ABSTRACT
This paper describes a new Asian hard clam, Meretrix taiwanica sp. n., found in the Tamsui River estuary in northern Taiwan. This species was formerly identified as M. lusoria, and was considered to be a descendant of a clam that originated in Japan. A molecular phylogenetic analysis, however, demonstrated that Meretrix taiwanica sp. n. is closely related to M. petechialis and M. lusoria. Meretrix taiwanica is distinguished by its smaller socket width and shorter posterior dorsal margin. This species is distributed throughout the coast of Taiwan on the southern coast of China.
Introduction
Asian hard clams of the genus Meretrix belong to the family Veneridae, and are commercially important bivalves in East Asia, Southeast Asia and East Africa (Yoosukh and Matsukuma Citation2001). They inhabit the lower reaches of sandy beaches, estuaries and tidal flats, and there are 15 recognised species in the genus (MolluscaBase Citation2022): M. astricta (Reeve, 1864), M. attenuata (Dunker, 1863), M. aurora (Hornell, 1917), M. casta (Gmelin, 1791), M. lamarckii (Deshayes, 1853), M. lusoria (Röding, 1798), M. lyrata (G.B. Sowerby II, 1851), M. marisarabicum (C. Martin & Matsukuma, 2020), M. meretrix (Linnaeus, 1758), M. morphina (Lamarck, 1818), M. petechialis (Lamarck, 1818), M. planisulcata (G.B. Sowerby II, 1854), M. subtrigona (Dunker, 1857), M. tigris (Lai & Nien, 2008), and M. vestita (Reeve, 1864).
Taiwan’s cultivated Asian hard clam species has been identified as M. lusoria for almost a century (Chen Citation1984). With a harvest of 49,501 tons and an economic value of more than US$155 million in 2019 (Fisheries Agency Citation2020), it is a major commercial aquaculture species in Taiwan.
Taxonomic research on Meretrix in Taiwan, including M. petechialis, M. lamarckii and M. lyrata, was first conducted by Kuroda (Citation1941). Two additional species from Taiwan, M. meretrix and M. lusoria, were added by Wu (Citation1980). An additional species, M. tigris, was described by Lai and Nien (Citation2008), but recent research indicates that the colour pattern of the shell of M. tigris is only a different pattern of the common hard clam, with no others having been found thus far (Hsu et al. Citation2020). Of these species, M. lyrata can be easily identified by the unique commarginal ribs of its shell; the shell of M. lamarckii, which usually inhabits shallow subtidal sandy flats and beaches, is thick, triangularly ovate, and sometimes has faint concentric stria. However, because M. meretrix, M. lusoria and M. petechialis have simple shell shapes with diverse colour patterns and their external morphological differences are relatively small, using morphological characteristics to identify them is difficult; these similarities can lead to frequent erroneous identifications, and notations to this effect are found in shell books, reports and references (Yamakawa et al. Citation2008).
With the development of molecular technologies, allozyme (Yamakawa et al. Citation2008) and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) (Yamakawa and Imai Citation2013) have been applied to studies of hard clam taxonomies of Japan, China, South Korea and Taiwan. The results suggested that M. lusoria and M. petechialis are closely related, having small genetic distances, and are allopatric (Yamakawa et al. Citation2008). The Taiwanese Meretrix is genetically different from M. lusoria and M. petechialis (Yamakawa and Imai Citation2013). Moreover, Hsiao et al. (Citation2019) revealed that Asian hard clams found in the Tamsui River estuary in Taiwan formed a distinctively different cluster from M. lusoria and M. petechialis in phylogenetic analysis based on the mitochondrial DNA cytochrome c oxidase I (COI) gene. The topology revealed that Meretrix taiwanica sp. nov. and M. petechialis are more closely related to one another than to M. lusoria. Eighty years after the distribution of Meretrix was initially reported in Taiwan, our results indicate that Taiwanese Meretrix represents a distinct evolutionary lineage that is both genetically and morphologically distinct from its congeners and is thus described herein as a new species.
