerous chemical fungicides [Citation8–10]. However, due to the high pathogenic variability of M. oryzae [Citation11], rice blast still remains difficult to control even after integrating the above methods. Therefore, it is necessary to develop new methods for controlling this disease.
Nowadays, chemical fungicides like tricyclazole and isoprothiolane are still the primary agents applied in the rice filed [Citation12]. However, the use of chemical fungicides is gradually minimized by the local government due to the adverse effects on the environment and human health [Citation13]. As an alternative approach, the application of antagonistic microbes and their metabolites as biocontrol agents has gained more attention for the sustainable and environmentally friendly features [Citation14]. These bioactive agents could decrease the occurrence of fungal diseases by inhibiting the growth, reproduction, and infection of pathogenic microbes [Citation15]. Several microbial strains have been reported to suppress M. oryzae, including Bacillus velezensis ZW-10 [Citation16], Pseudomonas mosselii BS011 [Citation17], Bacillus amyloliquefaciens Rdx5 [Citation18] and Streptomyces bikiniensis HD-087 [Citation19]. Furthermore, various antimicrobial peptides (AMPs) produced by antagonistic microbes have been identified successively, such as MgAPI16 [Citation20], Iturin A [Citation21] and AFP1 [Citation22]. Based on this, more macromolecules against M. oryzae are likely to be discovered.
In a previous study, we identified a novel protein AMEP412 from Bacillus subtilis that exhibited antimicrobial activity against Streptomyces scabiei [Citation23].
The aim of the present study was to evaluate the antifungal properties of AMEP412 against M. oryzae, and to evaluate its potential as an antifungal agent against rice blast. The antifungal activity of AMEP412 against M. oryzae was determined both in agar plates and in rice seedlings. The localization of AMEP412 on the hyphae and spores of M. oryzae was monitored by fluorescent labeling. The molecular structure model of AMEP412 was constructed and analyzed to predict the underlying mechanism.
Materials and methods
Strains, culture media and rice cultivar
A M. oryzae strain, deposited in the Agricultural Culture Collection of China (ACCC 36020), was used as the pathogen. Complete medium (yeast extract 6 g/L, acid-hydrolyzed casein 3 g/L, enzyme-hydrolyzed casein 3 g/L, sucrose 10 g/L, agar 10 g/L, pH 7.2) was selected for the growth of aerial hyphae, and oats medium (oats 30 g/L, agar 10 g/L, pH 7.2) was used for the growth of conidia. Unless stated otherwise, the size of the culture plate was 60 × 60 mm. B. subtilis strain BU412, deposited in the China Center for Type Culture Collection (CCTCC M2016142), was used for preparing AMEP412 protein sample following the previous method [Citation24]. Oryza sativa cv. Nipponbare (NPB), a standard Japanese cultivar, was cultured for pathogenicity assays.
Identification of antifungal activity against M. oryzae
The antifungal activity of AMEP412 against M. oryzae was assayed using the Oxford cup method [Citation25]. Oat medium agar plates (90 × 90 mm) were coated with M. oryzae spore suspension (100 μL, 2 × 105 spores/mL), and then AMEP412 protein sample (100 μL, 0.05 mg/mL) was added into an Oxford cup on the agar plate, with 100 μL sterile distilled water as control. After incubation at 28 °C for 7 days, the inhibition zone formed on the plate was observed.
Inhibition of aerial hyphae growth
To study the effect of AMEP412 on aerial hyphae growth of M. oryzae, the mycelial growth inhibition method was adopted [Citation26]. A series of complete medium agar plates containing different concentrations of AMEP412 (0.0125, 0.025, 0.05 mg/mL) were prepared. A growth control was prepared with an AMEP412-free complete medium. Thereafter, a 0.6-cm-diameter M. oryzae block was inversely placed to the center of each agar plate. The agar plates were cultured at 28 °C for 5 days, and the colony diameters were measured and photographed. The inhibition rate was calculated using the following formula: (DC-DT)/(DC-0.6)×100, where DC is the colony diameter in the control plate and DT is the colony diameter in the AMEP412 plate. The half maximal effective concentration EC50 (concentration for 50% of maximal effect) value of AMEP412 was obtained from the inhibition curves fitted by probit regression analysis [Citation27, Citation28].
Inhibition of conidial development
To clarify the specific inhibitory effect of AMEP412 on sporulation of M. oryzae, the number and morphology of conidia were observed. First, 6-mm M. oryzae mycelium blocks were inoculated on oat medium agar plates containing different concentrations of AMEP412 (0, 0.0125, 0.025 and 0.05 mg/mL), which were cultured at 28 °C for 7 days to generate conidia. Then all the hyphae and spores on agar plates were scraped off by same amount of 0.1% Tween 20, separately. The hyphae were removed through cloth filter to gain spore suspension. The spore morphology was observed under a compound microscope (Olympus BX63), while the spore number was calculated using blood cell counting plate.
