Abstract
Pulsed electromagnetic fields (PEMF) have been used in bone fracture healing for many years. However, it is still not clear which frequencies are more effective. Therefore, the aim of this study was to investigate the effect of single frequency of 4 Hz and a package of multiple frequencies (220 Hz, 727 Hz, 880 Hz and 10 kHz) on bone fractures of rats. Rats were randomly divided into three groups: sham, R4 and RM. A transverse osteotomy was created in the right medial tibias diaphysis of each rat under anesthesia. The right tibia of the rats in the R4 and RM groups was exposed to 4 Hz, and a package of multiple frequencies, respectively. The rats in both irradiation groups were exposed to a pulsed magnetic field with an amplitude of 10 mT for 1 h/day during 1 month under anesthesia with ketamine (90 mg/kg, i.p.) and xylazine hydrochloride (9 mg/kg, i.p.). The rats in the sham group were kept under the same experimental conditions without any field exposure. At the end of the study, the right tibia of each rat was removed and bone healing was evaluated histopathologically and radiologically, and the concentrations of some elements were measured, such as Na, Mg, K, Cr, Mn, Fe, Zn, Se, Ca and P. The results showed that 4 Hz exposure was more effective in bone fracture healing than the other frequencies in this study. Further studies need to be conducted to determine the mechanisms underlying the effect of 4 Hz PEMF.
Introduction
Studies on the effects of pulsed magnetic fields on bone fractures demonstrate that pulsed electromagnetic fields (PEMF) can significantly accelerate wound healing and enhance the repair ability of bone tissue, as an effective non-invasive method for addressing non-infected tibia union abnormalities [Citation1–3]. Some other studies indicate that PEMF play a role in bone healing with the same principles as mechanical stress applications, and this exogenous physical stimulus can promote osteogenic differentiation in several types of cells [Citation4, Citation5].
Some of the tools and equipment produced as a result of the developments prepared by the results of numerous studies on the subject have been approved by the American Food and Drug Administration (FDA) and turned into commercial products. Studies conducted for this purpose can be categorically grouped as capacitive, inductive and implant systems [Citation6]. In the capacitive system, an electric field is applied by placing metal plates (on the skin) on both sides of the fracture area. In the inductive system, the broken area is placed in Helmholtz coils and exposed to a magnetic field. In the implant system, electric current is applied to the fracture area with the help of electrodes placed on the broken bones. The application with the Helmholtz coil system is more preferred because it is both non-invasive and more practical.
Many studies have tried to explain the mechanism underlying the biological effect of low-frequency electromagnetic field. Some studies have determined that low-frequency electromagnetic waves affect the membrane potential of erythrocytes, increase oxygen transport and accelerate blood flow by widening the vessels, thus creating both a pain and edema-reducing effect [Citation7–9]. In addition, there are studies showing that these field applications reduce the infection process [Citation10]. Some studies suggest that the analgesic effect of low-frequency electromagnetic waves is due to presynaptic inhibition or a decrease in the excitability of pain fibers [Citation11]. It is also suggested that low-frequency electromagnetic waves applied in such traumas positively affect hormonal activity and antibodies [Citation12].
Recent studies have shown that pulsed magnetic fields trigger endogenous mechanisms that affect the resonance mechanism of charged carriers in the membrane and membrane potential. In these studies, voltage-gated calcium channels, which cause nitric oxide release and activation of signaling pathways, were specifically targeted [Citation13]. Pulsed electromagnetic field (PEMF) has been tested for potential use in the treatment of pathologies such as rheumatoid arthritis, knee osteoarthritis, fibromyalgia and skin ulcers [Citation14, Citation15]. PEMF is also frequently applied in the treatment of bone fractures, inflammation, arthritis, pain, edema and chronic wounds. Although there are many basic and clinical data supporting pulsed magnetic field applications as a treatment modality, scientific data regarding the therapeutic effect of whole body pulsed electromagnetic field application are not sufficient [Citation15]. In addition, studies have pointed out that treatment modalities related to electromagnetic field (EMF) applications are controversial due to the contradictory findings reported in clinics [Citation16–19]. Although the effect of EMF on osteogenesis is strongly dependent on the optimal parameters (frequency, intensity, waveforms, treatment times, etc.), it is very difficult to determine the optimal treatment parameters even in the very low frequency range (0-300 Hz). One of the main reasons for this difficulty is the insufficient information about the mechanisms of action of EMF.
In the systems used in bone fracture studies, signals that do not change in intensity over time or signals that vary over time but in a continuous mode can be applied. However, after the studies, it was concluded that applying the signal in pulsed mode is more effective and efficient. Today’s existing devices and studies are based on signals generated in pulsed mode [Citation20].
When the studies and devices developed are examined, it is seen that the frequencies used are between a few tens of Hertz and a few kilohertz. However, each study was investigated based on one or more frequencies in this frequency range. One of the most important reasons why low-frequency electromagnetic waves, which have significant effects at the cellular level, cannot be used with the desired efficiency in this area is that the selected frequencies cannot be made specific. This shows that this extremely important and safe method is still not used effectively. Therefore, this study was focused on the 4 Hz and multi-frequency PEMFs, which had not been used or were quite limited in bone fracture healing studies. Our first priority in this study was to use PEMFs with specific frequencies that can be effective in bone fracture healing, which can affect bone tissues and cells, and to develop a new approach to medical applications. Finally, the aim of this study was to investigate the effect of the selected frequencies on bone fractures of rats and on some elements in bone tissue.