Material and methods
Sample collection
Specimens of the Meretrix species were collected from Taiwan. The adductor muscle tissue (approximately 0.5 × 0.5 cm) of specimens was collected and stored in 95% alcohol prior to analysis. The holotype and paratypes were collected with a bull raker at the Tamsui River estuary in New Taipei City, Taiwan ( and ). In order to examine the status of Meretrix species, we also collected samples from Hsinchu, Changhua, Tainan and Kinmen in western Taiwan.
Figure 1. The type locality of Meretrix taiwanica sp. n. in the Tamsui River estuary, northern Taiwan.
Figure 2. A, Environment of type locality; B, local fisherman catches Asian hard clams in single small boat; C, use of a bull raker as harvesting gear; D, harvested wild Asian hard clams.

Specimens of M. petechialis were obtained from a supermarket in Mie Prefecture, Japan; the packaging was labelled ‘imported from China’. Specimens of M. lusoria were obtained from Shirakawa, Kumamoto Prefecture, Japan. All reference samples were deposited at the Molecular Systematic Laboratory, Marine Fisheries Division, Fisheries Research Institute.
The name Cytheraea formosa (G.B. Sowerby II, Citation1851) is not accepted and is synonymised with M. lusoria. Two syntype specimens are kept at the British Museum of Natural History (BMNH) (BMNH 20120227). To clarify the relationships of the two syntypes with the present species, we used two-dimensional digital images from BMNH to obtain relevant morphological data for comparison.
Morphological examinations
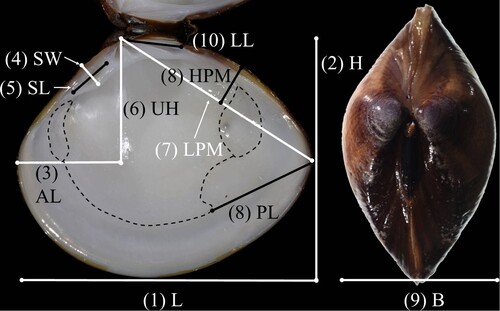
Shell measurements, partially consistent with those outlined by Torii et al. (Citation2010), included shell length (L), shell height (H), anterior shell length (AL), socket width (SW), socket length (SL), upper shell height (UH), length of posterior dorsal margin (LPM), height of posterior dorsal margin (HPM), shell breadth (B), ligament length (LL), and pallial sinus length (PL) (). The last 10 measurements were divided by shell length for standardisation (). Characters of the right shell valve were photographed with a digital camera and then measured with image analysis software, ImageJ v. 1.51 K (Abramoff et al. Citation2004).
Figure 3. Morphometric measurements of right valve and shell. L: shell length, H: shell height, B: shell breadth, AL: anterior shell length, SW: socket width, SL: socket length, UH: upper shell height, LPM: length of posterior dorsal margin, HPM: height of posterior dorsal margin, LL: ligament length, and PL: pallial sinus length.
Canonical discriminant analysis (CDA) was used to assess the 10 standardised characteristics using R-plot (R Core team, Citation2015). The method of multivariate analysis was partly based on Torii et al. (Citation2010).
Molecular methods
Mitochondrial COI was selected as the target gene. DNA was extracted using a Gentra Puregene Tissue Kit (Gentra, Minneapolis, MN, USA). The LCO-1490 (forward primer, 5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′) and HCO-2198 (reverse primer, 5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) primers were set and used to amplify the COI barcode (Folmer et al. Citation1994). The amplification of the COI gene followed the protocol described in our previous study (Hsiao et al. Citation2019).
The PCR products were examined through electrophoresis on 1% agarose gel to confirm that the correct length of fragment had been obtained; they were subsequently eluted using the QIAquick Gel Extraction kit (Qiagen, Hilden, Germany). The purified PCR products were sent to MB Mission Biotech (Taipei, Taiwan) for DNA sequencing.
The Meretrix species, the collecting locality of the samples and GenBank accession numbers of specimens that were used in this study are listed in . The sequence data were manually edited and automatically assembled using BioEdit 7.2.5 (Hall Citation1999), and sequence divergence statistics were then calculated using Clustal X programs (Thompson et al. Citation1997). The phylogenetic relationships among the samples were inferred using both neighbour-joining (NJ) and maximum parsimony (MP) methods. NJ trees were constructed using MEGA X (Kumar et al. Citation2018) under the Kimura two-parameter model of base substitution with 1000 bootstrap repetitions. The MP tree topology was estimated using the tree bisection–reconnection algorithm. Bayesian phylogenetic trees were created using MrBayes v. 3.2 (Ronquist et al. Citation2012) with 107 cycles and an HKY85 + G substitution model, as determined by jModelTest v. 2 (Darriba et al. Citation2012).
