ABSTRACT
Larvae (glochidia) of the freshwater mussel, Westralunio albertisi were obtained from the marsupia of a preserved female at the Western Australian Museum. Glochidial shells of W. albertisi are subtriangular and scalene in shape outline with a convoluted surface dotted with pores and have a protruding ventral apex. Glochidial shells (n = 60) measured 260.0 μm long (L) (±1.11 SE), 233.1 μm high (H) (± 1.32 SE), with a size of 246.6 μm (± 0.99 SE), hinge length (Hg) of 183.6 μm (± 0.99 SE), larval tooth length (LTL) of 53.6 μm (± 0.84 SE) and angle of obliquity (Á) of 12.9° (± 0.28 SE). Larval teeth are singular in each valve, lack microstylets, have convex or concave basal protuberances on opposing valves and terminate as blunt outward facing spoon-like to peg-like cusps. In comparison to Westralunio carteri, W. albertisi is smaller (d.f., 178; P < 0.001) for L (t = 33.85), H (t = 12.80), Size (t = 28.98), Hg (t = 21.32), H/L (t = −15.06) and Hg/L (t = −4.21). Westralunio albertisi also has a more acute Á than W. carteri (d.f., 71; t, 10.17; P < 0.001), but displays no difference in LTL (d.f., 6; t, 0.16, P = 0.44).
Introduction
Present day freshwater mussels (Bivalvia: Palaeoheterodonta: Unionida) inhabit inland waters of every contin-ent apart from Antarctica, with an estimated 958 species (Graf and Cummings Citation2021). Extant Hyriidae occur in the Southern Hemisphere, found in Australasia (Australia, New Guinea, New Zealand and Solomon Islands) and South America, comprised of 15 genera and 93 species (Marshall et al. Citation2014; Walker et al. Citation2014; Graf and Cummings Citation2021; Klunzinger et al. Citation2022; Ponder et al. Citation2022). Like Unionidae and Margaritiferidae, hyriids bear glochidia larvae, many of which are parasitic on fishes, although some South American taxa have foregone parasitism (Walker et al. Citation2014; Miyahira et al. Citation2017). Glochidia develop from embryos brooded in the marsupia of female ctenidia and parasitic glochidia are released when they reach a mature stage, ready to attach to a host fish or amphibian (Ponder et al. Citation2020). Glochidia release mechanisms are variable and sometimes highly specialised (Barnhart et al. Citation2008). In Australasian Hyriidae, glochidia are released functionally, in mesoconglutinates, which resemble host prey items (Melchior et al. Citation2021a), simply broadcast into the water column (Melchior et al. Citation2021a, Citation2021b; Klunzinger et al., unpublished data) or in amorphous mucus conglutinates, a passive host entanglement strategy (Klunzinger et al. Citation2012, Citation2013; Klunzinger Citation2020; Melchior et al. Citation2021a, Citation2021b).
Glochidia morphology and morphometry have proven to be useful in systematics and taxonomy (e.g., Surber Citation1912; Mansur Citation1999; Mansur and Silva Citation1999; Vale et al. Citation2005; Pimpão et al. Citation2012; Cruz and Quesada Citation2017; Lopes-Lima et al. Citation2018; Miyahira et al. Citation2019; Chernyshev et al. Citation2020; Pfeiffer and Graf Citation2020). For example, Pimpão et al. Citation2012 distinguished several species of Brazilian Hyriidae using glochidial characters and revised the taxonomy of some genera and subgen-era on this basis.
The genus Westralunio Iredale, 1934 is comprised of four extant species and two subspecies: Westralunio carteri Iredale, 1934 (ZooBank LSID urn:lsid:zoobank.org:act:6B740F4D-40C3-4D6A-8938-B0FD7FD1F6D7); Westralunio inbisi inbisi Klunzinger, Whisson, Zieritz, Benson, Stewart & Kirkendale, Citation2022 (ZooBank LSID urn:lsid:zoobank.org:act:325089AF-F7C1-42AA-82E5-4223EB6B7F1B); Westralunio inbisi meridiemus Klunzinger, Whisson, Zieritz, Benson, Stewart & Kirkendale, Citation2022 (ZooBank LSID urn:lsid:zoobank.org:act:BEBCEDC1-D944-4CFF-A332-4087C97CBC14), from southwestern Australia, and Westralunio flyensis (Tapparone Canefri, Citation1883) and Westralunio albertisi Clench, 1957, both from the Fly River system of western Papua New Guinea (McMichael and Hiscock Citation1958; Walker et al. Citation2014; Klunzinger et al. Citation2022). Glochidia of W. carteri were described by Klunzinger et al. Citation2013, but details on the morphology of glochidia from the remaining Westralunio taxa are unknown. Shell morphology and morphometric measurements of W. albertisi are described herein.