The pathogenicity of above spores was tested on detached rice leaves. The middle segments (7 cm long) of rice leaves were obtained and three wounds were made by stabbing the main vein with the narrow end of a 1-mL pipette tip on three layers of filter paper. The leaves were then incubated in 6-Benzylaminopurine (6-BA) solution (1 μg/mL) for 1 day in the dark at 28 °C. Next, 5 μL of M. oryzae spore suspension (2 × 105 spores/mL) containing 0.05 mg/mL AMEP412 was dripped onto the wounds. After 1 day of dark culture and 6 days of 12-h light/12-h dark culture at 28 °C, the lesion areas on the leaves were assessed.
Fluorescence localization assay
For localization observation, AMEP412 was labeled using fluorescein isothiocyanate (FITC) as previously described [Citation24]. Briefly, AMEP412 protein was incubated with FITC in carbonate buffer (0.05 mol/L, pH 9.0) for 12 h at 4 °C. The resultant mixture of FITC and protein was applied to an equilibrated Superdex 75 10/300 GL column and eluted with 20 mmol/L Tris-HCl (pH 7.5) to remove the free FITC molecules. Then the FITC-protein complex was incubated with the hyphae and spores suspension of M. oryzae (without AMEP412 treatment) at 28 °C for 2 h. The binding of the FITC-protein complex to hyphae and spores was observed using a confocal laser scanning microscope (Leica TCS SP8) at an excitation wavelength of 495 nm.
Spatial structure prediction and analysis of AMEP412
To further understand the mechanism underlying the interaction between AMEP412 and M. oryzae, the spatial structure of AMEP412 (WP_017418614.1) was predicted by AlphaFold2 [Citation29]. The three-dimensional structure was analyzed and displayed using Pymol to reveal the details [Citation30], including the polymer and monomer morphology, the distribution of positively charged and negatively charged amino acids, and the hydrophobic core.
Biocontrol effect on rice seedling
To further understand the biocontrol effect of AMEP412 on M. oryzae, the pathogenicity test on rice seedlings was performed [Citation31]. Rice plants were grown in a greenhouse (with 12-h light photoperiod at 30 ± 4 °C) using plastic pots with field soil (Institute rice field, Shenzhen, China, pH 6.5). Seedlings at the four-leaf stage were sprayed with M. oryzae spore suspension (2 × 105 spores/mL) containing 0.05 mg/mL AMEP412, with spore suspension as control. Each plant received 1 mL of the suspension. The seedlings were coated with nylon membrane to maintain high humidity. After 1 day of dark culture and 6 days of 12-h light/12-h dark culture at 28 °C, the lesion areas on the leaves were assessed.
Statistical analysis
All the above experiments were repeated three times, and consistent data were observed. Statistical analyses were performed using SPSS (version 19.0; SPSS Inc). All data were expressed as means ± standard deviation (SD). Means were compared among the groups by one-way analysis of variance (ANOVA), followed by pairwise comparisons of groups using least significant difference (LSD) tests. Differences were considered statistically significant at the level of p < 0.05.
Results
AMEP412 exhibited antifungal activity against M. oryzae
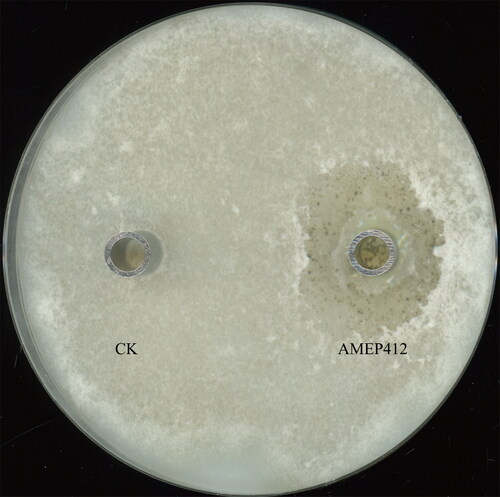
The antifungal activity of AMEP412 against M. oryzae was first determined. As shown in , AMEP412 (0.05 mg/mL) treatment led to a visible inhibition zone (diameter of 32.845 ± 0.99 mm) on the agar plate. The growth of aerial hyphae was totally inhibited in the center and partially suppressed in the outer region, suggesting that AMEP412 had antifungal activity against M. oryzae.