Table 1. Table of species, sample collecting localities and GenBank accession numbers that were used in this study.
Results
Systematics
Superfamily Veneroidea Rafinesque, 1815
Family Veneridae Rafinesque, 1815
Genus Meretrix Lamarck, 1799
Meretrix taiwanica Hsiao & Chuang, sp. n.
http://zoobank.org:act:AB345172-30F1-4CDD-AADD-CEADB57EB3D6
and .
Meretrix lusoria.— Kuo Citation1964: 32, left shell in figure; Wu Citation1980: 112, 109, pl. III, fig. N; Wu and Liu Citation1989: 51–52; The Malacological Society of Taiwan 1994: 95, pl. 61, fig. 386; Lam Citation1999: 51–52, figs 1, 7; Wu Citation1997: 76–77 (with 2 figs); Wu and Jian Citation2008: 195, fig. 108 (not of Röding, Citation1798).
Meretrix meretrix.—Wu and Liu Citation1989: 49–61; Higo and Goto Citation1993: 661; Wu and Jian Citation2008: 196, fig. 109 (not of Linnaeus, Citation1758).
Meretrix petechialis.—Kuroda Citation1941: 65–216; Kuo Citation1964: 32, middle shell in figure (not of Lamarck, 1818).
Meretrix formosana.—Kuo Citation1964: 32, right shell in figure (not of G.B. Sowerby II, Citation1851).
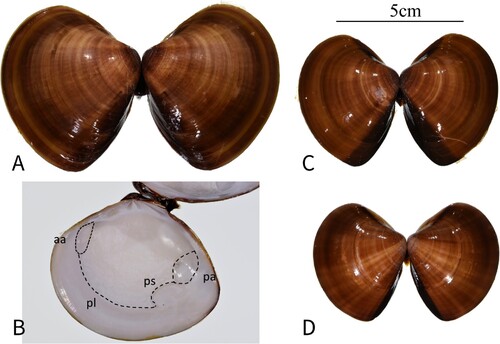
Holotype. Tamsui River estuary, New Taipei City, Taiwan, 25°09'02.2"N, 121°27'11.8"E, coll. S.T. Hsiao and S.C. Chuang, 15 May 2019 (FRIM10028, paired valves, length 67.9 mm, height 57.2 mm) (A, B; ).
Table 2. Measurements (in mm) of type specimens of Meretrix taiwanica sp. n.
L: shell length; H: shell height; AL: anterior shell length; SW: socket width; SL: socket length; UH: upper shell height; LPM: length of posterior dorsal margin; HPM: height of posterior dorsal margin; B: shell breadth; LL: ligament length; PL: pallial sinus length.
Paratypes. Tamsui River estuary, New Taipei City, Taiwan, 25°09'51.3"N, 121°25'51.8"E, coll. S.T. Hsiao and S.C. Chuang, 21 Jun. 2018 (FRIM10029, paired valves, length 56.5 mm, height 45.2 mm) (C; ); Tamsui River estuary, New Taipei City, Taiwan, 25°09'52.5"N, 121°26'28.0"E, coll. S.T. Hsiao and S.C. Chuang, 21 Jun. 2018 (FRIM10030, paired valves, length 49.4 mm, height 37.8 mm) (D; ).