Materials and methods
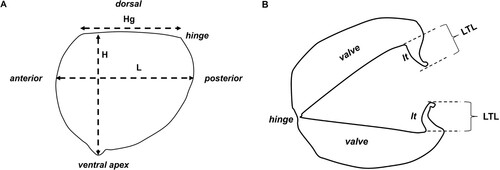
Glochidia were dissected from the marsupia of ethanol preserved W. albertisi (Western Australian Museum Registration No. S72148) by means of a pipette, then placed on a glass slide and examined under a light microscope to determine stage of maturation. Because several glochidia appeared to be open and some were floating freely in ethanol within the preservation jar and had what appeared to be fully formed larval teeth, it was assumed that they had been released and were presumably mature (see Klunzinger et al. Citation2012, Citation2013 and Klunzinger Citation2020 for description of glochidial maturation). Several subsamples of glochidia were siphoned from the gravid female and specimen jar using a glass pipette and deposited onto specimen stubs with double sided carbon tape and allowed to dry overnight. The following day, specimens were photographed using a Hitachi TM 3030 Plus Scanning Electron Microscope (SEM) and associated software at the Western Australian Museum. Terminology used to describe glochidial morphology follows Jones et al. (Citation1986), Mansur (Citation1999), Pimpão et al. (Citation2012), Klunzinger et al. (Citation2013), Klunzinger (Citation2020) and Melchior et al. (Citation2021a). Glochidia were measured to the nearest 1 µm from scale-imprinted SEM images for length (L), height (H), hinge length (Hg) and larval tooth length (LTL) and, to the nearest 1 degree (°), for angle of obliquity (Á) (see ). A size metric was determined as the mean of L and H for each individually measured glochidium (Klunzinger Citation2020; Melchior et al. Citation2021a).
Figure 1. Morphometric measurements of glochidia shells. A, L, length and Hg, hinge length, measured from anterior to posterior; H, height, measured from hinge to ventral apex; B, LTL, larval tooth length, measured from the base of the junction with each valve to the terminal point of each larval tooth (lt); C, Á, angle of obliquity, measured in degrees (°), as the angle between the line that joins the ventral point (a) to the point perpendicular to the hinge (b) and the line that joins the ventral point (a) to the middle of the hinge line (c).
Data on glochidial shell measurements (L, H, and Hg) of W. carteri (Klunzinger et al. Citation2013) were used for comparison; ‘size’ was also determined from the data of Klunzinger et al. (Citation2013) and unpublished SEM images of shell outlines of this species were measured for Á and LTL. Where two opposing larval teeth could be accurately measured from an individual glochidium, the reported LTL value was the mean of the two larval teeth. However, in the case where only one larval tooth could be measured on an individual glochidium it was reported as the LTL value.
Shell measurements of W. albertisi and W. carteri glochidia were compared statistically by independent samples t-test using SPSS 29.0 statistical software. Means with P < 0.05 were considered significantly different.
Results
The glochidial shell morphology of W. albertisi is illustrated in . The valves are operated by a single adductor muscle attached to the medial portion of each valve. The shells of W. albertisi glochidia are subtriangular and scalene in shape with a somewhat convoluted to smooth surface dotted with pores which lack surface spikes. The ventral apex of the shell protrudes and is located off-centre, closest to the posterior region of the glochidial shell. Larval teeth are singular in each valve, slightly curved towards the adductor muscle with a concave protuberance on the base of the right valve tooth and a convex protuberance on the base of the left valve, terminating in an outward facing blunt spoon-like to peg-like cusp. A summary of morphometric measurements of glochidia shells of W. albertisi is presented in along with its comparative congener W. carteri. Data for individually measured glochidia of both species are available in the supplementary data. Samples of glochidia of W. albertisi examined (n = 60) had a mean L of 260.0 µm (± 1.11 SE) μm, mean H of 233.1 µm (± 1.32 SE) μm, mean ‘size’ of 246.6 µm (± 0.99 SE), Hg of 183.6 µm (± 0.99 SE), LTL of 53.6 µm (± 0.84 SE) and Á of 12.9° (± 0.28 SE). Westralunio albertisi is significantly smaller than its congener, W. carteri, in L, H, Size, Hg, H/L and Hg/L (). Westralunio albertisi also has a more acute Á than W. carteri, giving a less scalene sub-triangular shape outline, but there is no difference in LTL between the two species ().