AMEP412 inhibited the growth of aerial hyphae
To further evaluate the antifungal activity of AMEP412 against aerial hyphae, agar plates with different concentrations of AMEP412 in complete medium were prepared for the incubation of M. oryzae blocks. AMEP412 induced a significant difference in colony growth from day 2 to day 5. As the AMEP412 concentration increased, the colony growth was inhibited to a greater degree (). Without AMEP412 treatment, M. oryzae had vigorous growth, compact morphology, fast expansion rate, dense aerial hyphae, and white pigment secretion. With AMEP412 treatment, M. oryzae showed abnormal colony morphology, slow expansion rate, sparse aerial hyphae, and poor pigment secretion. As shown in , compared with control, the colony diameters showed significant difference from day 2 to day 5 on all AMEP412 treated agar plates. After 5 days of incubation, the assayed concentrations (0.0125–0.05 mg/mL) of AMPE412 resulted in inhibition rates ranging from 21.25 to 54.40% (). Based on the inhibition curve fitted by probit regression analysis, the EC50 value of AMEP412 was calculated as 0.041 mg/mL. These results indicated that AMEP412 had a significant inhibitory effect on the growth of aerial hyphae.
Figure 2. Inhibition of M. oryzae aerial hyphae growth by AMEP412. M. oryzae mycelium blocks were grown on complete medium agar plates containing different concentrations of AMEP412 at 28 °C for 5 days.
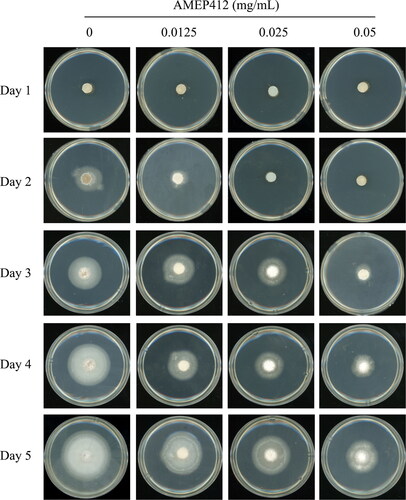
Table 1. Colony diameter (cm) of M. oryzae grown on complete medium containing different concentrations of AMEP412 at 28 °C for 5 days.
Table 2. Radial growth inhibition of M. oryzae after 5 days of incubation and EC50 value.
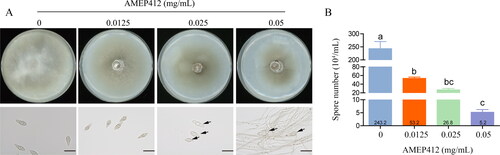
AMEP412 inhibited the development of conidia
The development of M. oryzae conidia has a significant effect on its pathogenicity [Citation32]. To determine whether AMEP412 affected spore development, oat medium agar plates containing different concentrations of AMEP412 (0, 0.0125, 0.025, and 0.05 mg/mL) were used for culture of conidia. As shown in , the colony growth was significantly inhibited by AMEP412. With the increase of AMEP412 concentration, the colony diameter decreased in gradient, which was consistent with the results on complete medium agar plates (). Moreover, the spore development was affected by AMEP412 (0.025 and 0.05 mg/mL). Deformed spores were observed, which had abnormal transverse septa and irregular external shapes. AMEP412 also severely affected the sporulation of M. oryzae. As the AMEP412 concentration increased (0.0125, 0.025, and 0.05 mg/mL), the number of spores decreased by 78.13%, 88.98%, and 95.72%, respectively (). These results indicated that AMEP412 had inhibitory effects on the development of the conidia, including the production rate and morphological development.
Figure 3. Inhibition of M. oryzae conidia development by AMEP412. M. oryzae mycelium blocks were grown on oat medium agar plates containing different concentrations of AMEP412 at 28 °C for 7 days. Growth phenotype (A); spore morphology (B). Scale bar = 20 μm. Deformed spores were indicated by black arrows. Data are mean values from three replicates with SD.
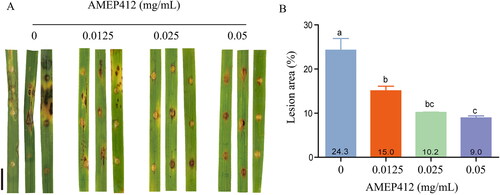
AMEP412 inhibited the pathogenicity of conidia
To further evaluate the antifungal activity of AMEP412 against M. oryzae conidia, the pathogenicity of AMEP412-treated spores was determined by treating detached rice leaves. Compared to the control group (0 mg/mL AMEP412 treatment), the lesion area caused by AMEP412-treated conidia (0125, 0.025, and 0.05 mg/mL) decreased by 38.2%, 58.0%, and 63.0%, respectively (). These results indicated that AMEP412 not only impaired the conidial development in terms of the number of spores and their morphology, but it also reduced the pathogenicity of the conidia against rice leaves.