Material examined
Type material. See above. Other material. M. taiwanica sp. n.: New Taipei City, coll. S.T. Hsiao and S.C. Chuang, 26 Jul. 2018 (FRIM10031, 16 paired valves); New Taipei City, coll. S.T. Hsiao and S.C. Chuang, 20 Oct. 2018 (FRIM10032, 35 paired valves); New Taipei City, coll. S.T. Hsiao and S.C. Chuang, 20 Jul. 2018 (FRIM10035, 7 paired valves); Tainan, coll. S.T. Hsiao, 18 Aug. 2020 (FRIM10036, 3 paired valves); Changhua, coll. S.T. Hsiao, 18 Aug. 2020 (FRIM10037, 3 paired valves); Hsinchu, coll. S.T. Hsiao and S.C. Chuang, 20 Jun. 2018 (FRIM10038, 4 paired valves); Kinmen, coll. S.T. Hsiao, 6 Nov. 2018 (FRIM10039, 2 paired valves). M. petechialis: collected at supermarket, Mie Prefecture, Japan, coll. S.T. Hsiao and J.H. Wu, 7 Nov. 2018 (FRIM10033, 17 paired valves). M. lusoria: collected on Shirakawa, Kumamoto Prefecture, Japan, coll. Y. Henmi, 20 Jun. 2019 (FRIM10034, 24 paired valves). Cytheraea formosa: BMNH catalogue number: 20120227, 2 individual images from BMNH, length 70.8–89.2 mm, unavailable name-bearing types (https://data.nhm.ac.uk/object/cadd6f3c-b54d-412f-9ec9-df01d37420f1/1623715200000).
Type locality
Taiwan, New Taipei City, Tamsui River estuary (25°09'02.2"N; 121°27'11.8"E), water depth approximately 2.41 m, sandy to muddy bottoms.
Distribution
Western coast of Taiwan, southern coast of China to northern Vietnam.
Description
Shells in our collection medium-sized, length of up to 86.69 mm. Solid, triangular-ovate shaped, inequivalve, have a rounded ventral margin, fairly straight dorsal edge; posterior margin length approximately 0.65–0.78 times shell length. Umbones elevated well above the hinge line. Shells topped with ligaments behind the umbones. Umbones slightly biased towards the front. Shell posterior margin longer than anterior margin, the posterior has an escutcheon, usually a dark-brown colour, anterior sides have a clear lunule.
Shell exterior smooth, having fine growth lines but no grooves on the surface. Shells have highly variable colour patterns roughly divided into yellow-white, reddish-brown, dark-brown mottling, W-shaped markings and dark green.
Shell interior thin and shiny, creamy-white colour, posterior sections of deep purple; hinge area with fine sculpture, with three cardinal teeth. Teeth attached to each other at the top of the shell and extend to outer edge in arrow shapes. Length of socket is approximately 0.04–0.044 times shell length. One anterior lateral tooth. One small, clear oblong anterior adductor scar, length 0.20–0.24 times shell length. One large, oval posterior adductor scar approximately 0.21–0.25 times shell length. Pallial sinuses shallow, reaching end of cardinal tooth. Pallial line almost reaches middle of posterior adductor scar; length of the pallial sinus approximately 0.21–0.44 times shell length.
Etymology
We name this species ‘taiwanica’ in reference to the collection locality in Taiwan. Additionally, this species is the most abundant and widely distributed hard clam in Taiwan.
Diagnosis
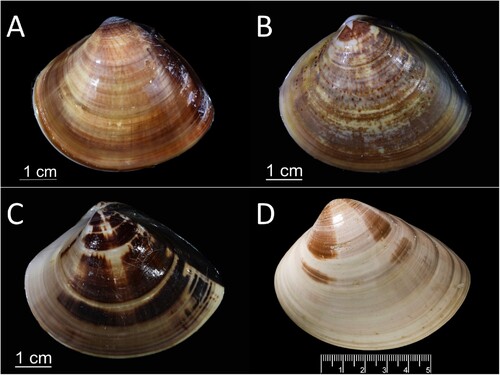
Shell surface of M. lyrata has commarginal ribs; ventral margins of M. taiwanica sp. n., M. lamarckii and M. lusoria smooth, flat and round, respectively. Meretrix taiwanica sp. n. similar in shape to M. lusoria and M. petechialis (A–C) but differs in several morphological features. Posterior dorsal margin of M. lusoria relatively straight, causing rear shell angle to be sharper, socket narrower. Shell-shaped posterior margin of M. petechialis rounded,with wider socket. Posterior margin of M. taiwanica sp. n. rounded with narrower socket than M. petechialis.