Materials and method
Ethics statement
Experimental protocols were approved by the Dicle University Prof. Dr. Sabahattin Payzın Health Sciences application and research center animal experiments local ethics committee (Protocol Number; 2021/01, date of the approval:27/01/2021).
Animals and animal care
The experiments were performed on 21 male Sprague–Dawley rats obtained from the Medical Science Application and Research Center of Dicle University. All animals (4 months of age and weighing 272-352 g) were kept in a standard controlled environment (22 ± 1° C, 40–70% humidity, 12:12 h light: dark cycle, ventilation 10 changes/hour, sound level up to 75 dB, daytime light intensity up to 200 lux) and given rat chow and water ad libitum.
The rats were assigned into three groups (sham group, n: 7 and two experimental groups: n: 7 for each group). The rats in the experimental groups were randomly divided into two groups named R4 and RM. In the first experimental group, R4, the right tibia of the rats were exposed to 4 Hz PEMF with a magnetic field amplitude of 10 mT, while the right tibia of the rats in the second experimental group, RM, were exposed to PEMF with multi frequencies, consecutively, 220 Hz, 727 Hz, 880 Hz and 10 kHz, with a magnetic field amplitude of 10 mT for 1 h/day (7 days in a week) during 1 month in a Plexiglas cage under general anesthesia with ketamine (90 mg/kg, i.p.) and xylazine hydrochloride (9 mg/kg, i.p.) (). For the sham group, the experimental procedure was similar: the rats were kept in a Plexiglas cage identical to that for the exposed group, but the PEMF generator was turned off.
PEMF exposure and experimental design
In this study, a solenoid with about 500 turns and 2 cm height was used as coil to create the magnetic field. The block diagram of the pulsed electromagnetic field (PEMF) system is shown in .
Figure 2. Block diagram of the control cards of PEMF devices used in this study. 4 Hz single frequency(a), and AD9850 based multifrequency oscillator systems (B).
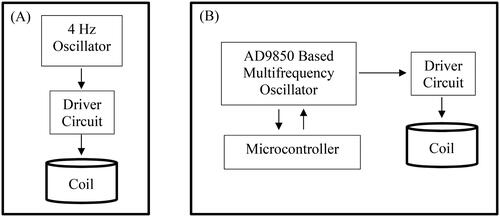
As seen in , the coil was driven by using an oscillator and a driver circuit. Two sets of PEMF devices were designed and used in this study. In the first set, a square wave oscillator adjusted to a single frequency of 4 Hz was used and the coil was driven with this signal during operation. In the second set, a combination of frequencies, 220 Hz, 727 Hz, 880 Hz and 10 kHz, were used to drive the coil, consecutively, with a burst frequency of 10 Hz. Each frequency was applied for 10 min, with the total exposure time adjusted to 1 h for the complete set, similar to the protocol of the 4 Hz group. An AD9850 DDS signal generator and a microcontroller-based control card were used to generate the signals and to execute the protocol as outlined. The amplitude of the signals was adjusted to create an average magnetic field of 10 mT in the coil. The coils were placed vertically on the right feet of the rats (in the area of the tibia). The magnetic field intensities were measured once per week as an average of 10 mT in different seleneoids of by using a Bell 7030 Gauss/Teslameter (F.W. Bell Inc, Orlando, FL, USA) to ensure homogenity of the field during the course of the experiment. All field measurements were performed by persons not involved in the animal experiments. Observers were not aware of which group of rats was PEMF or sham-exposed, i.e. the whole study was performed blind. At the end of the 1-month PEMF exposure, the rats in the whole group were sacrificed by exsanguination under ketamine anesthesia (90 mg/kg, i.p.) and xylazine hydrochloride (9 mg/kg, i.p.) and, in order to maintain anesthetic depth, supplemental ketamine (50 mg/kg, i.p.) was administered considering the reflex responses; then the right tibia were removed for histopathologic and element analysis.
Surgical technique
Tibial osteotomy is a model for fracture healing. Since there are many similarities between human and rat bone (cellular and tissue levels, trabecular bone, etc.), the rat tibia is used as a nonhuman skeletal site to measure bone haling changes. As previously described [Citation21], a fracture was created with transverse osteotomy (open surgery) in each rat’s right medial tibial diaphysis under ketamine (90 mg/kg, i.p.) and xylazine hydrochloride (9 mg/kg, i.p.) general anesthesia. After osteotomy, intramedullary pinning was retrogradly applied by moving along the proximal portion of the bone fracture. Postoperatively, anteroposterior and lateral radiographs were taken to confirm the transverse fracture in the midshaft of the tibia. The contralateral left tibia was left intact.
