?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
During a series of sampling conducted in Nobaria, El-Beheira, Egypt in 2022 and 2023, defoliation disorders were identified in tomato fields. Three soil samples were collected and subjected to fungal isolation and identification. Molecular analysis revealed the presence of three fungal species, namely Fusarium verticillioides, Fusarium proliferatum and Alternaria tenuissima, with their Genbank accession numbers being OP889680, OP889682 and OP886855, respectively. The efficacy of an effective microorganism (EM) product as an antifungal agent was screened and its bioactive substances were analyzed. The bioactive substances in the EM product included antioxidant compounds, such as phenolics (58 mg/mL) and tannins (17 mg/mL), which contributed to total antioxidant activity of 73% as measured by FRP, PMA and DPPH assays. The EM product was tested for its antifungal activity against the three isolated fungal pathogens. The sensitivity of A. tenuissima, F. verticillioides and F. proliferatum was assessed by measuring the colony growth when exposed to the EM product. It showed concentration-dependent growth inhibition, completely stopping growth at 7% concentration, with inhibition percentages of 93, 100 and 100% for each pathogen. Gene expression in treated fungi was studied, revealing down-regulation of the chitinase gene in F. verticillioides, F. proliferatum and A. tenuissima, compared to controls. Up-regulation of PR4 and PR5 genes was noted in F. verticillioides, while a significant up-regulation of peroxidase and PPO genes was observed in A. tenuissima. This suggests the EM product holds promise as a fungicide alternative.
Introduction
Fungal populations in soil play a crucial role in shaping soil diversity and composition, impacting nutrient availability and cycling [Citation1]. Agriculture on a global scale is faced with numerous pathogenic threats that have a severe impact on crop productivity and the quality of yields. These threats primarily arise from various pathogens such as bacteria, viruses, fungi, and nematodes [Citation2]. Of particular concern are the underground fungi, which far outnumber their above-ground counterparts [Citation3]. These soil-borne fungi have the ability to persist in the soil for extended periods owing to the development of specialized survival structures, such as sclerotia, melanized hyphae, chlamydospores, and oospores [Citation4].
Focusing on one of the most prevalent soil-borne fungus, Fusarium, it can be held responsible for a range of plant maladies including Fusarium head blight (FHB), Fusarium root rot (FRR), and Fusarium crown rot (FCR), among others [Citation5, Citation6]. The impact of this fungus extends to a diverse array of crops including grain cereals, maize, a variety of vegetables, banana, lilies, and trees. Each crop exhibits unique disease symptoms, resulting in substantial reductions in yield [Citation7, Citation8]. In addition to Fusarium, Alternaria sp. also contributes to crop losses both pre- and post-harvest. Alternaria has been identified as a major cause of post-harvest damage in tomatoes, often leading to leaf blight symptoms. Furthermore, Alternaria invasions in vegetables contribute to a late blight symptom, affecting both foliage and fruits [Citation9–12].
Tomato (Solanum lycopersicum L.) is widely recognized as one of the essential vegetable crops worldwide, valued for its versatility in both fresh consumption and various processed forms [Citation13]. In the context of Egypt, tomato production accounts for approximately 16% of total vegetable cultivation, albeit representing only 32% of the overall vegetable growth [Citation14]. Due to fungal diseases, the production of tomatoes faces significant challenges. It is noteworthy that plants employ a range of immune strategies to combat these diseases, beginning with the identification of pathogens and activation of defence signalling pathways. Subsequently, they produce antifungal substances, including pathogenesis-related (PR) proteins [Citation15].
In order to effectively control and manage the global spread of plant diseases caused by fungi, a variety of strategies are implemented. Unfortunately, the widespread use of pesticides in agriculture, aimed at combating both pre- and post-harvest pathogens, has resulted in numerous harmful outbreaks and the emergence of fungicide resistance among fungal infections [Citation16]. Additionally, these chemical pesticides leave residues that can enter groundwater, lakes, and marine sources through various environmental processes, posing a significant threat to organisms inhabiting these bodies of water [Citation12, Citation17, Citation18].
As a result, current research efforts are focused on seeking new and safe alternatives to reduce the presence of pesticide residues in soil. Natural extracts have emerged as one such alternative, offering a safer, non-toxic, and cost-effective substitute for the excessive use of synthetic chemical fungicides, thereby promoting the creation of chemical-free environments [Citation19].
Various microbial inoculants have proven effective in acting as biological control agents or bio-fertilizers to manage plant pathogens [Citation20]. Teruo Higa developed the microbial combination known as 'Effective Micro-organisms' (EM) at the University of Ryukyus in Okinawa, Japan in 1970, and it has since become a widely used microbial solution [Citation21]. EM consists of various beneficial microorganisms, including lactic acid bacteria like Lactobacillus plantarum L. casei and Streptococcus lactis. It also includes photosynthetic bacteria such as Rhodopseudomonas palustrus and Rhodobacter spaeroides, yeasts like Saccharomyces cerevisiae and Candida utilis, actinomycetes such as Streptomyces albus and S. griseus, and fermenting fungi including Aspergillus oryzae, Penicillium sp. and Mucor hiemalis [Citation22].
EM products have been widely recognized for their contributions to soil health, crop production and plant protection. Numerous studies have demonstrated the positive effects of EM application, enhanced growth and improving crop quality in various plants including tomato [Citation23], corn [Citation24] and wheat [Citation25]. Furthermore, the beneficial effects of EM in controlling plant phytopathogens have been reported, such as its role in managing Candidatus phytoplasma solani in periwinkle plants[Citation26] and controlling Phytophthora cinnamomi in lupin [Citation27].
Consequently, our research prioritized the isolation and identification of pathogenic fungi that have a detrimental impact on tomato-crops in 'Nobaria', Egypt. Additionally, we aimed to determine the bioactive substance(s) and antioxidant properties of a commercially available EM product from Japan. Furthermore, our objective was to assess the in-vitro antifungal efficacy of the EM product against three soil-borne phytopathogenic fungi (F. verticillioides, F. proliferatum and A. tenuissima) isolated from local soil. This investigation is a crucial step towards developing potential strategies for application in the affected area.
Materials and methods
Soil sampling, fungal isolation, culture media and growth conditions
A total of three representative soil samples (visibly affected by plant morphological disorders) were collected from cultivated tomato fields in El-Beheira Governorate, Egypt, specifically in the Nobaria region. The sampling process took place in 2022 and 2023 at a depth of 20 cm. The coordinates of the sampling location are GPS: (30°39′57.1″N, 30°15′07.4″E). Carefully sealed in clear bags and labelled accordingly, the soil samples were transported to the laboratory for subsequent pathogen isolation and soil analysis. The collected soil samples underwent careful air-drying and were subsequently processed to reduce the particle size to approximately 2 mm.
To maximize the likelihood of capturing fungi attached to soil particles, the isolation procedure involved diluting the soil samples. Subsequently, these diluted samples were incubated on potato dextrose agar (PDA) supplemented with 10 mg/L of chlortetracycline to inhibit the growth of bacteria. The resulting isolates were further purified on PDA at a temperature of 28 °C. Ultimately, three distinct fungal isolates were identified. These isolates were then maintained on slants at 4 °C to enable further investigations in the future.