Figure 6. Shells of hard clams A, Meretrix taiwanica sp. n.; B, M. petechialis; C, M. lusoria; D, Cytheraea formosa (unavailable name-bearing types, syntype, digital image cited from BMNH HP collection, 20120227).
To compare the posterior margin sizes of M. taiwanica sp. n., M. petechialis and M. lusoria, we used the ratio of the length of each species’ posterior dorsal margin to its shell length (LPM/L) (). For M. taiwanica sp. n. (N = 70), the range was 0.65–0.78, with an average of 0.74. The range for M. petechialis (N = 17) was 0.69–0.78 with an average of 0.74, and for M. lusoria (N = 24) the range was 0.73–0.81 with an average of 0.75. The syntypes of the two Cytheraea formosa in Sowerby (Citation1851) both had a ratio of 0.77. In measured specimens, the LPM/L of M. taiwanica sp. n. and M. petechialis did not significantly differ from each other (P = 0.6446), whereas they were shorter than M. lusoria (P = 0.00437, P < 0.01, respectively) and perhaps C. formosa.
Table 3. Ten characters standardised against shell length calculated in M. taiwanica sp. n. (N = 70), M. petechialis (N = 17), M. lusoria (N = 24) and C. formosa (N = 2) (unavailable name-bearing types). Avg.: mean, Std.: standard deviation, L: shell length, H: shell height, B: shell breadth, AL: anterior shell length, SW: socket width, SL: socket length, UH: upper shell height, LPM: length of posterior dorsal margin, HPM: height of posterior dorsal margin, LL: ligament length, and PL: pallial sinus length.
Multivariate analysis
Because the shell breadths of the two syntypes of C. formosa were not measurable, these measurements were excluded in the first CDA. The results of the analysis demonstrated that the two C. formosa specimens were distinct from the samples of the other three species (A). However, because of the small sample sizes, those two were excluded in the second CDA.
Figure 7. Two-dimensional scatterplots of canonical discriminant analysis. A, Analysis including the species Meretrix taiwanica sp. n., M. petechialis, M. lusoria and C. formosa; B, analysis including the species Meretrix taiwanica sp. n., M. petechialis and M. lusoria.
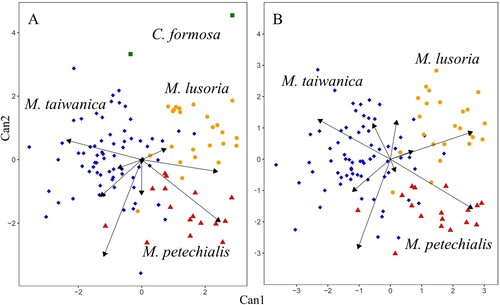
Meretrix taiwanica sp. n., M. petechialis and M. lusoria were clearly separated by this CDA using the standardised 10 morphological characteristics (; B). The proportions of variation explained by canonical variates 1 and 2 were 61.4% and 38.6%, respectively. The absolute values of SW/L, UH/L, HPM/L, LL/L and PL/L were more than 0.5 in the centroid of canonical variates 1 and 2 (). These results demonstrated that M. taiwanica sp. n. has a rounder posterior dorsal margin than M. lusoria and a smaller socket width than M. petechialis.
Table 4. Centroids of canonical variates (Can.) 1 and 2 for 10 standardised characters of Meretrix taiwanica sp. n., M. petechialis, and M. lusoria. L: shell length, H: shell height, B: shell breadth, AL: anterior shell length, SW: socket width, SL: socket length, UH: upper shell height, LPM: length of posterior dorsal margin, HPM: height of posterior dorsal margin, LL: ligament length, and PL: pallial sinus length.
Molecular and phylogenetic analyses
Phylogenetic analysis of the COI gene with new samples of Meretrix taiwanica sp. n. resulted in a tree concordant with that of Hsiao et al. (Citation2019, fig. 2), with all species supported ().
Figure 8. Phylogenetic tree constructed for Meretrix spp. and reference sequences based on the cytochrome c oxidase I gene barcode sequence. Branch support values estimated by bootstrap pseudo-replicates in maximum parsimony and neighbour-joining are displayed above each branch. A minus sign (-) indicates that the bootstrap values were <75%.