Histopathological analysis
Histopathological evaluation was performed under a Zeiss brand Axiolab 5 model light microscope. All broken and intact tibiae and muscle tissues in the right and left fracture area were fixed in 10% (v/v) buffered formalin solution. After a 24-hour fixation period, the bone tissues were decalcified in 10% formic acid for 24 h, the screws were removed from the right tibia, and after tissue follow-up, all samples were embedded in paraffin. A 4-µm thick section was taken from a Leica brand rotary microtome and stained with hematoxylin and eosin (H&E). Fracture healing was evaluated by scoring out of 10 points. A histopathological healing scale was used () [Citation22]. Slides were evaluated by a pathologist to ensure standardization. In pairwise group comparisons, a decrease in the score was determined as a negative effect on fracture healing, while an increase was considered a positive effect. Inflammatory activity in the fracture-healed tissue was scored according to complete filling of the 10-point objective. Inflammatory cells were recorded. The number of vascular structures observed with a 10-gauge objective in the fracture-healed tissue was recorded.
Table 1. Histopathological scoring for the assessment of fracture healing.
Element analysis of tibia
The determination of element levels in this research was conducted at the Science and Technology Application and Research Centre of Dicle University (DÜBTAM) laboratories. Except for Calcium (Ca) and Phosphorus (P), the other elements of interest were quantified with a Model 7700x inductively coupled plasma mass spectrometer (ICP-MS) (Agilent, USA). Samples of weighed tibia were exposed to microwave digestion with 3 mL of HNO3 and 1 mL of H2O2 for each sample by use of CEM Mars Xpress (Matthews, NC, USA) in accordance with a previously published procedure [Citation23, Citation24]. Ultrapure water (Merck Millipore Direct-Q8, Germany) with a resistivity of 18MΩ cm was used to prepare the solutions for the experimental study. Acetylene (Cas no.: 74-86-2) and Argon gas (Cas no.: 7440-37-1) which had 99.999% purity were obtained from a provincial local company. We utilized previously proposed operating parameters of the ICP-MS system to determine elements in the biological matrix. The instrument repeated the calibration two times during all analysis. Each sample was analyzed three times by one injection. The helium mode was employed for the quantification of all elements detected using the ICP-MS instrument. Internal Standard Mix was purchased from Agilent (Lot#:20-40VYY2/USA). As an internal standard,45Scandium (Sc) was used for Sodium (Na), Magnesium (Mg), Potassium (K), Chrome (Cr), Manganese (Mn), Iron (Fe), while 72Germanium (Ge) was for Zink (Zn) and Selenium (Se). [Citation25]. Calcium was determined by a model 240FS flame atomic absorption spectrophotometer (AAS) (Agilent, USA). The phosphorus concentration was assessed using the molybdenum blue phosphorous method and an ultraviolet (UV)-visible spectrophotometer (model 160 Shimadzu, Japan) operating at 830 nm. This is a sensitive method for the determination of phosphorus. The element levels detected at high concentrations were expressed in milligrams per gram (mg/g), while elements detected at low concentrations were expressed in micrograms per kilogram (μg/kg, ppb). Calibration standard solutions (Multi-element calibration Standard 2 A, Agilent Technology) were used at the concentration of 100 mg/L elements (Na, Mg, K, Cr, Mn, Fe, Zn and Se, with 4% (v/v) HNO3). One of the other standards used for the analysis was calcium (Ca) analytical standard, 1.000 g/L Ca+2 in hydrochloric acid (Merck, Germany). Nitric acid (HNO3, 65% v: v) and hydrogen peroxide (H2O2, 30% v:v) were purchased from Merck (Darmstadt, Germany).
Radiologic analysis of tibia
Radiography of the animals was performed under general anesthesia (ketamine (90 mg/kg, i.p.) and xylazine hydrochloride (9 mg/kg, i.p.) on the 1st and 28th days when the experimental model was created. Bidirectional radiography (40 kV, 100 mA and 2.20 mAS) of the rats, craniocaudal and mediolateral, were taken. Radiographic images were taken with a fixed x-ray device (EPX-F5000 120 KV X-Ray) with a power of 5 kW, 100 kV/100 mA and images were digitally transferred (Fujifilm T2 CR).
The radiologic images of the tibia were scored by a modified radiological union scale for tibia (mRUST), which numerically evaluates progression to union after intramedullary (IM) nailing of tibia fractures [Citation26]. mRUST scoring system is a validated metric for evaluating bony healing in humans and animals utilizing plain radiographs, which are relatively inexpensive and do not require animal sacrifice [Citation27].
Data analysis
Data are presented as mean values and standard deviations. SPSS 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of the differences between exposed samples and controls. Shapiro-Wilk test was used for normal distribution. Data were analyzed by Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks and post hoc multiple comparison tests using a Tukey-HSD procedure. All hypothesis tests used a criterion level of p = 0.05.
Results
Histopathologic assessment of the tibia
Statistical evaluation regarding histopathological scoring is seen in . According to the histopathological scoring regarding bone fracture healing, we observed that the 4 Hz EMF exposure had more pronounced potential to improve bone fracture healing according to the sham group (p = 0.000) () (). However, we did not observe a statistical difference in the healing of bone fractures between the multiple frequency PEMF and sham groups (p = 0.069) (, ). Considering the healing in bone fractures of rats, it was also observed that 4 Hz was more effective than multi-frequency PEMF (p = 0.004) (, ).