Figure 4. Inhibition of M. oryzae conidia pathogenicity by AMEP412. AMEP412 treated spores were inoculated on detached rice leaves and cultured at 28 °C for 7 days. Lesion symptoms on detached leaves (A); quantitative analysis of lesion area (B). Scale bar = 1 cm. Data are mean values from three replicates with SD.
AMEP412 localized on aerial hyphae and spores
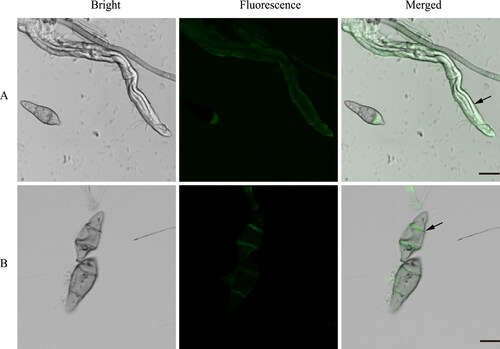
To further understand the mechanism underlying the interaction between AMEP412 and M. oryzae, FITC-labeled AMEP412 protein samples were incubated with M. oryzae aerial hyphae and spores. After incubation for 2 h, a large number of aerial hyphae and spores emitted green fluorescence (). The magnified photographs showed that AMEP412 localized to the mycelium and led to irregular bulging and wrinkling (). The cytoplasm and septa of spores were stained with green fluorescence, and some of them exhibited abnormal appearance (), indicating the possible mechanism of action. These results suggested that AMEP412 inhibited the growth of M. oryzae by impairing the integrity and permeability of the cell wall of the hyphae and spores.
Molecular structure prediction and analysis of AMEP412
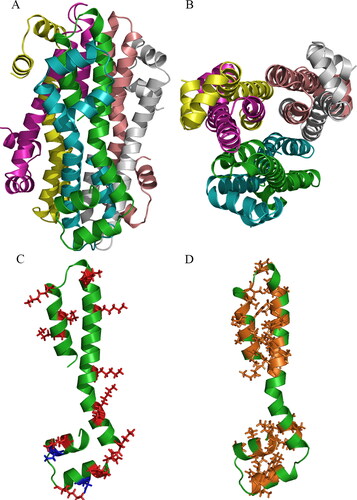
Comparing the calculated and apparent molecular weight of AMEP412 [Citation24], a hexamer structure of AMEP412 was modeled by AlphaFold2. With the help of Pymol software, the structure details were visualized in . The complex was composed of six AMEP412 polypeptides, in which each color represented a single peptide (). Furthermore, two peptides bound in a head-to-tail style and repeated three times, forming a hollow α-helix barrel (). The positively charged (13, colored in red) and negatively charged (2, colored in blue) amino acids distributed in the outer aquatic environment (), while the hydrophobic amino acids (38, colored in orange) gathered in the inner part to form a hydrophobic core (). The predicted AMEP412 polymer had a large amount of positive net charge and hydrophobic face, which facilitated the attachment and insertion to the cell membrane [Citation33, Citation34].
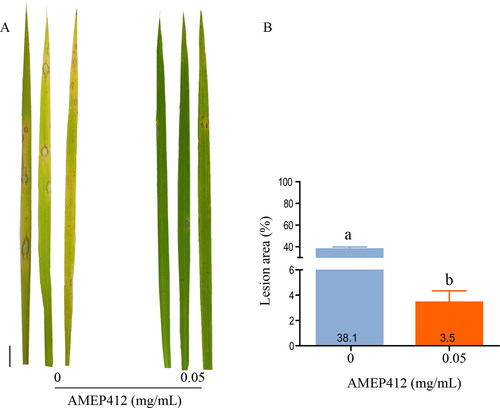
AMEP412 reduced blast symptoms on rice leaves
To further explore the biocontrol potential of AMEP412 on M. oryzae, rice seedlings were treated with a spore suspension containing 0.05 mg/mL AMEP412 for pathogenicity testing. As shown in , the disease symptoms were significantly controlled by AMEP412, where the lesion area decreased by 90.8% on rice leaves. This demonstrated that AMEP412 could effectively suppress the disease symptoms caused by M. oryzae on rice seedlings. Thus, AMEP412 could be promising for development as a biocontrol agent against rice blast.
Figure 7. Biocontrol effect of AMEP412 against M. oryzae on rice seedlings. M. oryzae spore suspension was sprayed on rice seedlings and cultured at 28 °C for 7 days. Lesion symptoms on rice leaves (A); quantitative analysis of lesion area (B). Scale bar = 1 cm. Data are mean values from three replicates with SD.