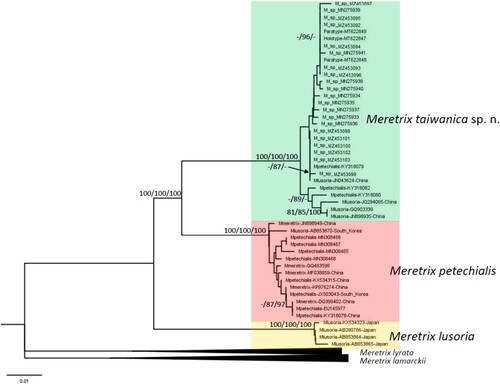
In the clade of M. petechialis, some sequences from The National Center for Biotechnology Information (NCBI) (JN898949, GQ463598, MF038859, KP976274 and DQ399402) were identified as M. meretrix and one sequence (AB853870) was identified as M. lusoria. The most samples collected from China and South Korea were M. petechialis but had previously been misidentified as belonging to other taxa.
Our results support the presence of a new species (herein named Meretrix taiwanica) in a monophyletic clade with M. petechialis, M. lusoria, M. lyrata and M. lamarckii. The average nucleotide sequence divergence is 7.59% between M. taiwanica sp. n. and M. petechialis and 9.53% between M. taiwanica sp. n. and M. lusoria. Nucleotide diversity is 1.13% within M. taiwanica sp. n. The topology of the phylogenetic tree indicates that M. taiwanica sp. n. and M. petechialis are more closely related to one another than to M. lusoria.
All of the samples that we collected from western Taiwan, including the holotype and paratypes, were clustered together in a single clade as Meretrix taiwanica sp. n., excluding M. petechialis and M. lusoria.
Discussion
Sowerby (Citation1851) described the new species Cytheraea formosa, and this species is currently listed as a synonym of M. lusoria. The source of the specimen was Hugh Cuming’s collection. The type specimen has not been specified, and thus the type locality is unknown. According to Sowerby II’s description of C. formosa in p.620, ‘the shell is thinner, more oblique and more elongated than C. meretrix, which it otherwise greatly resembles’. The shells of M. taiwanica sp. n. and C. formosa exhibit clear morphological differences (A, D).
The syntypes deposited in the BMNH are supposed to be from Japan, based on the original label with the M.C. (Museum Cuming) sticker and according to the museum’s curator, although ‘Formosa’ is a previous name for Taiwan. However, no distribution of M. taiwanica sp. n. has been found in Japan. Therefore, C. formosa should be considered a variant and synonym of M. lusoria.
Habe (Citation1977) posited that the Asian hard clams produced in Taiwan are Meretrix meretrix, which has a range centred on the Indian Ocean. Meretrix meretrix has been reported to be primarily distributed in the South China Sea (Zhang et al. Citation2012), Thailand (Supmee et al. Citation2020), Southeast Asia (Trang et al. Citation2018) and along the East African coast (Branch et al. Citation2010). The shells of M. meretrix are oval and broader in shell breadth (Supmee et al. Citation2020) than those of M. taiwanica sp. n. Based on our results, M. taiwanica sp. n., M. petechialis and M. lusoria cannot be accurately distinguished using single morphological characteristics. However, these species can be distinguished from each other through multivariate analysis using multiple shell morphological characteristics.
Wang et al. (Citation2017) indicated that M. petechialis in China has two different population groups: the northern lineage (including the Bohai Sea, Yellow Sea and East China Sea) and the south lineage (including the East China Sea and South China Sea). However, we discovered that the mtDNA haplotypes of this southern lineage form a highly supported monophyletic clade with those of M. taiwanica sp. n. On the other hand, the haplotypes of the northern lineage clustered in the same clade as those of M. petechialis. Therefore, the southern lineage as distinguished from the northern lineage by Wang et al. (Citation2017) represents populations of M. taiwanica sp. n. in southern China rather than those of M. petechialis.