Fungal morphological and molecular characterization
The three fungal isolates were cultivated on PDA at a controlled temperature of 28 °C ± 2 for a duration of 8 days. During this time, careful examination of the mycelia and spores was conducted under a light microscope in accordance with established identification protocols [Citation28–31].
To facilitate molecular identification, the fungi were cultured in potato dextrose broth media for a period of 6 days. Following incubation, a fungal disk was collected, and the total genomic DNA was isolated from the samples using a DNA isolation kit (Cat. No. 4992725, Tiangen Biotech, Beijing, China). The DNA samples were separated by electrophoresis on 1.0–1.2% agarose in 0.5X TBE buffer according to Sambrook and Fritsch [Citation32]. After adding 0.5 μg/mL ethidium bromide, the electrophoresis run was performed using an apparatus with a power supply and visualized by ultraviolet transilluminator and Gel documentation system (Chemi.DocTM XRS + with Image LabTM Software, BIO-RAD, USA). The purity and integrity of DNA were appropriate, and the OD260/280 was 1.82. Subsequently, the fungus DNA was subjected to PCR amplification using ITS specific primers (ITS1 – TCCGTAGGTGAACCTGCGG and ITS4 – TCCTCCGCTTATTGATATG) primers, according to White et al. [Citation33], synthesized by Shanghai-Sangon Biolo. Engin. Tech. & Ser. Co., Ltd. The 25 μL PCR reaction components contained: 12.5 μL master mix (cat. No. 2112006, Applied Biotechnology, Egypt), 1 μL DNA (30 ng), 1 μL for each primer (10 pmol⁄μL), and the volume was completed up to 25 μL with sterile H2O. The PCR program was applied using a thermocycler (MJ Research, Inc., PTC-100™ Programmable thermal controller, USA) as follows: initial denaturation at 95 °C for 2 min; 35 cycles of 94 °C for 1 min; annealing at 55 °C for 1 min; extension at 72 °C for 1 min and a final extension step at 72 °C for 5 min. Then, 5 μL of PCR products were separated via 1.5% (w/v) agarose gel electrophoresis in 0.5x TBE buffer. The molecular weight of bands was estimated using DNA marker/marker size (cat. No. 3428 A, lot.AM51847A, TAKARA, Japan). Finally, the gel was photographed using a gel documentation system. The resulting purified DNA samples were sent to Macrogen Company (Republic of Korea; https://www.macrogen.com/en/main) for sequencing analysis.
DNA sequencing and phylogenetic analysis
The DNA sequencing process was performed on the ITS PCR amplicons, and the resulting sequences were aligned using DNA Blast. Subsequently, the clean sequences were submitted to the NCBI GenBank database (https://www.ncbi.nlm.nih.gov) to obtain accession numbers. The obtained DNA sequences were then compared to numerous fungal strains of the same species available in the GenBank database.
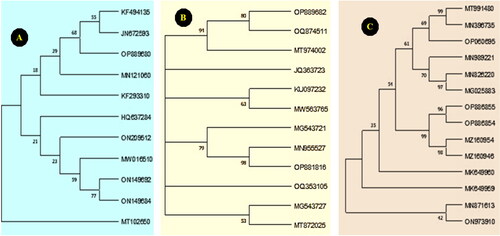
To elucidate the evolutionary relationships, phylogenetic trees were constructed using the maximum-likelihood (ML) technique in MEGA 11 software [Citation34]. This approach facilitated the visualization and analysis of the genetic relatedness among the fungal isolates.
Soil sampling and preparations
A total of three representative soil-samples were prepared for further analysis. Various soil parameters including pH, anions, cations, and total nitrogen content were determined as follows. Soil pH was determined in a 1:2.5 (w:v) suspension of soil in water using a glass electrode following the method outlined by Houba et al. [Citation35]. The soil anions and cations were analysed using a flame photometer (model PFP.7 Jenway) according to Richards [Citation36]. Soil total nitrogen was measured via combustion using a LECO analyzer (model LECO CNS 2000) according to Rashad et al. [Citation37]. The organic material in soil was analyzed according to Wang et al. [Citation38]. These analyses provided valuable information regarding the composition and nutrient status of the soil samples.
EM product bioactive substance analysis
The EM product was acquired from DIMIKRO (EM-active, Brand: DIMIKRO, DIMIKRO-Effective microorganisms Co.; https://dimikro.de/DIMIKRO), and the content of bioactive substances were analyzed. Quantitative analysis of saponin content was conducted using the method adapted from Hiai et al. [Citation39]. Additionally, the determination of tannin content was carried out utilizing the method described by Broadhurst and Jones, [Citation40].
Furthermore, the assessment of flavonoids, phenolics, ascorbic acid and total amino acids was performed in a sequential manner following the respective methods outlined in Broadhurst and Jones, [Citation40], Jindal and Singh, [Citation41], Oser and Hawk, [Citation42] and Lee and Takahashi, [Citation43]. To assess the antioxidant capacity of the EM product, three different methods were employed. These included the ferric reducing power (FRP) assay according to Oyaizu [Citation44], the phosphomolybdate assay (PMA) according to Brand-Williams et al. [Citation45] and the diphenyl-1-picrylhydrazyl (DPPH) assay following Bondet et al. [Citation46] and Jayaprakasha et al. [Citation47], as per the protocols detailed.
These evaluations provided insights into the antioxidant and free-radical scavenging properties of the EM product, shedding light on its potential benefits in terms of oxidative stress reduction and protection against harmful free radicals [Citation45, Citation46].
Gas chromatography-mass spectrometry (GC–MS) analysis
The composition of the EM product was examined using a Trace GC Ultra Gas Chromatograph-DSQ II Mass Spectrometer (CAT#: STEM-MS-0288-LGZ, Thermo-Fisher-Scientific, Waltham, USA; https://www.ste-mart.com/trace-gc-ultra-gas-chromatograph-dsq-ii-mass-spectrometer-thermo-fisher-117441.htm?gad_source=1&gclid=CjwKCAjw74e1BhBnEiwAbqOAjHUTjwNPSPv8-rTCsvtrEUGsg74dvVtqSFgVpASx-SkIBsYbsbgbQBoCpOYQAvD_BwE) and programming settings and sample preparation were completed according to Okla et al. [Citation48]. The analysis was conducted with a direct capillary column TG–5MS with dimensions of 30 m × 0.25 mm × 0.25 µm film thickness. The analytical procedure involved initially setting the column oven temperature at 45 °C, followed by a gradual increase of 5 °C/min up to 250 °C, where it was held for 2 min before further increasing to 280 °C at a rate of 10 °C/min. The injector and MS transfer line temperatures were maintained at 250 °C throughout the analysis. Helium was utilized as the carrier gas at a constant flow rate of 1 mL/min. A solvent delay of 2 min was applied, and 1 µL of diluted samples was automatically injected using an Autosampler AS1310 in the split mode configuration. Electron ionization (EI) mass spectra were recorded at 70 eV ionization energy over a mass-to-charge ratio (m/z) range of 40–600 in full scan mode. The ion source temperature was set at 200 °C.