Figure 3. Histopathologic image of rat tibia, H&E stain, X100, (a)- sham group with bone healing score 5 (predominantly chondroid tissue with slightly immature bone). (B, C)-RM group with bone healing score 6 (Equal amounts of chondroid and immature bone); (D) -R4 group with bone healing score 10 (healing with mature bone). Scale bar = 100µm (The rats were assigned into three groups (sham group, and two experimental group named R4 and RM). In the first experimental group, R4, the right tibia of the rats were exposed to 4 Hz PEMF with a magnetic field amplitude of 10 mT, while the right tibia of the rats in the second experimental group, RM, were exposed to PEMF with multi frequencies, consecutively, 220 Hz, 727 Hz, 880 Hz and 10 kHz, with a magnetic field amplitude of 10 mT for 1 h/day (7 days in a week) during 1 month).
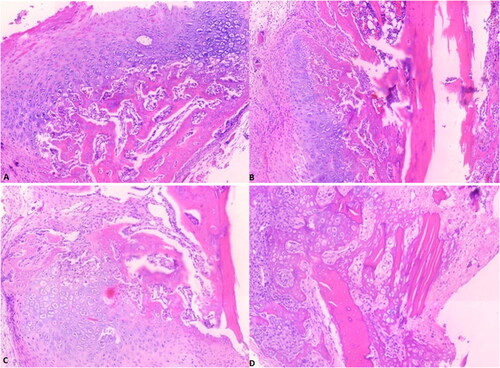
Table 2. Statistical analysis of histopathological scoring for evaluation of bone fracture healing in tibia. One-way analysis of variance and Tukey HSD multiple comparison test were applied. Data shown in the table are expressed as means ± SD.
Radiologic evaluation of the tibia using mRUST score
Three orthopedic surgeons determined an mRUST score for each X-ray image. The images were presented in random order, and surgeons were blinded to the time of radiograph. The mRUST assigns an integer score to each cortex imaged on the AP (medial and lateral cortices) and lateral (anterior and posterior cortices); mRUST score for each X-ray image as follows; 1 = no healing; 2 = callus present, no bridging; 3 = bridging callus, fracture line visible; 4 = bridging callus with no fracture line visible [Citation27]. Statistical analysis of mRUST scoring for fracture healing in the tibia bone is shown in . According to the radiological mRUST scoring performed for the tibia, 4 Hz and multiple frequency PEMFs exposure improved the bone fracture healing compared to the sham group. In the statistical comparison made according to the sham group, the bone healing in the 4 Hz PEMF exposure group was found to be significant (p = 0.005) (, ). However, statistically significant difference was not observed between the multifrequency PEMF and sham group (p = 0.113) (, ). Additionally, statistically significant difference was not observed between the 4 Hz and multifrequency PEMF group in terms of bone fracture healing (p = 0.376) (, ). The radiographical results also indicated that 4 Hz exposure was more effective than other groups in terms of bone fracture healing.
Figure 4. Representative radiography of a rat belonging to the sham group (A) and to the RM group (B) and the R4 group (C). The rats were assigned into three groups (sham group, and two experimental group named R4 and RM). In the first experimental group, R4, the right tibia of the rats were exposed to 4 Hz PEMF with a magnetic field amplitude of 10 mT, while the right tibia of the rats in the second experimental group, RM, were exposed to PEMF with multi frequencies, consecutively, 220 Hz, 727 Hz, 880 Hz and 10 kHz, with a magnetic field amplitude of 10 mT for 1 h/day (7 days in a week) during 1 month.
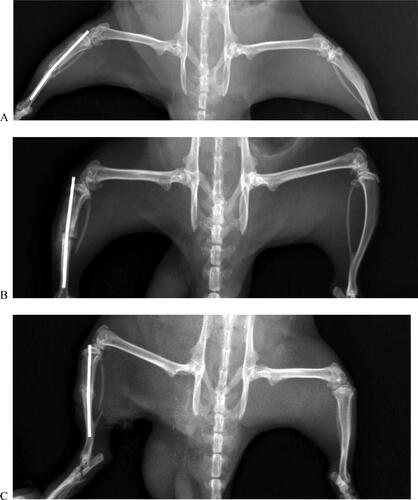
Table 3. Results of mean modified radiologic union scale for tibia fractures (mRUST) and statistical analyses for whole groups. One-way analysis of variance and Tukey HSD multiple comparison test were applied. Data shown in the table are expressed as means ± SD.
Elemental analysis of the tibia
In order to investigate the effect of the 4 Hz and multifrequency PEMF on the levels of some elements, Na, Mg, K, Cr, Mn, Fe, Zn, Se, Ca and P, in the tibia bone tissue, an elemental analysis was performed at the end of the study. The statistical analysis showed that there was no statistically significant change in the Cr, Mn, Fe, Se, Ca and P levels in both experimental groups compared to the sham group (). However, an increase was observed in the level of P, which is an essential element for bone tissue, and this increase was very high in the 4 Hz group (). Likewise, Ca, which is another element important for ossification, increased, especially in the 4 Hz group, although it was not statistically significant (). Significant decreases in Na, Mg and Zn levels were detected in both experimental groups (). There was a significant decrease in the K level only in the RM group ().