Figure 2. Scanning electron microscopy of glochidia of Westralunio albertisi. A, Shell shape subtriangular, scalene with ventral edge apex protruding and located off-centre and closest to the anterior end of a left valve; shell surface convoluted to smooth and dotted with pores; B, Ventral view, valves open, anterior end to right; position of adductor muscle (am) indicated; larval teeth enlarged in C and D, convex basal protuberance (cxp) and concave basal protuberance (cvp) indicated by arrows for each larval tooth; C, Right larval tooth, terminating to a relatively broad, blunt outward facing peg- to spoon-like cusp; D, Left larval tooth, terminating to a relatively broad, blunt spoon-like cusp.
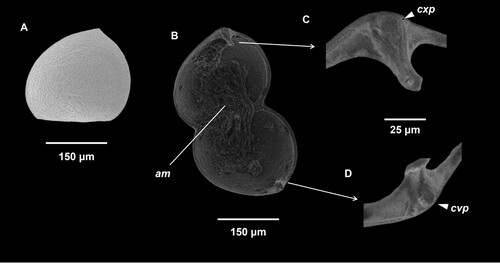
Table 1. Glochidia dimensions in freshwater mussels (Bivalvia: Hyriidae) from this study. Values presented are means ± standard errors with n representing the number of samples within each taxon measured for each variable. Ranges are provided in parentheses. H, height; L, length; Hg, hinge length; LTL, larval tooth length; Á, angle of obliquity. Taxonomy follows Walker et al. (Citation2014). Shell measurements for W. carteri, except LTL and Á, were also reported by Klunzinger et al. Citation2013. Data of individually measured glochidia are provided in supplementary material. Results of independent samples t-test are provided with degrees of freedom (d.f.), t-statistic and P-value.
Discussion
The glochidia of W. albertisi are morphologically distinct from those known from other species of freshwater mussels. In particular, the larval teeth of this species have a unique morphology. The cusps of individual larval teeth curve outwards, unlike the glochidia of other Hyriidae (e.g., Parodiz and Bonetto Citation1963; Walker Citation1981; Humphrey Citation1984; Widarto Citation1993; Mansur Citation1999; Mansur and Silva Citation1999; Vale et al. Citation2005; Pimpão et al. Citation2012; Klunzinger et al. Citation2013; Klunzinger Citation2020; Melchior et al. Citation2021a) which curve inward towards the dorsal hinge line or medially. Statistically, W. albertisi glochidia are clearly smaller and less scalene in shape outline than those of W. carteri.
Results of this study and Klunzinger et al. (Citation2013) show that the glochidia of W. albertisi and W. carteri are distinct from other Velesunioninae in having glochidia with unique larval tooth morphology. Unlike Alathyria jacksoni Iredale, 1934, Velesunio ambiguus (Philippi, 1847), Velesunio angasi (G.B. Sowerby II, 1867) and Alathyria pertexta pertexta Iredale, 1934, which have singular s-shaped larval teeth terminating to sharp cusps pointing inward towards the dorsal hinge (Parodiz and Bonetto Citation1963; Walker Citation1981; Humphrey Citation1984; Widarto Citation1993; Klunzinger Citation2020), W. albertisi has larval teeth with blunt, spoon-like cusps which face outward, pointing away from the dorsal hinge. Westralunio carteri differs from other velesunionine glochidia in having straighter, more lanceolate larval teeth terminating sharply and bluntly on opposing valves with multiple cusps, and shell outlines are more scalene in shape, with larger angles of obliquity. However, like other Velesunioninae, the glochidia of both Westralunio share similar larval tooth basal protuberance structure, have generally smooth shells dotted with pores which lack shell surface spikes, and have shells greater than 250 μm in length, with the valves held together by a singular adductor muscle. Jones et al. (Citation1986) showed that Cucumerunio novaehollandiae (Gray, 1834), Hyridella australis (Lamarck, 1819) and Hyridella depressa (Lamarck, 1819) have bifurcated larval teeth, terminating in two cusps, set apart, giving a ‘double hooked’ appearance. This larval tooth structure was also shown in an image of Hyridella drapeta (Iredale, 1934) published online by Ponder et al. Citation2022. This indicates that larval tooth bifurcation appears to be a common tribal feature among most Australian Hyridellini (Hyriidae: Hyriinae), although Atkins (Citation1979) showed that larval teeth of H. drapeta from southwestern Victoria are s-shaped, like A. jacksoni, A. pertexta pertexta, V. angasi and V. ambiguus, discussed above.