Subsequent to data acquisition, the constituents were identified by their retention times and through comparison of their mass spectra with entries in the NIST and Wiley libraries[Citation49]. Match factors from the Xcalibur 3.0 data system of the GC/MS, including the Standard Index (SI) and Reverse Standard Index (RSI), were used as threshold values to validate the compounds. A match value of ≥650 was considered acceptable to confirm the identified compounds [Citation48, Citation50].
Antifungal efficacy
The antifungal activity of the EM product was assessed using the radial mycelial growth method. Various concentrations of EM (3.0, 2.0, 1.0, 0.5, 0.2 and 0.1%) were incorporated into potato dextrose agar (PDA) media, while the control treatment consisted of replacing EM with sterile deionized water. Each plate of PDA was inoculated with a 0.5 cm fungal disc and incubated for one week at 28 ± 2 °C. The resulting fungal radial growth was measured and compared to the control to determine the degree of inhibition. The proportion of inhibition of mycelial growth in the treatment was calculated using this formula;
(1)
(1)
Where; RGI represents the radial growth inhibition (%), C is the control pathogen radial growth (mm), and T is the treated pathogen radial growth (mm) [Citation51].
Molecular assessments
Total RNA extraction, cDNA synthesis and RT-PCR
After collecting fungal mycelium from the petri dishes, RNA samples were extracted from both untreated and treated fungi using the RNeasy Mini Kit (Cat. No. 74904, Qiagen, Netherlands) following the manufacturer's instructions. Subsequently, complementary DNA (cDNA) was synthesized through reverse transcription of 20 μL reaction-mixture consisting of 1 μL RNA, 0.3 μL reverse transcriptase enzyme (Cat. No. M0253S, M-MuLV RT, Biolabs, New England, Ipswich, MA, USA), 5 μL primer using oligo (dT) with random hexamer primer; GCACGACGCTTGTATTGCTC according to Hafez et al. [Citation52] (Cat. No. RAPD10, Shanghai-Sangon Biolo. Engin. Tech. & Ser. Co., Ltd), 2 μL dNTPs, 2 μL 5x reaction buffer (Tris-HCl, KCl, MgCl2 and dithiothreitol/DTT) provided with reverse transcriptase enzymes. The remaining volume was filled with distilled water (H2O) and sterilized. Reverse transcription was carried out in a thermocycler (MJ Research, Inc., PTC-100™ Programmable thermal controller, USA), with an initial activation cycle at 42 °C for 1 h, followed by inactivation at 95 °C for 5 min. The resulting cDNA was stored at −20 °C for future analysis.
For RT-qPCR analysis, the SYBR Green PCR Master Mix (Fermentas, USA) was used, and reactions were performed in triplicate. A 25-μL reaction mixture was prepared, consisting of 100 pmol of each primer (PR1, PR3, PR4, PR5, peroxidase and polyphenol oxidase), 12 μL SYBR green, 1 μl of template cDNA (50 ng), and H2O to finalize the volume to 25 μL (). The PCR reactions were carried out using a Rotor-Gene 6000 (QIAGEN, ABI System, USA). The amplification program included an initial denaturation step at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. The housekeeping gene beta actin (β-actin) was used as a reference gene, following the protocol described by Saleha [Citation53]. Quantification and calculation of relative gene expression levels were performed according to Livak and Schmittgen, [Citation54].
Table 1. Specific primer sequences used in RT-qPCR.
Statistical analysis
A randomized experiment was employed, and the resulting data were analyzed in triplicate using one-way analysis of variance (ANOVA) with the assistance of the software program 'CoSTAT' (VERSION 6.45; http://cohortsoftware.com/costat.html) running on a Windows operating system. The reported outcomes represent the mean values obtained from six replicated measurements, which were divided into two separate experiments with three replicates each. Statistical significance was determined at a significance level of p ≤ 0.05.
Results
Observed symptoms in the sampled plants
A comprehensive investigation was conducted in 'Nobaria', El-Beheira, Egypt during the years 2022 and 2023, defoliation-related abnormalities were identified in the tomato fields. These abnormalities were visually evident through wilting, chlorosis, early blight manifestation in tomato seedlings, and root rot, as depicted in . Given the suspected involvement of fungal pathogens in the soil, the presence of fungi in the soil samples was assessed.
Isolation and identification of the fungal isolates
Morphological identification
visually portrays the morphological features of the mycelial growth of three isolated fungi cultivated on PDA medium, as observed from the lower surface. Fusarium verticillioides () displayed the development of pink to violet pigments with age. Fusarium proliferatum () exhibited a white, cottony appearance with varying densities of mycelia. Alternaria tenuissima () could be distinguished by its characteristic dark olive colour on PDA.
Figure 2. The morphological appearance of fungal mycelia on PDA; A (Fusarium verticillioides); B, (Fusarium proliferatum), and C, (Alternaria tenuissima).

Further scrutiny was directed towards the spores of each fungal isolate. The spores of F. verticillioides, recognized as microconidia, were found to originate in lengthy chains and clusters. They were oval to club-shaped, possessed a flattened base, and were devoid of septation. These spores were produced from monophialides and, on some occasions, occurred in V-shaped pairs, resembling a configuration reminiscent of a rabbit ear. In contrast, F. proliferatum microconidia were abundant and exhibited an oval, club-shaped morphology with a thin wall. These microconidia bore a tapering and curved apical cell. Furthermore, the spores of A. tenuissima were typically present in extended chains of 3-12. They exhibited a dark brown colour and displayed an obpyriform, ovoid or elliptical shape. These spores possessed 1-6 transverse septa and 0-3 longitudinal septa, while featuring a cylindrical, light brown beak.
Molecular identification
The internal-transcribed-spacer (ITS) region of fungal ribosomal DNA sequences (rDNA) has gained significant attention in the field of fungal identification and phylogenetics. This region serves as a valuable molecular marker due to its high-sequence variability among fungi, the presence of conserved flanking regions, and its easy accessibility in public reference sequence databases. Consequently, ITS has been widely recognized as a 'fungal barcode' and is commonly used in various fungal studies [Citation55].
PCR amplification of the ITS section in the genomic DNA of the isolated fungi was performed. By comparing the resulting PCR end-products with a DNA ladder, the molecular size of the three PCR products was determined to be approximately 550-600 bp. The DNA sequence of the ITS region of the fungi was obtained from Macrogen Company (Seoul, Korea). This sequence was then compared to the universal database available in the NCBI using the BLAST tools. As a result, the fungal isolates showed a high degree of similarity to Fusarium verticillioides, Fusarium proliferatum and Alternaria tenuissima.
The ITS sequences of the fungal isolates were submitted to the GenBank database and assigned accession numbers (OP889680, OP889682 and OP886855, respectively). A phylogenetic tree was constructed using the obtained fungal nucleotide sequences, along with fungal sequences retrieved from the GenBank database. This phylogenetic tree demonstrated a close relationship between the fungal isolates and F. verticillioides, F. proliferatum, and A. tenuissima ().