Discussion
In China, the annual application of chemical fungicides for controlling rice blast has not only led to an increase in the resistance of pathogens, but also has caused harm to the environment and human health [Citation35]. For the high quality and sustainable development of agriculture, the Chinese government has published serials of policies to encourage the replacement of chemical fungicides by biofungicides [Citation36]. In this context, the identification and development of new biofungicides are highly valued. AMEP412 is a novel antimicrobial protein isolated from B. subtilis, a common microbe used as a biocontrol agent. Moreover, the live bacteria and by-products also have proper antifungal activity [Citation37, Citation38]. Thus, the development of AMEP412 as a biofungicide has innate advantages on safety and efficacy.
As M. oryzae is the causal agent of rice blast, its infection process has been well clarified [Citation39, Citation40]. When attached to the rice leaf, the conidium can sense the hydrophobicity of the cuticle and consequently form an appressorium with a hard and hydrophobic surface, which can penetrate through the leaf cuticle. Once incide the cell, hyphae multiply rapidly and then lead to disease development and visible blast symptoms. It has been reported that either inhibiting the growth of aerial hyphae or disturbing conidial development can suppress disease caused by M. oryzae [Citation41–43]. This study showed that AMEP412 had antifungal activity against both the growth of aerial hyphae and the formation of conidia, which guaranteed its application effect at different developmental stages of M. oryzae. Moreover, AMEP412 successfully controlled blast symptoms on rice seedlings at the concentration of 0.05 mg/mL. In our previous study, the AMEP412 concentration in the fermentation product was optimized to as high as 2.39 mg/mL [Citation44], so the product could be diluted up to about 40-fold for field application, which was acceptable for developing a biocontrol agent.
Recent research has shown that cell wall integrity is a key factor in the pathogenicity of M. oryzae [Citation45]. In this study, the fluorescence localization assays showed that both aerial hyphae and conidia were stained with a FITC-AMEP412 complex. The hyphae exhibited abnormal swelling and lysis, while the conidia exhibited structural malformation. Each normal conidium is separated by two septa into three single-nucleated cells, and the division of these cells is tightly linked to the infection process [Citation46–48]. Notably, after treatment with AMEP412, obvious green fluorescence was observed in the two septa, suggesting that the barrier function was destroyed. Subsequently, the division and infection ability of the conidia were both suppressed, as indicated by the decreased number of spores and impaired pathogenicity.
It appears that the mechanism of M. oryzae inhibition is rather complex. Chemical fungicides usually inhibit the development and pathogenicity of M. oryzae by interrupting the inner metabolic pathways. For example, tricyclazole inhibits the formation of melanin and isoprothiolane inhibits the transmethylation of phospholipid choline [Citation49, Citation50]. AMPs mainly impair the integrity of biological membranes by forming water-permeable and transmembrane-oriented pores. The electrostatic forces attract the cationic AMPs to the negatively charged surface, and the hydrophobic interaction further facilitates the insertion [Citation51]. In this study, the molecular structure model revealed that six AMEP412 peptides formed a stable α-helix barrel with noticeable cationic and amphipathic features, which well explained the fluorescent localization and pathogenicity test results.
In addition to the role of AMPs, AMEP412 could also interact with plants as a protein elicitor. In our previous studies, AMEP412 exhibited the ability of triggering plant defense response and promoting resistance against biotic [Citation24] and abiotic [Citation52] stresses. Induced resistance has been proved as an effective strategy for the biocontrol of rice blast [Citation53]. Therefore, an attempt was made to determine whether AMEP412 could reduce the disease symptoms caused by M. oryzae through eliciting the innate resistance of rice seedlings, which provided the potential of enhancing the biocontrol effect of AMEP412 through an additional approach.
Limitations
This study demonstrated the antifungal activity of AMEP412 against M. oryzae. However, there are still a few problems to be elucidated in subsequent research. For example, the specificity and control range of AMEP412 could be examined by introducing more fungal strains which cause rice blast. A comparison test with known fungicides could be executed to further evaluate the effectiveness of AMEP412. For better understanding the antifungal mechanism, the expression of defense gene in M. oryzae should also be determined by transcriptome analysis and qRT-PCR verification.
