The front cover images on this issue of Molluscan Research show the trochid Ethminolia vitiliginea (Menke, 1843), shell diameter 6.0 mm, sieved from shallow sub-littoral sand at Portarlington, southern Port Phillip Bay, Victoria (NMV F248148). It was collected on 1st December 2012 during fieldwork by the Micro-Mollusc Workshop, a three-day activity of the Malacological Society of Australasia (MSA) held immediately before its triennial conference – Molluscs 2012 – in Melbourne (see Stephens Citation2013; Vafiadis Citation2013). Additional views of this same snail are shown below in . This was the third live specimen of E. vitiliginea that I have seen, the others being from Portarlington (in 2007) and Clifton Springs (in 2011), both of which are shown in .
Figure 1. Ethminolia vitiliginea (NMV F248148). All images show the same specimen, shell diameter 6.0 mm, from shallow sub-littoral sand, Portarlington, Port Phillip Bay, Victoria, 1 December 2012. Enlarged ventral images show different views of the head and anterior foot. (Photos: P. Vafiadis).

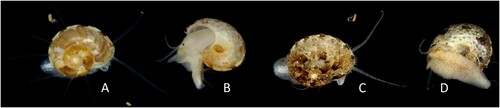
Figure 2. Ethminolia vitiliginea. A, B: Shell diameter 4.5 mm, from shallow sub-littoral silt adjacent to rocky reef, Portarlington, Port Phillip Bay, Victoria, 21 April 2007 (specimen lost); C, D (NMV F248149): Shell diameter 6.0 mm, habitat not recorded, Clifton Springs, Port Phillip Bay, Victoria, 23 April 2011. (Photos: P. Vafiadis).
Ethminolia vitiliginea is endemic to the temperate coastline of southern Australia, occurring from Fremantle, Western Australia to Bass Strait (along the Victorian and northern Tasmanian coasts) (May Citation1923; Wilson Citation1993). It is an interesting snail for two key reasons. First, it is an active, flamboyant animal whose external features have not, to my knowledge, been previously documented, although Hickman and McLean (Citation1990: 130, Fig. 85A, G) have figured its radula. Second, members of its subfamily (Umboniinae), and the closely allied trochid subfamily Solariellinae, are known to swim (Herbert Citation1987, Citation1992; Hickman and McLean Citation1990; Hickman Citation1998).
Swimming in Ethminolia vitiliginea was briefly mentioned in Vafiadis and Hales (Citation2015: 204) during general discussion on vetigastropod swimming, and is expanded on here, together with a description of its external morphology. To ground this discussion, brief mention is made of the main differences in the shells and radulae of Umboniinae and Solariellinae, whose shells can look similar. The Umboniinae are generally small to medium-sized, with shell often depressed (but sometimes tall-conical), often umbilicate (but closed by callus in some), have a beaked protoconch apex, and a radula with reduced elaboration of the central and lateral teeth and many well-developed marginal teeth (Hickman and McLean Citation1990; Herbert Citation1992). The Solariellinae are small, have an open umbilicus, lack an apical beak, and their radula has fewer rows, well-developed central and lateral teeth and a small number of marginal teeth (Herbert Citation1987).
Ethminolia vitiliginea has long, micro-papillate cephalic tentacles (as long as the shell breadth) that gently taper to a blunt point, with eyes on stalks at their lateral bases. The snout bears two transverse rows of crowded tentacle-like processes, one at mid-length and the other distally, which are micro-papillate. The neck lobes are broad and prominent. The left neck lobe has a densely digitate margin whilst the right is smooth-margined and can be rolled to form an exhalant pseudo-siphon. There are four pairs of long epipodial tentacles of the same structure as the cephalic tentacles, each with a distinct epipodial sense organ at the lateral base (as a short papilla with a black spot at the apex – in the images, an identical structure is also seen on the dorsal foot below the left neck lobe). The propodium is bi-lobed with angled corners, the sole is broader behind this, and bluntly rounded posteriorly. The dorsal metapodium looks symmetrically thickened in the midline – this was not studied in detail, but could be a posterior extension and fusion of the epipodial folds (as described by Herbert Citation1992). The sole is smooth. The extended foot is a little longer than the maximal shell diameter. The operculum is round, translucent, pale yellow, multi-spiral and externally concave.
These features are consistent with known soft tissue details in other members of the Umboniinae (Herbert Citation1992; Hickman and McLean Citation1990). Hickman and McLean (Citation1990: 125) note the umboniine snout processes to be ‘setaform’ (as observed here) and a unique feature of the subfamily, possibly sensory in function. The snout processes in Solariellinae are located distally around the mouth and have a mechanical function, raking the sediment and grasping food particles (Hickman and McLean Citation1990). A right post-optic lobe was not seen – it is a feature of Solariellinae (Herbert Citation1992), and some Solariellinae only have 3 pairs of epipodial tentacles (Herbert Citation1987; Citation1992). The umboniine left neck lobe can be rolled to form an inhalant pseudo-siphon, with the digitate margin at its apex (with digits being branched in some species) acting as a net to stop silt entering the mantle cavity (Hickman and McLean Citation1990). Rolling of the left neck lobe was not seen in E. vitiliginea but may reflect its study in a bare petri dish free of substrate. In contrast, the neck lobes in Solariellinae are relatively reduced, and many (but not all) members of that group have a metapodial fin – an extensible fold of tissue from the left metapodium – which helps the animal to swim (Herbert Citation1987). No metapodial fin was seen in studied South African Umboniinae, although all such species studied alive were known to swim (Herbert Citation1992). It was also not seen in E. vitiliginea. Burrowing is known in both Solariellinae and Umboniinae, facilitated by the bifid propodium (Herbert Citation1987, Citation1992; Hickman and Mclean Citation1990) but was not studied in E. vitiliginea. The posterior foot of E. vitiliginea is more rounded compared to South African umboniine species (see illustrations in Herbert Citation1992).