Physical and chemical properties of collected soil samples
presents a set of physicochemical characteristics of the three soil samples obtained from Nobaria, El-Beheira Governorate, Egypt. This region is known to experience various plant disorders, particularly in tomato fields. The soil samples exhibited slightly alkaline properties with a pH value of 8.35. The electroconductivity of the soils was measured at 2.52 dS m−1, indicating a relatively high salt concentration. The dominant cations in the soil included Ca2+, Mg2+, Na+ and K+, with mean concentrations of 6.38, 0.48, 19.89 and 0.69 mg/L respectively (). Additionally, the presence of anions such as HCO3, Cl-and SO42− was observed, with mean concentrations of 4.23, 12.89, and 6.75 meq/L respectively. The soil analysis results revealed low levels of organic material (R = 1.32%) and nitrogen content (R = 0.04%), indicating poor fertility conditions (). Furthermore, these soils demonstrated a low water-holding capacity of 50%.
Table 2. Physicochemical properties of three collected soil samples.
Bioactive substance screening of EM product
To assess the antimicrobial potential of the EM product, a bioactive substance screening was conducted to determine the presence of antioxidant compounds and other groups of bioactive constituents that may be indicative of its activity. The results presented in demonstrate the presence of various secondary metabolites, including saponins (2.4 mg/mL) and tannins (12.7 mg/mL), as well as significant amounts of antioxidant compounds such as phenolics, flavonoids, ascorbic acid and amino acids (59, 23, 16 and 17 mg/mL, respectively). Furthermore, the antioxidant capacity of the EM product was evaluated using three different methods (FRP, PMA and DPPH), which all showed a highly significant antioxidant capacity and/or free-radical scavenging potential.
Table 3. Bioactive substances screening of some active compounds of the EM product.
Bioactive compounds identified in EM product by GC-MS
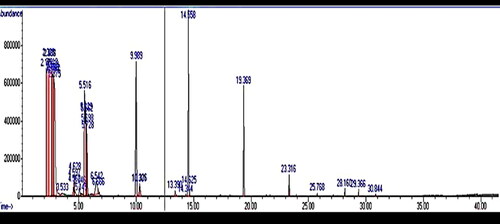
The GC-MS analysis of the EM product revealed the presence of several active compounds in the product. and provide details on the various extract components identified at different retention times (RT). Among the 20 active components identified, notable relative abundance levels were observed for 2-Pentene, 2,3-dimethyl (3.9%), 3-Hydroxymandelic acid, ethyl ester (2.95%), and 3-Penten-2-one, 4-methyl (2.72%). At an RT of 18.16 min, 3-Chloro-N, N-dimethyl-6-phenyl-11H-indolo[3,2-c]quinoline-11-ethanamine, 5-oxide was found to have a relative abundance of 0.32%. Additionally, at an RT of 28.15 min, 6-(2-Aminophenyl)-1,2,4-triazine-3,5(2H,4H)-dione tritms had a relative abundance of 1.35%.
Table 4. Bioactive substances identified in the EM product by GC-MS.
Effect of EM product as an antifungal agent in vitro
The results presented in illustrate a gradual reduction in fungal radial growth with increasing EM concentration in a concentration-dependent manner. The most effective concentration of EM product (3%) resulted in the least fungal radial growth, with reductions of 93%, 100% and 100% observed in F. verticillioides, F. proliferatum, and A. tenuissima respectively, compared to the untreated control. On the other hand, as the extract concentration decreased through serial dilution, fungal growth gradually increased until reaching a value close to that of the control at a concentration of 0.1%, accounting for a 20%, 23%, and 21% decrease in F. verticillioides, F. proliferatum and A. tenuissima growth respectively, compared to the control.
Table 5. Effect of different EM concentrations against mycelial growth of three different phytopathogenic fungi. Data are the mean values of three replicates ± SD. The results with identical letters in each column are not significantly different at p < 0.05.
Expression of fungal defence genes in response to the EM treatments
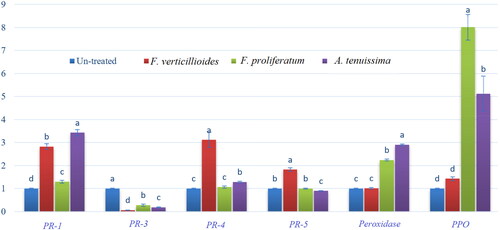
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) was employed to determine the transcript levels of five PR genes (PR-1, PR-3, PR-4, PR-5 and PR-9), as well as the PPO gene, which is known for its involvement in membrane defence. The fungal mycelia grown on PDA plates treated with EM product were compared to untreated controls. The results presented in demonstrate that PR-1 had higher levels of expression in EM-treated F. verticillioides (2.83-fold), F. proliferatum (1.30-fold) and A. tenuissima (3.43-fold) compared to the untreated controls. Conversely, the expression of PR-3 was significantly down-regulated in all treated fungal isolates, with down-regulation observed in F. verticillioides, F. proliferatum and A. tenuissima, respectively, relative to their control counterparts. In contrast to the PR-3 pattern, PR-4 exhibited a significant up-regulation in F. verticillioides (3.12-fold) and A. tenuissima (1.29-fold), while in F. proliferatum, the expression level remained relatively stable, showing no significant changes when compared to the control. The expression level of PR-5 increased in F. verticillioides (1.83-fold) and decreased in A. tenuissima, while in F. proliferatum, the expression level remained similar under EM treatment compared to the control. Furthermore, EM treatment resulted in a substantial increase in the expression level of PR-9 (peroxidase) and PPO in F. proliferatum and A. tenuissima, while the treatment had no significant effect on the expression level of these genes in F. verticillioides compared to the untreated control. The obtained increase percentages were 2.24-fold and 2.9-fold for PR-9 and 8.01-fold and 5.12-fold for PPO in F. proliferatum and A. tenuissima, respectively.
Figure 5. Effects of EM product on the expression levels of PR-1, chitinase/PR-3, PR-4, PR-5, peroxidase and PPO genes compared to housekeeping gene (reference gene/ß-actin). Relative expression of control and three EM-treated fungi (F. verticillioides, F. proliferatum, and A. tenuissima). Data are means of three replicates ± SD and different letters on columns indicate statistically significant variations at LSD ≤ 0.05.
Discussion
Plant diseases cause significant economic crop losses worldwide. Among these diseases, fungal diseases pose a continuous threat to food and feed safety, potentially dating back to the domestication of agricultural crops [Citation56]. Due to soil contamination with antimicrobial chemicals, such as fungicides and pesticides, there is an increasing demand for safe and eco-friendly alternatives [Citation57–59].