Table 4. Elemental analysis of tibia and statistical analyses for whole groups. Data shown in the table are expressed as means ± SD. One-way analysis of variance and Tukey HSD multiple comparison test were applied. *p < 0.05, **p < 0.01 and indicate statistically significant differences from the sham group.
Discussion
While the rats in one of the experimental groups in this study were exposed to pulsed multifrequency PEMFs with a frequency range of 220 Hz to 10 kHz, the animals in the other experimental group were exposed to magnetic field with a single frequency of 4 Hz. The healing of bone fractures in the tibia of rats was evaluated histopathologically and radiographically. Additionally, elemental analysis of the tibia bone tissue was performed. As a result of the histopathological and radiographic evaluation, it was determined that the application of 4 Hz PEMF was more effective than multifrequency pulsed electromagnetic field in terms of bone healing.
Many studies have been conducted on the effect of pulsed electromagnetic fields on bone fracture healing, bone microarchitecture, osteoblast and osteoclast cells and osteoporosis. However, these studies cover a wide spectrum, from very high-frequency pulsed electromagnetic fields to very low frequency pulsed electromagnetic fields. There is no full consensus regarding the use of frequency in studies on the effect of pulsed electromagnetic fields on bone tissue. However, studies on PEMF show that signal characteristics such as waveform signal intensity and application time change [Citation28]. Many studies have found that various parameters, including frequency and treatment duration, alter cell and tissue response to PEMF therapy [Citation28]. Cai et al. [Citation29] studied the effect of pulsed electromagnetic fields and mechanical vibration on the skeletal structure in adult and elderly rats. The authors have determined that non-invasive whole-body vibration and pulsed magnetic field applications can be used in the treatment of age-related osteopenia and osteoporosis [Citation29]. Oltean-Dan et al. [Citation30] investigated the effect of high-frequency pulsed electromagnetic fields on bone fracture healing, and found that high-frequency electromagnetic field application (10 min a day for two weeks starting from the first day after the operation) increased bone consolidation (quality) in the early period of fracture healing [Citation30]. Additionally, Oltean-Dan et al. [Citation30] suggested that the molecular mechanisms underlying the effect of high-frequency pulsed electromagnetic fields on bone fracture healing in humans should be elucidated. In a study, the effect of application time and sinusoidal electromagnetic field on peak bone mass was investigated [Citation31]. In that study, after comparing the effects of different exposure times on osteogenic differentiation and mineralization of osteoblasts, it was concluded that 1.5 h per day was the optimal exposure time for 50 Hz 1.8 mT sinusoidal electromagnetic fields to increase the maximum bone mass of young rats [Citation31]. As a result of the study, the authors concluded that optimal therapeutic effects can only be achieved with optimum exposure time [Citation31]. Pulsed electromagnetic fields are claimed to increase bone mass, microarchitecture, and strength by enhancing canonical Wnt signaling-mediated bone formation in rats with spinal cord injury [Citation32]. Shao et al. [Citation32] indicated that whole body exposure to pulsed electromagnetic field significantly reduced the deterioration in trabecular and cortical bone mass and microarchitecture caused by spinal cord injury. Moreover, according to that study, it has been suggested that pulsed electromagnetic field stimulation, as an easy and non-invasive biophysical technique, could have promising therapeutic potential for osteoporosis caused by spinal cord injury in clinics [Citation32]. Cai et al. [Citation33] investigated whether pulsed electromagnetic field would change the negative effects of glucocorticoids on bone architecture, bone strength and porous implant osseointegration by improving bone anabolic functions. According to the results of the study, it was observed that low-intensity pulsed electromagnetic field therapy could partially block the harmful effects of glucocorticoids on spongy and cortical bone architecture and mechanical properties [Citation33]. With this study, Cai et al. [Citation33] claimed that PEMF could be an effective alternative for the treatment of bone disorders associated with glucocorticoids. Zhou et al. [Citation34] determined that application of 50-Hz 1.8-mT sinusoidal electromagnetic field increased the bone mass of growing mice. Another study investigated the effect of pulsed electromagnetic field on bone fracture healing as a potential treatment method. In that study, it was determined that 5 and 10 mT PEMF (15 Hz) treatment changed the biomechanical properties of bone and increased bone mineral density, serum Ca and ALP levels in animal models of bone fracture [Citation1]. Liu et al. [Citation1] found that PEMF at 5 and 10 mT can significantly accelerate wound healing and enhance the repairing ability of bone tissue. Topal et al. [Citation35] reported that heparin application caused bone loss and osteoporosis, but 0.8 mT, 7.3 Hz, 1 h a day, 28-day PEMF application reduced these effects.