Conclusions
In a previous study, we reported on the antimicrobial activity of AMEP412 against S. scabiei, a pathogen of potato common scab. Here, we characterized the antifungal effect of AMEP412 on the rice blast pathogen M. oryzae. AMEP412 significantly inhibited the growth of aerial hyphae, and disrupted the quantity, morphology and pathogenicity of spores. The fluorescent localization assay suggested that AMEP412 may destroy the cell membrane integrity and permeability, leading to deformed aerial hyphae and spores. The molecular structure model displayed cationic and amphipathic features, which further demonstrated the above results. The pathogenicity assay on rice seedlings confirmed the disease prevention effect of AMEP412, which could be developed as an ideal fungicide for the biocontrol of rice blast.
Authors’ contributions
JY carried out experiments. MG analyzed data. QW and QL designed research. JY and QL wrote the manuscript. QW and QL acquired the funding. MG and QL revised the paper. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors thank the reviewers, whose comments and suggestions helped us to improve this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data that support the findings of this study are available from the corresponding author (QW and QL) upon reasonable request.
Additional information
Funding
References
- Devanna BN, Jain P, Solanke AU, et al. Understanding the dynamics of blast resistance in rice-Magnaporthe oryzae interactions. J Fungi (Basel). 2022;8(6):584. doi: 10.3390/jof8060584.
- Huang M. The decreasing area of hybrid rice production in China: causes and potential effects on Chinese rice self-sufficiency. Food Sec. 2022;14(1):267–272. doi: 10.1007/s12571-021-01199-z.
- Younas MU, Wang G, Du H, et al. Approaches to reduce rice blast disease using knowledge from host resistance and pathogen pathogenicity. Int J Mol Sci. 2023;24(5):4985. doi: 10.3390/ijms24054985.
- Wilson RA, Talbot NJ. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol. 2009;7(3):185–195. doi: 10.1038/nrmicro2032.
- Nalley L, Tsiboe F, Durand-Moratv A, et al. Economic and environmental impact of rice blast pathogen (Magnaporthe oryzae) alleviation in the United States. PLoS One. 2016;11(12):e0167295. doi: 10.1371/journal.pone.0167295.
- Maclean JL, Dawe DC, Hardy B, et al. Rice Almanac: source book for the most important economic activity on earth. 3rd ed. Wallingford, UK CABI Pub: International Rice Research Institute (IRRI); 2002.
- Feng S, Cao Y, Xu T, et al. Rice leaf blast classification method based on fused features and one-dimensional deep convolutional neural network. Remote Sens. 2021;13(16):3207. doi: 10.3390/rs13163207.
- Zhang Q. Strategies for developing green super rice. Proc Natl Acad Sci USA. 2007;104(42):16402–16409. doi: 10.1073/pnas.0708013104.
- Hirooka T, Ishii H. Chemical control of plant diseases. J Gen Plant Pathol. 2013;79(6):390–401. doi: 10.1007/s10327-013-0470-6.
- Kongcharoen N, Kaewsalong N, Dethoup T. Efficacy of fungicides in controlling rice blast and dirty panicle diseases in Thailand. Sci Rep. 2020;10(1):16233. doi: 10.1038/s41598-020-73222-w.
- Bezerra GA, Chaibub AA, Oliveira MIS, et al. Evidence of Pyricularia oryzae adaptability to tricyclazole. J Environ Sci Health B. 2021;56(10):869–876. doi: 10.1080/03601234.2021.1971913.
- Wu X, Chen Y, Chen C, et al. Combining the microbial agent Rhodopseudomonas palustris strain PSB-06 with fungicides for controlling rice blast. Front Sustain Food Syst. 2022;6:1072156. doi: 10.3389/fsufs.2022.1072156.
- Fei L, Hao L. In vitro and ex vivo antifungal activities of metconazole against the rice blast fungus Pyricularia oryzae. Molecules. 2024;29(6):1353. doi: 10.3390/molecules29061353.
- Shen W, Liu R, Wang J, et al. Characterization of a broad-spectrum antifungal strain, Streptomyces graminearus STR-1, against Magnaporthe oryzae. Front Microbiol. 2024;15:1298781. doi: 10.3389/fmicb.2024.1298781.
- Ayilara MS, Adeleke BS, Akinola SA, et al. Biopesticides as a promising alternative to synthetic pesticides: a case for microbial pesticides, phytopesticides, and nanobiopesticides. Front Microbiol. 2023;14:1040901. doi: 10.3389/fmicb.2023.1040901.
- Chen Z, Zhao L, Chen WQ, et al. Isolation and evaluation of Bacillus velezensis ZW-10 as a potential biological control agent against Magnaporthe oryzae. Biotechnol Biotechnol Equip. 2020;34(1):714–724. doi: 10.1080/13102818.2020.1803766.