As a result, discussions on soil health have gained attention among soil scientists and in developed countries. This has sparked interest in restoring soil health to its pre-pesticide and chemical fertilizer use state. This investigation focused on studying infested soil through soil analysis, revealing high concentrations of ions. These elevated mineral concentrations can significantly impact microbial biodiversity, as well as sensitive and semi-sensitive crops. Additionally, the presence of these salts disrupts the interactions between plant roots and microbial communities [Citation37, Citation60, Citation61].
The antifungal efficacy of the effective microorganism (EM) extract can be attributed to its antioxidant compounds and activity. Saponins, which are steroidal or tri-terpenoid complexes commonly glycosylated at one end of the compound, exhibit soap-like properties in H2O [Citation62, Citation63]. Some saponins possess antimicrobial and anti-insect properties, acting as protective agents against potential pathogens [Citation64–66]. Furthermore, tannins, secondary metabolites with antioxidant properties, play a role in defending against oxidative damage [Citation67, Citation68].
In the present study, the antioxidant capacity of the EM product was found to be influenced by its total phenolic and flavonoid contents. Flavonoids, a significant natural group of phenolics, possess a wide range of chemical and biological characteristics, including radical scavenging properties and described roles as antioxidants, anticarcinogens, and antimicrobials [Citation69]. Moreover, the reducing capability of the EM product serves as a substantial indicator of its antioxidant potential, as it aids in converting free radicals into more stable products, effectively terminating free radical-generated chain reactions [Citation70]. In this study, the EM product demonstrated high antioxidant activity, with a DPPH scavenging rate of 73.1%.
To assess the effectiveness of EM (effective microorganism) on the tested fungi, the radial growth method was employed. The data, as shown in , revealed a significant reduction in radial fungal growth upon the application of EM in a concentration-dependent manner. Complete inhibition of fungal growth was observed at a concentration of 3% of the EM product. This reduction in growth may be attributed to the presence of secondary metabolites produced by various microbes, which act as antimicrobial agents. These findings align with the study of Montesinos, [Citation71], and also suggest the additional possibility that some bioactive compounds might have been added previously to the EM product. Furthermore, EM may contain chitinolytic enzymes that can lyse and degrade the cell wall of numerous pathogenic organisms [Citation22, Citation25]. Additionally, the microorganisms responsible for producing these enzymes possess the capability to eliminate some pathogens which pose a threat to global crop production. Therefore, these chitinolytic microorganisms/EM could serve as an alternative to chemical treatments and may be employed as natural substitutes for plant protection against phytopathogens. Compared to synthetic fungicides, these natural products do not contribute to environmental pollution. This aspect has significantly increased the attention towards the utilization of biological methods to combat plant pathogens and restore soil health to its natural state.
To elucidate the mechanism of action of EM on the treated fungi at the genetic level, RT-PCR was employed as the most accurate and widely used method for quantification of gene expression. Real-time PCR offers a large dynamic range and high sensitivity, enabling the detection of rare transcripts and small changes in gene expression levels [Citation72]. Furthermore, monitoring gene expression levels in response to stress offers valuable insights into the activity of antioxidant genes, providing a specific indication of how the organism is responding and defending against potential damage [Citation73]. Pathogenesis-related genes (PR genes), which are integral components of the defence system in various organisms, including plants and fungi [Citation55], are induced by stress or defence signalling molecules. These genes can serve as molecular markers for assessing the defence system [Citation74–76]. PR genes encode a variety of different molecules that vary in structure, mode of action, and specificity toward particular pathogens [Citation77]. In the present study, we evaluated the expression levels of PR1, chitinase (PR3), PR4, and PR5 as well as peroxidase (PR9), as important representative molecular markers to determine the induction or suppression of the fungal defence system. Our data demonstrated that the addition of the EM compound to the growth media of the tested fungi resulted in an increased expression of PR1 in both F. verticillioides and A. tenuissima. This observation may indicate an immune response of the fungi towards the application of EM.
To facilitate survival and penetration into host cells, fungi produce hydrolytic enzymes, including chitinases, which play a crucial role in initiating the infection process [Citation78]. Additionally, many fungi utilize chitinase enzymes as a defence mechanism to degrade substrates and gather necessary nutrients for survival in unfavourable environmental conditions [Citation79]. Moreover, chitinolytic agents have been widely successful as biocontrol agents for controlling various pathogenic fungi [Citation80, Citation81]. Consistent with these findings, our study also revealed that EM suppressed the expression of the chitinase gene in the studied fungi. This suppression may consequently affect the pathogenicity of the fungi and result in reduced radial growth.
Regarding PR-4 and PR-5, the results obtained showed a significant upregulation in F. verticillioides, while such increase was not observed in F. proliferatum or A. tenuissima. The elevated expression of PR-4 and PR-5 in F. verticillioides suggests a defensive response of this pathogen towards EM. However, the levels of PR-4 and PR-5 were probably not sufficient to counteract the effect of EM, leading to a decrease in fungal growth. PR-4, known as chitin-binding protein, and PR-5, known as thaumatin-like protein, are highly responsive in both plants and fungi, and their expression is induced under stress conditions [Citation81]. In addition to its role in stress regulation, PR-5 has been found to modulate transcription factors through close protein-protein interactions [Citation82].
Peroxidases are assumed to be the first evolved enzyme in the sequence of proteins expressed during the fungal catabolism of lignin [Citation82, Citation83]. Once stress affects the organism, it starts increasing the peroxidase level via inducing its gene transcription to face the harm imposed by EM inoculated in the growth media.
The obtained data revealed up-regulation of PPO in F. proliferatum or A. tenuissima, and the increase in levels observed after infection may be attributed to the defence reaction or as a consequence of disruption in the cellular metabolism of the treated fungal cell. may be attributed to the defence reaction, or may be a consequence of disruption of cellular metabolism in the treated fungal cell [Citation22]. Moreover, many researchers suggest that fungal PPO is similar to plant PPO, and can be activated by proteolysis or acid shock [Citation84, Citation85]. Furthermore, fungal PPO which has a role in melanin synthesis which may in-turn affect the pathogenesis has been reported [Citation86]. The appressorium's cell walls leverage melanin, a valuable pigment that enhances their osmotic potential and aids in breaching host cell walls, in addition to providing protection.
Moreover, the polyphenol oxidase (PPO) gene, which codes for the PPO enzyme that catalyses the conversion of phenol to quinones, is also produced by organisms when suffering attacks or infection [Citation87] and is also a key gene in the sensitivity of plants to infection.
The simultaneous increase in the expression levels of PR-1, PR-4 and PR-5 in F. verticillioides, as well as PR-1, peroxidase, and PPO in A. tenuissima, and peroxidase and PPO in F. proliferatum, could indicate an antagonistic response of the fungi under examination toward the indigenous microflora. This response may manifest as damage due to the presence of microorganisms that are known producers of proteins and peptides used for pathogen defence, as noted by Sathya et al. [Citation88]. Streptomyces species from the genus are recognized for their production of blasticidin, mildiomycin, and polyoxins, which play a role in combating pathogens and influencing their genetic pathways. Moreover, this finding demonstrated to what extent the studied fungi were capable of responding by inducing changes in the expression levels of some genes in an attempt to survive, but still cannot fully overcome the suppressing activity of the EM product. From the gene expression pattern, we could suggest that the defence pathway in these fungi probably depends on increasing the expression levels of some genes such as; PR-1, PR-4, PR-5, peroxidase and PPO. On the other hand, the EM mode of action depends on a wide-ranging decrease in chitinase gene expression. The results revealed a significant antagonistic interaction between EM and fungi, which evidently impacted fungi, resulting in a reduction in their morphological growth.