In a randomized controlled study, Shi et al. [Citation36] conducted a long-term study on patients with bone fractures using PEMFs, and showed that applying PEMF significantly changed the healing rates and reduced the overall pain duration in patients with long bone fractures [Citation36]. Despite these positive results, some studies have raised some issues regarding the effectiveness of PEMF therapy. Hannemann et al. [Citation18] observed that there was no significant difference in fracture healing in the PEMF group, and according to these findings, Hannemann et al. [Citation18] suggested that there is some controversy regarding the therapeutic effects of PEMF. Additionally, the evaluation of clinical trial results on bone repair involving PEMFs raises concerns about the reliability of the results. Inconsistencies in PEMF parameters and treatment protocols used in different studies may affect the comparability of studies. Additionally, individual differences between patients and other factors before and after treatment may also affect treatment outcomes. Therefore, despite existing research demonstrating the potential benefits of PEMFs in bone fracture repair, further standardized clinical trials are needed to confirm their effectiveness. Additionally, combining PEMF with other therapeutic interventions may synergistically improve bone fracture repair outcomes. However, to take full advantage of these synergistic effects, more in-depth research on specific mechanisms and optimal application methods is required [Citation37]. This means that optimized treatment strategies, which better accommodate individual patient differences may influence the success of this treatment [Citation37]. In their systematic review and meta-analysis study, Peng et al. [Citation38] determined that PEMF increased the bone fracture healing rate, relieved fracture pain and accelerated the healing time [Citation38]. Additionally, the authors concluded that larger and better quality randomized controlled trials and preclinical studies on optimal frequency, amplitude and duration parameters are needed [Citation38]. Mikaelyan et al. [Citation39] investigated the Na/Ca exchange mechanism as a target for antitumor effect of 4 Hz pulsing magnetic field (PMF). The authors concluded that the antitumor effect of 4 Hz PMF due to activation of cGMP-dependent Na/Ca exchange, and recommended to use 4 Hz PMF therapeutic purpose in clinics only at the early age of tumorigenesis [Citation39]. Ayrepetyan et al. [Citation40] suggested that metabotropic effect of 4 Hz-EMF treated physiological solution is due to the activation of cGMP-dependent Na/Ca exchange, leading to the decrease in the number of functional active receptors in the membrane through Na-K pump-induced cell shrinkage, and to increase the receptors affinity to acetylcholine [Citation40].
The histopathological findings and radiological findings obtained in the presented study indicated that PEMF stimulated bone fracture healing. These findings are consistent with studies reporting that PEMF application increases bone fracture healing. However, we observed in this study that 4 Hz exposure was more effective than PEMF with multifrequency. According to the result of our study, it can be suggested that a single frequency application may have greater therapeutic potential in the PEMFs application on bone fracture.
One of the mechanisms of PEMF in bone fracture healing is related to ionic changes in bone tissue and stimulation of some ion channels [Citation37]. In vitro and in vivo animal studies have shown that physical PEMF application exerts its therapeutic effect on bone healing by altering voltage-gated ion channels, increasing cytosolic calcium, enhancing early vascular reaction and promoting osteoblast differentiation and maturation, and relieving pain by regulating the release of inflammatory cytokines (Reviewed in [Citation38]). Bone tissue can store high levels of a wide variety of elements. Osteocytes in the bones help regulate the levels of elements in the bone, and these elements play a vital role in physiochemical and enzymatic reactions [Citation41]. In our study, in which we investigated the roles of both 4 Hz and multifrequency PEMFs in tibia fracture healing, it was observed that both magnetic fields may have the potential to cause statistically significant changes in the levels of some elements in the tibia. The elemental analysis in this study showed that the effect of PEMFs application on bone fracture healing under the experimental conditions applied in the study could also point to some molecular mechanisms other than elemental changes in bone tissue. Therefore, it is necessary to plan new studies.
Conclusions
Exposure to PEMF stimulated bone fracture healing, but it was observed that 4 Hz may be more effective in bone fracture healing than multifrequency PEMF in this study. It was also concluded that exposure to PEMF under the experimental conditions in the present study may stimulate bone fracture healing through some molecular mechanisms other than changes in some elements in bone tissue. Further molecular studies are required to determine the underlying mechanisms regarding the effect of PEMF exposure on bone fracture healing.
Author contributions
All authors contributed significantly to the work and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MZA, SD and AG made substantial contributions to the conception and design of the work. MBA, MG, NB, FA, TB, EA, SK, RA and HK were involved in project management, literature search, data acquisition, interpretation, drafting, and reviewing of the manuscript. MZA and SD were involved in visualization. All authors read and approved the final manuscript.
Acknowledgement
The authors thank Prof.Dr. Omer Yavuz (Department of Chemistry, Faculty of Science, Dicle University) and Prof.Dr. Sadık Yayla (Department of Surgery, Faculty of Veterinary Medicine, Dicle University) for their contribution to improve the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author [SD], upon reasonable request.
Additional information
Funding
References
- Liu Y, Hao L, Jiang L, et al. Therapeutic effect of pulsed electromagnetic field on bone wound healing in rats. Electromagn Biol Med. 2021;40(1):26–32. doi: 10.1080/15368378.2020.1851252.
- Assiotis A, Sachinis NP, Chalidis BE. Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature. J Orthop Surg Res. 2012;7:24. doi: 10.1186/1749-799X-7-24.