- Wu L, Xiao W, Chen G, et al. Identification of Pseudomonas mosselii BS011 gene clusters required for suppression of rice blast fungus Magnaporthe oryzae. J Biotechnol. 2018;282:1–9. doi: 10.1016/j.jbiotec.2018.04.016.
- Dong YL, Li H, Rong SH, et al. Isolation and evaluation of Bacillus amyloliquefaciens Rdx5 as a potential biocontrol agent against Magnaporthe oryzae. Biotechnol Biotechnol Equip. 2019;33(1):408–418. doi: 10.1080/13102818.2019.1578692.
- Law JWF, Ser HL, Khan TM, et al. The potential of streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front Microbiol. 2017;8:3. doi: 10.3389/fmicb.2017.00003.
- Rebollar A, López-García B. A novel cecropin a-derived peptide as specific inhibitor of appressoria in Magnaporthe oryzae. J Plant Pathol Microbiol. 2016;07(03):336. doi: 10.4172/2157-7471.1000336.
- Ma Z, Zhang S, Sun K, et al. Identification and characterization of a cyclic lipopeptide iturin A from a marine-derived Bacillus velezensis 11-5 as a fungicidal agent to Magnaporthe oryzae in rice. J Plant Dis Prot. 2020;127(1):15–24. doi: 10.1007/s41348-019-00282-0.
- Sagehashi Y, Ashizawa T, Takaku H, et al. Defensin AFP1 inhibits appressorium formation and penetration of rice cells by the rice blast fungus Magnaporthe oryzae. J Plant Pathol. 2019;101(4):1183–1186. doi: 10.1007/s42161-019-00312-8.
- Liu Q, Shen YR, Yin K. The antimicrobial activity of protein elicitor AMEP412 against Streptomyces scabiei. World J Microbiol Biotechnol. 2020;36(1):18. doi: 10.1007/s11274-019-2794-7.
- Shen YR, Li JW, Xiang JL, et al. Isolation and identification of a novel protein elicitor from a Bacillus subtilis strain BU412. AMB Express. 2019;9(1):117. doi: 10.1186/s13568-019-0822-5.
- Wang NN, Gao XN, Yan X, et al. Purification, characterization, and heterologous expression of an antifungal protein from the endophytic Bacillus subtilis strain em7 and its activity against Sclerotinia sclerotiorum. Genet Mol Res. 2015;14(4):15488–15504. doi: 10.4238/2015.November.30.27.
- Miao J, Zhao G, Wang B, et al. Three point-mutations in cytochrome B confer resistance to trifloxystrobin in Magnaporthe oryzae. Pest Manag Sci. 2020;76(12):4258–4267. doi: 10.1002/ps.5990.
- Chen Y, Yao J, Wang WX, et al. Effect of epoxiconazole on rice blast and rice grain yield in China. Eur J Plant Pathol. 2013;135(4):675–682. doi: 10.1007/s10658-012-0104-4.
- Zhou AA, Li RY, Mo FX, et al. Natural product citronellal can significantly disturb chitin synthesis and cell wall integrity in Magnaporthe oryzae. J Fungi (Basel). 2022;8(12):1310. doi: 10.3390/jof8121310.
- Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2.
- DeLano WL. The PyMOL molecular graphics system; 2002. http://www.pymol.org.
- Fan JB, Bai PF, Ning YS, et al. The monocot-specific receptor-like kinase SDS2 controls cell death and immunity in rice. Cell Host Microbe. 2018;23(4):498–510.e5. doi: 10.1016/j.chom.2018.03.003.
- Tang W, Jiang H, Zheng Q, et al. Isopropylmalate isomerase MoLeul orchestrates leucine biosynthesis, fungal development, and pathogenicity in Magnaporthe oryzael. Appl Microbiol Biotechnol. 2019;103(1):327–337. doi: 10.1007/s00253-018-9456-9.
- Yu L, Li K, Zhang J, et al. Antimicrobial peptides and macromolecules for combating microbial infections: from agents to interfaces. ACS Appl Bio Mater. 2022;5(2):366–393. doi: 10.1021/acsabm.1c01132.
- Talapko J, Meštrović T, Juzbašić M, et al. Antimicrobial peptides-mechanisms of action, antimicrobial effects and clinical applications. Antibiotics (Basel). 2022;11(10):1417. doi: 10.3390/antibiotics11101417.
- Zhang Y, Yang Y, Zhang L, et al. Antifungal mechanisms of the antagonistic bacterium Bacillus mojavensis UTF-33 and its potential as a new biopesticide. Front Microbiol. 2023;14:1201624. doi: 10.3389/fmicb.2023.1201624.
- Li Z, Hu R, Zhang C, et al. Governmental regulation induced pesticide retailers to provide more accurate advice on pesticide use to farmers in China. Pest Manag Sci. 2022;78(1):184–192. doi: 10.1002/ps.6622.