Conclusions
The findings from this study provide compelling evidence that EM contain a rich composition of antioxidant compounds such as phenolics and flavonoids. Also, this investigation demonstrated that the tested EM product exhibited a potent antioxidant capacity, and effectively reduced and inhibited the growth of plant fungal pathogens in vitro. Furthermore, it was observed that EM exerted a significant influence on the gene expression levels of the target fungal species, leading to a marked decrease in the mRNA levels of chitinase genes. These significant findings support the suggestion that microorganisms, such as EM, originating from diverse sources, play a crucial role in effectively combating plant fungal diseases. Consequently, further investigations conducted in natural conditions (in-vivo) are warranted to evaluate the efficacy of these EMs in the control of plant pathogenic fungi. Such research efforts aim to enhance crop yield productivity, while reducing reliance on chemical interventions, promoting the establishment of chemical-free and healthier agricultural environments.
Author contributions
AAS: Investigation, Formal analysis, Data curation, Writing – review & editing. MHE: Resources, Methodology, Data curation. FAE: Methodology, Investigation, Formal analysis. SES: Resources, Investigation, Methodology, Data curation, Writing- review & editing. SSK: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. EEH: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Investigation, Formal analysis, Conceptualization. All authors have read and approved the final version of the paper.
Informed consent statement
Not applicable.
tbeq_a_2387190_sm4024.docx
Download MS Word (103.3 KB)Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
Data are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Hannula SE, Van Veen JA. Primer sets developed for functional genes reveal shifts in functionality of fungal community in soils. Front Microbiol. 2016;7:1897. doi: 10.3389/fmicb.2016.01897.
- E. Sobhy S, G. Aseel D, M. Abo-Kassem E-E, et al. Priming of wheat plant with weed extracts, calcium and salicylic acid for contribution to alleviating the oxidative stress imposed by Fusarium graminearum and lead toxicity. Novel Res Microbiol J. 2023;7(2):1932–1965. doi: 10.21608/nrmj.2023.294938.
- Lehmann A, Zheng W, Ryo M, et al. Fungal traits important for soil aggregation. Front Microbiol. 2019;10:2904. doi: 10.3389/fmicb.2019.02904.
- Vorholt JA. Microbial life in the phyllosphere. Nat Rev Microbiol. 2012;10(12):828–840. doi: 10.1038/nrmicro2910.
- Zhao D, Yang X, Wu S, et al. First report of Fusarium proliferatum causing root rot of Gerbera in China. J Plant Dis Prot. 2020;127(2):279–282. doi: 10.1007/s41348-019-00281-1.
- Todorović I, Moënne-Loccoz Y, Raičević V, et al. Microbial diversity in soils suppressive to Fusarium diseases. Front Plant Sci. 2023;14:1228749. doi: 10.3389/fpls.2023.1228749.
- Hibar K, Daami-Rema M, Ayed F, et al. Fusarium crown and root rot of tomato and its chemical control. Int. J. Agric. Res. 2007;2(8):687–695. doi: 10.3923/ijar.2007.687.695.
- Kharayat BS. Diseases of field and horticultural crops and their management volume–II. New India Publishing Agency- Nipa, PHI Learning Pvt. Ltd; 2023. ND-110001ISBN: 978-93-91818-95-1
- Akhtar KP, Saleem MY, Asghar M, et al. New report of Alternaria alternata causing leaf blight of tomato in Pakistan. Plant Pathol. 2004;53(6):816–816. doi: 10.1111/j.1365-3059.2004.01099.x.
- Chao C-CT, Parfitt DE, Michailides TJ. Alternaria late blight (Alternaria alternata) resistance in pistachio (Pistacia vera) and selection of resistant genotypes. JASHS. 2001;126(4):481–485. doi: 10.21273/JASHS.126.4.481.
- Animashaun MO. The use of hot water treatment by small holders for the control of Alternaria alternata, the cause of black mould disease of tomato. Ph.D. Thesis, Department of Horticulture, Writtle College University of Essex; 2015. https://repository.essex.ac.uk/15389/1/Mufutau%20PhD%204%20Nov%202015%20Online%20repository.pdf.
- Sobhy S, Al-Askar AA, Bakhiet EK, et al. Phytochemical characterization and antifungal efficacy of camphor (Cinnamomum camphora L.) extract against phytopathogenic fungi. Separations. 2023;10(3):189. doi: 10.3390/separations10030189.
- Gupta P, Reddaiah B, Salava H, et al. Next‐generation sequencing (NGS)‐based identification of induced mutations in a doubly mutagenized tomato (Solanum lycopersicum) population. Plant J. 2017;92(3):495–508. doi: 10.1111/tpj.13654.
- Siam G, Abdelhakim T. Analysis of the tomato value chain in Egypt and establishment of an action plan to increase its efficiency. [Research Report].Centre International de Hautes Etudes Agronomiques Méditerranéennes - Institut Agronomique Méditerranéen de Montpellier (CIHEAM-IAMM); 2018.118. p.(hal-02143775)
- Shaheen N, Khan UM, Azhar MT, et al. Genetics and genomics of Fusarium wilt of chilies: a review. Agronomy. 2021;11(11):2162. doi: 10.3390/agronomy11112162.
- Heflish AA, Abdelkhalek A, Al-Askar AA, et al. Protective and curative effects of Trichoderma asperelloides Ta41 on tomato root rot caused by Rhizoctonia solani Rs33. Agronomy. 2021;11(6):1162. doi: 10.3390/agronomy11061162.
- Pragadheesh VS, Saroj A, Yadav A, et al. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind Crops Prod. 2013;49:628–633. doi: 10.1016/j.indcrop.2013.06.023.
- El-Gendi H, Al-Askar AA, Király L, et al. Foliar applications of Bacillus subtilis HA1 culture filtrate enhance tomato growth and induce systemic resistance against Tobacco mosaic virus infection. Horticulturae. 2022;8(4):301. doi: 10.3390/horticulturae8040301.
- Sobhy SE, Abo-Kassem E-EM, Sewelam NA, et al. Pre-soaking in weed extracts is a reasonable approach to mitigate Fusarium graminearum infection in wheat. J Plant Growth Regul. 2021;41(6):2261–2278. 1. doi: 10.1007/s00344-021-10442-y.
- Sharma K, Bruns C, Butz AF, et al. Effects of fertilizers and plant strengtheners on the susceptibility of tomatoes to single and mixed isolates of Phytophthora infestans. Eur J Plant Pathol. 2012;133(3):739–751. doi: 10.1007/s10658-012-9954-z.