Although they are morphologically similar, M. lusoria, M. petechialis, M. meretrix and M. taiwanica sp. n. have distinct geographical distributions. Meretrix lusoria is mainly distributed in Japan and South Korea. Meretrix petechialis is distributed from the south-western coasts of South Korea to the coast of China (Torii et al. Citation2010; Wang et al. Citation2017). The distribution of Meretrix meretrix includes coastal areas of South and Southeast Asia (Trang et al. Citation2018), and M. taiwanica sp. n. is distributed in Taiwan (the present study) and the southern coast of China to northern Vietnam (Wang et al. Citation2017).
The Taiwanese hard clam was previously identified as M. lusoria and is considered to be a descendant of a clam that originated in Japan and was introduced to Taiwan during the Japanese colonial period (Chen Citation1984). We examined specimens from the National Taiwan Museum, National Museum of Natural Science and Shihsanhang Museum of Archaeology collections. We discovered that all the Taiwanese specimens labelled as M. lusoria in those institutions are actually M. taiwanica sp. n. and that wild M. lusoria clams do not occur in Taiwan.
Acknowledgements
We give special thanks to Director General Chun-Ju Chen and Chief Hsin-Ming Yeh of the Fisheries Research Institute for their support and guidance. We are grateful to Mr Jenn-Shing Hwang for assistance with specimen collection. This study (2018–2019) was supported by the Fisheries and Fishing Port Affairs Management Office, New Taipei City Government, Taiwan, ROC. We acknowledge the Managing Editor and Associate Editor of Molluscan Research and the reviewers for their valuable comments that improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abramoff, M.D., Magalhaes, P.J. & Ram, S.J. (2004) Image Processing with ImageJ. Biophotonics International 11, 36–42.
- Branch, G.M., Griffiths, C.L., Branch, M.L. & Beckley, L.E. (2010) Two Oceans, a Guide to the Marine Life of Southern Africa. Struik Nature, Cape Town.
- Chen, H.C. (1984) Recent innovations in cultivation of edible molluscs in Taiwan, with special reference to the small abalone Haliotis diversicolor and the hard clam Meretrix lusoria. Aquaculture 39, 11–27. doi:10.1016/0044-8486(84)90256-4
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012) jModelTest2: more models, new heuristics and parallel computing. Nature Methods 9, 772. doi:10.1038/nmeth.2109
- Fisheries Agency (2020) Fisheries Statistical Yearbook 2019, Taiwan. Fisheries Agency, Council of Agriculture. Executive Yuan, Taipei, Taiwan.
- Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299.
- Habe, T. (1977) Systematics of Mollusca in Japan, Bivalvia and Scaphopoda 244–275. Tokyo: Zukan-No-Hokuryukan.
- Hall, T.A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41, 95–98.
- Higo, S. & Goto, Y. (1993) A Systematic List of Molluscan Shells from the Japanese Islands and the Adjacent Areas. Eru Malacological Publisher, Osaka.
- Hsiao, S.T., Chuang, S.C., Wu, Y.H., Chang, J.W., Chin, C.P., Yeh, H.M., Henmi, Y. & Chen, J.R. (2019) Preliminary studies for the habitat conditions of the asian hard clam Meretrix spp. in Tamsui Estuary, Taiwan. Proceedings of the 2019 Climate Change, Disaster Management and Environmental Sustainability International Conference, Kumamoto, Japan, pp. 474–482.
- Hsu, T.H., Huang, C.W. & Gong, H.Y. (2020) The past and present of Taiwanese hard clams. 2020 Annual Forum for the Fisheries Society of Taiwan, Tainan, Taiwan, p. 127. [In Chinese].
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35, 1547–1549. doi:10.1093/molbev/msy096
- Kuo, H. (1964) The investigation on Taiwan economic molluscs. The Council of Agriculture, Special Publication No. 38, 32–50.
- Kuroda, T. (1941) A catalogue of molluscan shells from Taiwan (Formosa). Memoirs of the Faculty of Science and Agriculture Taihoku Imperial University 22, 194–195.
- Lai, Y.L. & Nien, Y.G. (2008) A new species of Meretrix from Taiwan (Bivalvia: Veneridae). Bulletin of Malacology 31, 23–30.
- Lam, T.C. (1999) Natural history of Asian hard clam. The Pei-Yo 25, 44–53. [In Chinese].