The shell colour and patterning of Ethminolia vitiliginea is variable. The background colour of the animal itself is white to off-white. There are sparse, fine, transverse black lines on the cephalic and epipodial tentacles. The head bears a midline dark brown-black stripe, the snout is mottled with yellow and brown (distally being more confluent brown) and the peri-oral area is dull yellow. There are white patches on the right neck lobe. The dorsal foot in the 2012 animal is lightly mottled with irregularly shaped brown-grey patches, these becoming confluent on the epipodium between the epipodial tentacles. The dorsal foot also has white patches (mainly around the periphery, epipodium and metapodium) and is also mottled with fine brown-grey irregular patches (centrally distributed in the 2012 specimen, more evenly scattered in the 2011 specimen). Yellowish-orange mottles were also present on the sole and dorsal foot in the 2011 specimen.
Ethminolia vitiliginea is an active species. When crawling, the snout processes and eye stalks project beyond the anterior shell margin, the metapodium is visible behind, and the long cephalic tentacles, all epipodial tentacles and right neck lobe are prominently visible. The edge of the left neck lobe sometimes also protrudes beyond the front left apertural edge. This snail readily rights itself and is thus challenging to study. A rested animal can be induced to swim in short, explosive bursts (lasting 1 to 2 seconds) when prodded with blunt forceps – I was startled in first noticing this after trying to invert the animal for ventral inspection. The swimming is achieved by rapid, alternating movements of the foot as it is held extended from the shell. Foot movement during swimming was always vigorous and seemed to be an ‘all or nothing’ reflex. When swimming, it travels shell-first in the direction of movement, the thrashing foot trailing behind. Landing after swimming is sometimes aperture up, sometimes aperture down. Study in a petri dish could not inform whether, or how far, swimming could lift the animal above the substratum. Gentle prodding immediately after a burst of swimming could generate a further burst, but this was fatiguable and could not be reproduced in succession more than two to three times. I did not try to determine a recovery-time before which a burst of swimming could again be induced. Blurry but informative video of the 2007 specimen showing swimming behaviour is available as supplementary material. High resolution video studied in slow motion may help a better understanding of the dynamics of the swimming of E. vitiliginea. Swimming is postulated to be an escape response (Herbert Citation1992; Hickman and McLean Citation1990), although, especially in a current, it could also confer dispersal advantage to more suitable habitat.
Supplemental Material
Download Microsoft Video (AVI) (16.2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Herbert, D.G. (1987) Revision of the Solariellinae (Mollusca: Prosobranchia: Trochidae) in southern Africa. Annals of the Natal Museum 28(2), 283–382. Available at: https://www.researchgate.net/publication/259356008_Revision_of_the_Solariellinae_Mollusca_Prosobranchia_Trochidae_in_southern_Africa.
- Herbert, D.G. (1992) Revision of the Umboniinae in southern Africa and Mozambique (Mollusca: Prosobranchia: Trochidae. Annals of the Natal Museum 33(2), 379–459. Available at: https://www.researchgate.net/publication/260186142_Revision_of_the_Umboniinae_Mollusca_Prosobranchia_Trochidae_in_southern_Africa_and_Mozambique.
- Hickman, C.S. (1998) Superfamily Trochoidea. In: Beesley, P.L., Ross, G.J.B. & Wells, A. (Eds), Mollusca: the Southern Synthesis. Fauna of Australia. Vol. 5. Part B, pp. 671–692, CSIRO, Melbourne. Available at: https://www.biodiversitylibrary.org/page/60797430#page/181/mode/1up.
- Hickman, C.S. & McLean, J.H. (1990) Systematic Revision and Suprageneric Classification of Trochacean Gastropods. No. 35, Science Series, Natural History Museum of Los Angeles County, California, USA.
- May, W.L. (1923) An illustrated index of Tasmanian shells with 47 plates and 1052 species. (Supplementary to the checklist issued in 1921). John Vale, Government Printer, Tasmania.
- Stephens, L. (2013) Marine micro-mollusc workshop at Queenscliff. Newsletter of the Malacological Society of Australasia 146, 3–4. Available at: http://www.malsocaus.org/docs/newsletter/MSA146.pdf.
- Vafiadis, P. (2013) Report on the Malacological Society of Australasia (MSA) marine micromollusc workshop, Queenscliff, Victoria, Friday 30 November-Monday 3 December, 2012. Victorian Branch Bulletin of the Malacological Society of Australasia 267: 5–6. Available at: http://www.malsocaus.org/docs/vic/bulletin/Bulletin%20267.pdf.
- Vafiadis, P. & Hales, T.J. (2015) The living morphology and movement of the temperate Australian marine snail Sukashitrochus pulcher (Petterd, 1884) (Vetigastropoda: Scissurellidae). Molluscan Research 35(3), 196–205.
- Wilson, B. (1993) Australian marine shells. Prosobranch gastropods, Part 1. Odyssey, Kallaroo, Western Australia.