- He Y, Zhu M, Huang J, et al. Biocontrol potential of a Bacillus subtilis strain BJ-1 against the rice blast fungus Magnaporthe oryzae. Can J Plant Pathol. 2019;41(1):47–59. doi: 10.1080/07060661.2018.1564792.
- Prasanna Kumar MK, Amruta N, Manjula CP, et al. Characterisation, screening and selection of Bacillus subtilis isolates for its biocontrol efficiency against major rice diseases. Biocontrol Sci Techn. 2017;27(4):581–599. doi: 10.1080/09583157.2017.1323323.
- Yan X, Talbot NJ. Investigating the cell biology of plant infection by the rice blast fungus Magnaporthe oryzae. Curr Opin Microbiol. 2016;34:147–153. doi: 10.1016/j.mib.2016.10.001.
- Yoshida S, Koitabashi M, Nakamura J, et al. Effects of biosurfactants, mannosylerythritol lipids, on the hydrophobicity of solid surfaces and infection behaviours of plant pathogenic fungi. J Appl Microbiol. 2015;119(1):215–224. doi: 10.1111/jam.12832.
- Rebollar A, López-García B. PAF104, a synthetic peptide to control rice blast disease by blocking appressorium formation in Magnaporthe oryzae. Mol Plant Microbe Interact. 2013;26(12):1407–1416. doi: 10.1094/MPMI-04-13-0110-R.
- Spence C, Alff E, Johnson C, et al. Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol. 2014;14(1):130. doi: 10.1186/1471-2229-14-130.
- Yin ZY, Feng WZ, Chen C, et al. Shedding light on autophagy coordinating with cell wall integrity signaling to govern pathogenicity of Magnaporthe oryzae. Autophagy. 2020;16(5):900–916. doi: 10.1080/15548627.2019.1644075.
- Liu Q, Shen YR, Yin K. Optimised production of protein elicitor AMEP412 by Bacillus subtilis BU412 through response surface methodology. Biotechnol Biotechnol Equip. 2021;35(1):1058–1064. doi: 10.1080/13102818.2021.1953402.
- Feng WZ, Yin ZY, Wu HW, et al. Balancing of the mitotic exit network and cell wall integrity signaling governs the development and pathogenicity in Magnaporthe oryzae. PLoS Pathog. 2021;17(1):e1009080. doi: 10.1371/journal.ppat.1009080.
- Saunders DG, Dagdas YF, Talbot NJ. Spatial uncoupling of mitosis and cytokinesis during appressorium-mediated plant infection by the rice blast fungus Magnaporthe oryzae. Plant Cell. 2010;22(7):2417–2428. doi: 10.1105/tpc.110.074492.
- Chen Y, Wu XY, Chen CY, et al. Proteomics analysis reveals the molecular mechanism of MoPer1 regulating the development and pathogenicity of Magnaporthe oryzae. Front Cell Infect Microbiol. 2022;12:926771. doi: 10.3389/fcimb.2022.926771.
- Rogers AM, Egan MJ. Septum-associated microtubule organizing centers within conidia support infectious development by the blast fungus Magnaporthe oryzae. Fungal Genet Biol. 2023;165:103768. doi: 10.1016/j.fgb.2022.103768.
- Salman EK, Ghoniem KE, Badr ES, et al. The potential of dimetindene maleate inducing resistance to blast fungus Magnaporthe oryzae through activating the salicylic acid signaling pathway in rice plants. Pest Manag Sci. 2022;78(2):633–642. doi: 10.1002/ps.6673.
- Zhao J, Chen Y, Ding Z, et al. Identification of propranolol and derivatives that are chemical inhibitors of phosphatidate phosphatase as potential broad-spectrum fungicides. Plant Commun. 2024;5(1):100679. doi: 10.1016/j.xplc.2023.100679.
- Zhang C, Yang M. Antimicrobial peptides: from design to clinical application. Antibiotics (Basel). 2022;11(3):349. doi: 10.3390/antibiotics11030349.
- Liu Q, Wang S, Du Y, et al. Improved drought tolerance in soybean by protein elicitor AMEP412 induced ROS accumulation and scavenging. Biotechnol Biotechnol Equip. 2022;36(1):401–412. doi: 10.1080/13102818.2022.2089596.
- Omoboye OO, Oni FE, Batool H, et al. Pseudomonas cyclic lipopeptides suppress the rice blast fungus Magnaporthe oryzae by induced resistance and direct antagonism. Front Plant Sci. 2019;10:901. doi: 10.3389/fpls.2019.00901.