- Caruso G, Massari L, Lentini S, et al. Pulsed electromagneticfield stimulation in bone healingand joint preservation: a narrative review of the literature. Appl Sci. 2024;14(5):1789. doi: 10.3390/app14051789.
- Victoria G, Petrisor B, Drew B, et al. Bone stimulation for fracture healing: what’s all the fuss? Indian J Orthop. 2009;43(2):117–120. doi: 10.4103/0019-5413.50844.
- Azadian E, Arjmand B, Khodaii Z, et al. A comprehensive overview on utilizing electromagnetic fields in bone regenerative medicine. Electromagn Biol Med. 2019;38(1):1–20. doi: 10.1080/15368378.2019.1567527.
- Griffin M, Bayat A. Electrical stimulation in bone healing: critical analysis by evaluating levels of evidence. Eplasty. 2011;11:e34.
- Fernandez-Cuadros ME, Perez Moro OS, Albaladejo-Florin MJ, et al. Delayed union of scaphoid fracture and effectiveness of pulsed electromagnetic fields: a case report and review of the literature. Middle East J Rehabil Health Stud. 2018;5(1):e63850. doi: 10.5812/mejrh.63850.
- Battecha KH, Soliman ES. Utilization of pulsed electromagnetic field and traditional physiotherapy in knee osteoarthritis management. IJPR. 2015;3(2):978–985. doi: 10.16965/ijpr.2015.119.
- Aktas I, Akgun K, Cakmak B. Therapeutic effect of pulsed electromagnetic field in conservative treatment of subacromial impingement syndrome. Clin Rheumatol. 2007;26(8):1234–1239. doi: 10.1007/s10067-006-0464-2.
- Tschon M, Veronesi F, Contartese D, et al. Effects of pulsed electromagnetic fields and platelet rich plasma in preventing osteoclastogenesis in an in vitro model of osteolysis. J Cell Physiol. 2018;233(3):2645–2656. doi: 10.1002/jcp.26143.
- Arokoski JP, Valta T, Kankaanpää M, et al. Activation of lumbar paraspinal and abdominal muscles during therapeutic exercises in chronic low back pain patients. Arch Phys Med Rehabil. 2004;85(5):823–832. doi: 10.1016/j.apmr.2003.06.013.
- Nguyen JV, Marks R. Pulsed electromagnetic fields for treating osteo-arthritis. Physiother. 2002;88(8):458–470. doi: 10.1016/S0031-9406(05)60848-6.
- Funk RH. Coupling of pulsed electromagnetic fields (PEMF) therapy to molecular grounds of the cell. Am J Transl Res. 2018;10(5):1260–1272.
- Ross CL, Ang DC, Almeida-Porada G. Targeting mesenchymal stromal cells/pericytes (MSCs) with pulsed electromagnetic field (PEMF) has the potential to treat rheumatoid arthritis. Front Immunol. 2019;10:266. doi: 10.3389/fimmu.2019.00266.
- Hug K, Röösli M. Therapeutic effects of whole-body devices applying pulsed electromagnetic fields (PEMF): a systematic literature review. Bioelectromagnetics. 2012;33(2):95–105. doi: 10.1002/bem.20703.
- Gupta A, Taly AB, Srivastava A, et al. Efficacy of pulsed electromagnetic field therapy in healing of pressure ulcers: A randomized control trial. Neurol India. 2009;57(5):622–626. doi: 10.4103/0028-3886.57820.
- Ryang We S, Koog YH, Jeong KI, et al. Effects of pulsed electromagnetic field on knee osteoarthritis: a systematic review. Rheumatology (Oxford). 2013;52(5):815–824. doi: 10.1093/rheumatology/kes063.
- Hannemann PF, van Wezenbeek MR, Kolkman KA, et al. CT scan evaluated outcome of pulsed electromagnetic fields in the treatment of acute scaphoid fractures: a randomized, multicenter, double-blind, placebo-controlled trial. Bone Joint J. 2014;96-B(8):1070–1076. doi: 10.1302/0301-620X.96B8.33767.
- Maziarz A, Kocan B, Bester M, et al. How electromagnetic fields can influence adult stem cells: positive and negative impacts. Stem Cell Res Ther. 2016;7(1):54. doi: 10.1186/s13287-016-0312-5.
- Gaynor JS, Hagberg S, Gurfein BT. Veterinary applications of pulsed electromagnetic field therapy. Res Vet Sci. 2018;119:1–8. doi: 10.1016/j.rvsc.2018.05.005.
- Bilgin HM, Çelik F, Gem M, et al. Effects of local vibration and pulsed electromagnetic field on bone fracture: A comparative study. Bioelectromagnetics. 2017;38(5):339–348. doi: 10.1002/bem.22043.
- Durgun M, Dasdag S, Erbatur S, et al. Effect of 2100 MHz mobile phone radiation on healing of mandibular fractures: an experimental study in rabbits. Biotech & Biotechnological Equip. 2016;30(1):12–120. doi: 10.1080/13102818.2015.1102612.
- Yuksel B, Kaya S, Kaya-Akyuzlu D, et al. Validation and optimization of an analytical method based on cold vapor atomic absorption spectrometry for the determination of mercury in maternal blood, cord blood, and placenta samples. AtSpectrosc. 2017;38(4):112–116. doi: 10.46770/AS.2017.04.006.