- Linnaeus, C (1758) Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata [10th revised edition], vol. 1: 824 pp. Laurentius Salvius: Holmiae.
- MolluscaBase (2022) MolluscaBase. Meretrix Lamarck, 1799. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p = taxdetails&id = 204011 [accessed 11 Dec. 2022].
- R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria. https://www.R-project.org/.
- Röding, P. F. (1798) Museum Boltenianum sive Catalogus cimeliorum e tribus regnis naturæ quæ olim collegerat Joa. Fried Bolten, M. D. p. d. per XL. annos proto physicus Hamburgensis. Pars secunda continens Conchylia sive Testacea univalvia, bivalvia and multivalvia. Trapp, Hamburg. viii, 199 pp.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 539–542. doi:10.1093/sysbio/sys029
- Sowerby, II, G.B. (1851) Monograph of the genus Cytheraea. In: G.B. Sowerby, II (Ed.), Thesaurus conchyliorum, or Monographs of Genera of Shells. (Vol. 2: pp. 611–648, pls 127–136). London: privately published.
- Supmee, V., Sangthong, P., Songrak, A. & Suppapan, J. (2020) Population genetic structure of Asiatic Hard Clam (Meretrix meretrix) in Thailand based on Cytochrome Oxidase subunit I gene sequence. Biodiversitas Journal of Biological Diversity 21, 2702–2709. doi:10.13057/biodiv/d210943
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F. & Higgins, D.G. (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25, 4876–4882. doi:10.1093/nar/25.24.4876
- Torii, H., Sato, S., Hamaguchi, M., Henmi, Y. & Yamashita, H. (2010) The comparison of shell morphology and genetic relationship between Meretrix lusoria and M. petechialis in Japan and Korea. Plankton and Benthos Research 5, 231–241. doi:10.3800/pbr.5.231
- Trang, V.T., Quynh, L.T., Thiet, C.C.C., Duc, N.H. & Ha, T.T.T. (2018) Genetic relationship of asiatic hard clam populations collected in northern coastal provinces in Vietnam based on mtDNA sequence analysis. Journal of Aquaculture & Marine Biology 7, 00184. doi:10.15406/jamb.2018.07.00184.
- Wang, X., Kong, L., Chen, J., Matsukuma, A. & Li, Q. (2017) Phylogeography of bivalve Meretrix petechialis in the Northwestern Pacific indicated by mitochondrial and nuclear DNA data. PLOS ONE 12, e0183221. doi:10.1371/journal.pone.0183221.
- Wu, W.L. (1980) The list of Taiwan bivalve fauna. Quarterly Journal of the Taiwan Museum 33, 65–206.
- Wu, W.L. (1997) The Economic Mollusks in Taiwan. The Council of Agriculture, Executive Yuan, Taipei.
- Wu, W.L. & Jian, S.J. (2008) The Mollusks of Taoyuan, Hsinchu, and Miaoli Area, Taiwan. Academia Sinica, Taipei.
- Wu, W.L. & Liu, H.P. (1989) Malacological research on Meretrix resources in Taiwan II. History review and evaluation on the studies of the Taiwan Meretrix. Bulletin of Malacology 14, 49–61.
- Yamakawa, A.Y. & Imai, H. (2013) PCR-RFLP typing reveals a new invasion of Taiwanese Meretrix (Bivalvia: Veneridae) to Japan. Aquatic Invasions 8, 407–415. doi:10.3391/ai.2013.8.4.04
- Yamakawa, A.Y., Yamaguchi, M. & Imai, H. (2008) Genetic relationships among species of Meretrix (Mollusca: Veneridae) in the western Pacific Ocean. Pacific Science 62, 385–394. doi:10.2984/1534-6188(2008)62[385:GRASOM]2.0.CO;2
- Yoosukh, W. & Matsukuma, A. (2001) Taxonomic study on Meretrix (Mollusca: Bivalva) from Thailand. Phuket Marine Biological Center Special Publication 25, 451–460.
- Zhang, S., Wang, H. & Xu, F. (2012) Taxonomic study on Meretrix (Bivalvia, Veneridae) from China seas. Acta Zootaxonomica Sinica 37, 473–479. [In Chinese with English abstract].