- Higa T, Parr JF. Beneficial and effective microorganisms for a sustainable agriculture and environment. International Nature Farming Research Center Atami, Japan; 1994. Vol. 1. https://www.em-pars.com/wp-content/uploads/2018/05/7-beneficial-and-effective-microorganisms-for-a-sustainab-.pdf
- Mayer J, Scheid S, Widmer F, et al. How effective are 'Effective microorganisms®(EM)'? Results from a field study in temperate climate. Appl Soil Ecol. 2010;46(2):230–239. doi: 10.1016/j.apsoil.2010.08.007.
- Ndona RK, Friedel JK, Spornberger A, et al. 'Effective micro-organisms'(EM): an effective plant strengthening agent for tomatoes in protected cultivation. Biol. Agric. Horticul. 2011;27(2):189–203. doi: 10.1080/01448765.2011.9756647.
- Xu H-L. Effects of a microbial inoculant and organic fertilizers on the growth, photosynthesis and yield of sweet corn. J. Crop Product. 2001;3(1):183–214. doi: 10.1300/J144v03n01_16.
- Hu C, Qi Y. Long-term effective microorganisms application promote growth and increase yields and nutrition of wheat in China. Eur J Agron. 2013;46:63–67. doi: 10.1016/j.eja.2012.12.003.
- Pierce S, et al. Evaluation of effective microorganisms® efficacy on 'Candidatus Phytoplasma Solani'-infected and healthy periwinkle plants. Mitt. Klosterneubg. Rebe Wein Obstbau Früchteverwert. 2016;66:89–92.
- García-Latorre C, Rodrigo S, Santamaria O. Protective effects of filtrates and extracts from fungal endophytes on Phytophthora cinnamomi in Lupinus luteus. Plants. 2022;11(11):1455. doi: 10.3390/plants11111455.
- Hunter B. 1998. Illustrated genera of imperfect fungi A comprehensive resource for recognizing, identifying, and learning various aspects of imperfect fungi. (41922).
- Alexopoulos CJ, Mims CW, Blackwell M. Introductory mycology (4th ed.). John Wiley & Sons; 1996;670–708. ISBN-10: ISBN: 0471522295, ISBN-13: 978-0-471-52229-4.
- KH, Domsch, W G, T-H, Anderson. Compendium of soil fungi. Eching: IHW-Verlag; 2007.
- Simmons EG. Alternaria an identification manual, fully illustrated and with catalogue raisonné. Utrecht (Netherlands) : CBS Fungal Biodiversity Centre; 2007;42:1796–2007.
- Sambrook J, Fritsch E. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997.
- White T.J, Bruns T, Lee S, Taylor J, Innis MA, Gelfand Sninsky DH, White JJ. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols, a guide to methods and applications. San Diego, CA: Academic Press; 1990:315–322.
- Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38(7):3022–3027. doi: 10.1093/molbev/msab120.
- Houba VJG, Temminghoff EJM, Gaikhorst GA, et al. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun Soil Sci Plant Anal. 2000;31(9-10):1299–1396. doi: 10.1080/00103620009370514.
- Richards LA. editor. Diagnosis and improvement of saline and alkali soils. BioScience. 1954;4(3):14–14.
- Rashad MA, et al. Arsenate phytoremediation-linked genes in Egyptian rice cultivars as soil pollution DNA geno-sensor. Bioscience Research. 2018;15(3):2207–2217.
- Wang X, Wang J, Zhang J. Comparisons of three methods for organic and inorganic carbon in calcareous soils of northwestern China. PLoS One. 2012;7(8):e44334. doi: 10.1371/journal.pone.0044334.
- Hiai S, Oura H, Hamanaka H, et al. A color reaction of panaxadiol with vanillin and sulfuric acid. Planta Med. 1975;28(2):131–138. doi: 10.1055/s-0028-1097841.
- Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. J Sci Food Agric. 1978;29(9):788–794. doi: 10.1002/jsfa.2740290908.
- Jindal K, Singh R. Phenolic content in male and female Carica papaya: a possible physiological marker for sex identification of vegetative seedlings. Physiol Plant. 1975;33(1):104–107. doi: 10.1111/j.1399-3054.1975.tb03774.x.
- Oser BL, Hawk PB. Hawk's physiological chemistry. New york: McGraw-Hill; 1965. https://www.abebooks.com/Hawks-Physiological-Chemistry-Oser-Bernard-McGraw-Hill/31259421554/bd
- Lee YP, Takahashi T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem. 1966;14(1):71–77. doi: 10.1016/0003-2697(66)90057-1.
- Oyaizu M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. JpnJNutrDiet. 1986;44(6):307–315. doi: 10.5264/eiyogakuzashi.44.307.
- Brand-Williams W, Cuvelier M-E, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5.
- Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH. free radical method. LWT-Food Science and Technology. 1997;30(6):609–615. doi: 10.1006/fstl.1997.0240.
- Jayaprakasha GK, Rao LJ, Sakariah KK. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98(4):720–724. doi: 10.1016/j.foodchem.2005.06.037.
- Okla MK, Alamri SA, Salem MZM, et al. Yield, phytochemical constituents, and antibacterial activity of essential oils from the leaves/twigs, branches, branch wood, and branch bark of Sour Orange (Citrus aurantium L.). Processes. 2019;7(6):363. doi: 10.3390/pr7060363.
- NIST/EPA/NIH Mass Spectral Library (NIST 14) and NIST Mass Spectral Search Program. (Version 2.0g); Standard Reference Data Program; U.S. Department of Commerce, National Institute of Standards and Technology: Gaithersburg, MD, May 2014.
- Salem MZM, Mansour MMA, Elansary HO. Evaluation of the effect of inner and outer bark extracts of sugar maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J Wood Chem Technol. 2019;39(2):136–147. doi: 10.1080/02773813.2018.1547763.
- Vincent J. Distortion of fungal hyphae in the presence of certain inhibitors. Nature. 1947;159(4051):850–850. doi: 10.1038/159850b0.
- Hafez EE, Abdelkhalek AA, El-Wahab ASE-DA, et al. Altered Gene expression: induction/suppression in leek elicited by iris yellow spot virus infection (IYSV) Egyptian isolate. Biotechnol. Biotechnol Equip. 2013;27(5):4061–4068. doi: 10.5504/BBEQ.2013.0068.
- Saleha Y. Gene expression and histopathology alterations during rat mammary carcinogenesis induced by 7, 12-dimethylbenz [a] anthracene and the protective role of Neem (Azadirachta indica) leaf extract. J Amer Sci. 2010;6(9):843–859.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262.
- Soliman SA, Al-Askar AA, Sobhy S, et al. Differences in pathogenesis-related protein expression and polyphenolic compound accumulation reveal insights into tomato–pythium aphanidermatum interaction. Sustainability. 2023;15(8):6551. doi: 10.3390/su15086551.
- Chellemi DO, Gamliel A, Katan J, et al. Development and deployment of systems-based approaches for the management of soilborne plant pathogens. Phytopathology. 2016;106(3):216–225. doi: 10.1094/PHYTO-09-15-0204-RVW.