- Yuksel B, Kaya-Akyuzlu D, Kayaalti Z, et al. Study of blood iron vs. blood lead levels in beta-thalassemia patients in turkey: an application of analytical toxicology. AtSpectrosc. 2017;38(2):71–76. doi: 10.46770/AS.2017.02.006.
- Arica E, Yuksel B, Yener I, et al. Icp-ms determination of lead levels in autopsy liver samples: an application in forensic medicine. AtSpectrosc. 2018;39(2):62–66. doi: 10.46770/AS.2018.02.002.
- Plumarom Y, Wilkinson BG, Willey MC, et al. Sensitivity and specificity of modified rust score using clinical and radiographic findings as a gold standard. Bone Jt Open. 2021;2(10):796–805. doi: 10.1302/2633-1462.210.BJO-2021-0071.R1.
- Alentado VJ, Knox AM, Staut CA, et al. Validation of the modified radiographic union score for tibia fractures (mRUST) in murine femoral fractures. Front Endocrinol (Lausanne). 2022;13:911058. doi: 10.3389/fendo.2022.911058.
- Huegel J, Choi DS, Nuss CA, et al. Effects of pulsed electromagnetic field therapy at different frequencies and durations on rotator cuff tendon-to-bone healing in a rat model. J Shoulder Elbow Surg. 2018;27(3):553–560. doi: 10.1016/j.jse.2017.09.024.
- Cai J, Shao X, Yan Z, et al. Differential skeletal response in adult and aged rats to independent and combinatorial stimulation with pulsed electromagnetic fields and mechanical vibration. Faseb J. 2020;34(2):3037–3050. doi: 10.1096/fj.201902779R.
- Oltean-Dan D, Dogaru GB, Apostu D, et al. Enhancement of bone consolidation using high-frequency pulsed electromagnetic fields (HF-PEMFs): An experimental study on rats. Bosn J Basic Med Sci. 2019;19(2):201–209. doi: 10.17305/bjbms.2019.3854.
- Zhu BY, Yang ZD, Chen XR, et al. Exposure duration is a determinant of the effect of sinusoidal electromagnetic fields on peak bone mass of young rats. Calcif Tissue Int. 2018;103(1):95–106. doi: 10.1007/s00223-018-0396-2.
- Shao X, Yan Z, Wang D, et al. Pulsed electromagnetic fields ameliorate skeletal deterioration in bone mass, microarchitecture, and strength by enhancing canonical Wnt signaling-mediated bone formation in rats with spinal cord injury. J Neurotrauma. 2021;38(6):765–776. doi: 10.1089/neu.2020.7296.
- Cai J, Shao X, Yang Q, et al. Pulsed electromagnetic fields modify the adverse effects of glucocorticoids on bone architecture, bone strength and porous implant osseointegration by rescuing bone-anabolic actions. Bone. 2020;133:115266. doi: 10.1016/j.bone.2020.115266.
- Zhou J, Gao YH, Zhu BY, et al. Sinusoidal electromagnetic fields increase peak bone mass in rats by activating Wnt10b/β-catenin in primary cilia of osteoblasts. J Bone Miner Res. 2019;34(7):1336–1351. doi: 10.1002/jbmr.3704.
- Topal O, Çina Aksoy M, Ciriş İM, et al. Assessment of the effect of pulsed electromagnetic field application on the healing of bone defects in rats with heparin-induced osteoporosis. Electromagn Biol Med. 2020;39(3):206–217. doi: 10.1080/15368378.2020.1762636.
- Shi HF, Xiong J, Chen YX, et al. Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: a prospective randomized controlled study. BMC Musculoskelet Disord. 2013;14(1):35. doi: 10.1186/1471-2474-14-35.
- Wang A, Ma X, Bian J, et al. Signaling pathways underlying pulsed electromagnetic fields in bone repair. Front Bioeng Biotechnol. 2024;12:1333566. doi: 10.3389/fbioe.2024.1333566.
- Peng L, Fu C, Xiong F, et al. Effectiveness of pulsed electromagnetic fields on bone healing: a systematic review and meta-analysis of randomized controlled trials. Bioelectromagnetics. 2020;41(5):323–337. doi: 10.1002/bem.22271.
- Mikaelyan Y, Eloyan N, Ayrapetyan S. The Na/Ca exchange as a target for antitumor effect of 4 Hz pulsing magnetic field. Electromagn Biol Med. 2020;39(3):218–226. doi: 10.1080/15368378.2019.1685542.
- Ayrapetyan SN, Hunanyan A, Hakobyan SN. 4 Hz EMF treated physiological solution depresses Ach-induced neuromebrane current. Bioelectromagnetics. 2004;25(5):397–399. doi: 10.1002/bem.20025.
- Ulku R, Akdag MZ, Erdogan S, et al. Extremely low-frequency magnetic field decreased calcium, zinc and magnesium levels in costa of rat. Biol Trace Elem Res. 2011;143(1):359–367. doi: 10.1007/s12011-010-8855-2.