- Pathak VM, Verma VK, Rawat BS, et al. Current status of pesticide effects on environment, human health and it's eco-friendly management as bioremediation: a comprehensive review. Front Microbiol. 2022;13(13):962619. PMID: 3 6060785; PMCID: PMC9428564. doi: 10.3389/fmicb.2022.962619.
- Vlaiculescu A, Varrone C. Sustainable and eco-friendly alternatives to reduce the use of pesticides. In Pesticides in the natural environment. Health Risks and Remediation, 1st Edition, Chapter (17), Elsevier; 2022;329–364. doi:10.1016/B978-0-323-90489-6.00014-8.
- Tiwari S, Dubey NK, Kumar C. Phytochemicals as an eco‐friendly source for sustainable management of soil‐borne plant pathogens in soil ecosystem. Agroecological Approaches for Sustainable Soil Management; 2023;303–318. doi:10.1002/9781119911999.ch13.
- Awaad HA. Salinity and its impact on sustainable crop production. in Salinity resilience and sustainable crop production under climate change. Cham: Springer; 2023:29–92. doi: 10.1007/978-3-031-48542-8.
- Awaad HA. 2023. Salinity resilience and sustainable crop production under climate change.
- Price KR, Johnson IT, Fenwick GR, et al. The chemistry and biological significance of saponins in foods and feeding stuffs. Crit Rev Food Sci Nutr. 1987;26(1):27–135. doi: 10.1080/10408398709527461.
- Francis G, Kerem Z, Makkar HPS, et al. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88(6):587–605. doi: 10.1079/BJN2002725.
- Gallardo F, Boethel D. Effects of the Allelochemical, α-tomatine, on the Soybean Looper (Lepidoptera: noctuidae). J Entomol Sci. 1990;25(3):376–382. doi: 10.18474/0749-8004-25.3.376.
- Desai SD, Desai DG, Kaur H. Saponins and their biological activities. Pharma Times. 2009;41(3):13–16.
- Aiswarya-Sudheer CK, Chattopadhyay I. Endophytic bacteria for drug discovery and bioremediation of heavy metals. In Endophytic association: what, why and how. Elsevier; 2023:159–181. doi: 10.1016/B978-0-323-91245-7.00015-8.
- Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23(1):21–48. doi: 10.3109/10408449309104073.
- Akbar MU, Aqeel M, Shah MS, et al. Molecular regulation of antioxidants and secondary metabolites act in conjunction to defend plants against pathogenic infection. S Afr J Bot. 2023;161:247–257. doi: 10.1016/j.sajb.2023.08.028.
- Ghafar M, et al. Flavonoid, hesperidine, total phenolic contents and antioxidant activities from Citrus species. Afr. J. Biotechnol. 2010;9(3):326–330. doi: 10.5897/AJB09.1229.
- Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104(1):21–29. doi: 10.1016/j.foodchem.2006.10.066.
- Montesinos E. Development, registration and commercialization of microbial pesticides for plant protection. Int Microbiol. 2003;6(4):245–252. doi: 10.1007/s10123-003-0144-x.
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1(3):1559–1582. doi: 10.1038/nprot.2006.236.
- Liu D, Shi L, Han C, et al. Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS One. 2012;7(9):e46451. doi: 10.1371/journal.pone.0046451.
- Sinha M, Singh RP, Kushwaha GS, et al. Current overview of allergens of plant pathogenesis related protein families. ScientificWorldJ. 2014;2014:543195. ID: 543195. doi: 10.1155/2014/543195.
- Edreva A. Pathogenesis-related proteins: research progress in the last 15 years. Gen Appl Plant Physiol. 2005;31(1-2):105–124.
- Aseel DG, Sobhy S, Samy MA, et al. Comparative analysis of the expression profiles of pathogenesis-related genes in tomato systemically infected with tobacco mosaic and cucumber mosaic viruses. IJPB. 2023;14(2):458–473. doi: 10.3390/ijpb14020035.
- Anisimova OK, Shchennikova AV, Kochieva EZ, et al. Pathogenesis-related genes of PR1, PR2, PR4, and PR5 families are involved in the response to Fusarium infection in garlic (Allium sativum L.). Int J Mol Sci. 2021;22(13):6688. doi: 10.3390/ijms22136688.
- Homthong M, Kubera A, Srihuttagum M, et al. Isolation and characterization of chitinase from soil fungi, Paecilomyces sp. Agric Nat Res. 2016;50(4):232–242. doi: 10.1016/j.anres.2015.09.005.
- da Silva MV, Santi L, Staats CC, et al. Cuticle-induced endo/exoacting chitinase CHIT30 from Metarhizium anisopliae is encoded by an ortholog of the Chi3 gene. Res Microbiol. 2005;156(3):382–392. doi: 10.1016/j.resmic.2004.10.013.
- Nur Mawaddah S, Aw MZ, Sapak Z. The potential of Pseudomonas fluorescens as biological control agent against sheath blight disease in rice: a systematic review. Food Res. 2023;7(Supplementary 2):46–56. doi: 10.26656/fr.2017.7(S2).11.
- Patil N, Raghu S, Mohanty L, et al. Rhizosphere bacteria isolated from medicinal plants improve rice growth and induce systemic resistance in host against pathogenic fungus. J Plant Growth Regul. 2023;43(3):770–786. doi: 10.1007/s00344-023-11137-2.
- Abdin MZ, Kiran U, Alam A. Analysis of osmotin, a PR protein as metabolic modulator in plants. Bioinformation. 2011;5(8):336–340. doi: 10.6026/97320630005336.
- Prasad B, Menon V. 2023. 4 Role of oxidative enzymes. Microbial Oxidative Enzymes: biotechnological Applications: p. 63.
- Leufken CM, Moerschbacher BM, Dirks-Hofmeister ME. Dandelion PPO-1/PPO-2 domain-swaps: the C-terminal domain modulates the pH optimum and the linker affects SDS-mediated activation and stability. Biochim Biophys Acta. 2015;1854(2):178–186. doi: 10.1016/j.bbapap.2014.11.007.
- Jukanti A. Polyphenol oxidases (PPOs) in plants. Singapore: Springer; 2017. ISBN: 978-981-10-5747-2. doi: 10.1007/978-981-10-5747-2.
- Janusz G, Kucharzyk KH, Pawlik A, et al. Fungal laccase, manganese peroxidase and lignin peroxidase: gene expression and regulation. Enzyme Microb Technol. 2013;52(1):1–12. doi: 10.1016/j.enzmictec.2012.10.003.
- Vanitha SC, Niranjana SR, Umesha S. Role of phenylalanine ammonia lyase and polyphenol oxidase in host resistance to bacterial wilt of tomato. J Phytopathol. 2009;157(9):552–557. doi: 10.1111/j.1439-0434.2008.01526.x.
- Sathya A, Vijayabharathi R, Gopalakrishnan S. Plant growth-promoting actinobacteria: a new strategy for enhancing sustainable production and protection of grain legumes. 3 Biotech. 2017;7(2):102. doi: 10.1007/s13205-017-0736-3.