Given that glochidia of W. albertisi were obtained from museum preserved material, the natural larval release mechanism could not be observed and the presence or absence of a larval thread in this species could not be confirmed. However, if the species follows a similar strategy to its other velesunionine counterparts, it may be reasonable to assume that the presence of a larval thread and release by amorphous mucus conglutinates (see Walker Citation1981; Klunzinger et al. Citation2012, Citation2013; Klunzinger Citation2020) and a passive entanglement infection strategy is likely. Given that A. jacksoni, V. angasi, V. ambiguus and W. carteri are host generalists, using multiple species of host fishes for parasitism and, presumably, dispersal (Walker Citation1981; Humphrey Citation1984; Widarto Citation1993; Klunzinger et al. Citation2012), it is quite possible that W. albertisi is also a host fish generalist, but this remains unknown. Furthermore, the relatively large size of W. albertisi suggests that it is likely a fin parasite, unlike small glochidia which are usually gill parasites; fin parasites, more often than not, use a passive entanglement mechanism for attachment to their host (Barnhart et al. Citation2008; Melchior et al. Citation2021a, Citation2021b).
The position of W. albertisi and W. flyensis within the genus Westralunio is unclear (Walker et al. Citation2014; Klunzinger et al. Citation2022). Phylogenetic analyses reported by Graf et al. (Citation2015) and Santos-Neto et al. (Citation2016) showed that W. carteri is separate to other Velesunioninae. Additionally, Zieritz et al. (Citation2013) and Klunzinger et al. (Citation2022) showed that the shell sculpture pattern exhibited by juvenile W. carteri, and indeed glochidia characteristics of W. carteri, might be used to argue that the genus does not belong in the same subfamily as other Velesunioninae. Unpublished genetic data (Jones, Klunzinger, Wilson and Kirkendale, in prep.) also suggest that W. albertisi and W. flyensis may be a separate genus to Australian Westralunio taxa and glochidia data on W. albertisi, presented herein, will likely be useful in arguing for a new taxonomic arrangement.
Supplemental Material
Download MS Excel (31.7 KB)Acknowledgements
I acknowledge the efforts of Dr. Andrew Storey who originally obtained the specimens held at the Western Australian Museum which allowed for the examination of glochidia in this study. I especially thank Lisa Kirkendale, Corey Whisson and Janelle Ritchie who allowed me access and training on the use of the SEM at the Western Australian Museum. I especially thank the editor and anonymous reviewer for their comments which significantly improved earlier versions of this publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Atkins, L. (1979) Observations on the glochidial stage of the freshwater mussel Hyridella (Hyridella) drapeta (Iredale) (Mollusca: Pelecypoda). Australian Journal of Marine and Freshwater Research 30, 411–416.
- Barnhart, M.C., Haag, W.R. & Roston, W.N. (2008) Adaptations to host infection and larval parasitism in Unionoida. Journal of the North American Benthological Society 27, 370–394.
- Chernyshev, A.V., Sayenko, E.M. & Bogatov, V.V. (2020) Superspecific Taxonomy of the Far Eastern Unionids (Bivalvia, Unionidae): Review and Analysis. Review and analysis. Biology Bulletin 47, 267–275.
- Cruz, R.A. & Quesada, R. (2017) Taxonomic position and glochidia from the freshwater mussel, Anodonta luteola (Unionoida: Unionidae), in Costa Rica. Cauadernos de Investigación UNED 9, 39–43.
- Graf, D.L. & Cummings, K.S. (2021) A ‘big data’ approach to global freshwater mussel diversity (Bivalvia: Unionoida), with an updated checklist of genera and species. Journal of Molluscan Studies 87(eyaa034), 1–36.
- Graf, D.L., Jones, H., Geneva, A.J., Pfeiffer, I.I.I., Klunzinger, J.M. & W, M. (2015) Molecular phylogenetic analysis supports a Gondwanan origin of the Hyriidae (Mollusca: Bivalvia: Unionida) and the paraphyly of Australasian taxa. Molecular Phylogenetics and Evolution 85, 1–9.
- Humphrey, C.L. (1984) Biology and ecology of the freshwater mussel Velesunio angasi (Bivalvia: Hyriidae) in the Magela Creek, Alligator Rivers region, Northern Territory. Ph.D. Thesis. University of New England, Australia.
- Jones, H.A., Simpson, R.D. & Humphrey, C.L. (1986) The reproductive cycles and glochidia of freshwater mussels (Bivalvia: Hyriidae) of the Macleay River, northern New South Wales, Australia. Malacologia 27, 185–202.
- Klunzinger, M.W. (2020) Description of the glochidia of Alathyria pertexta pertexta Iredale, 1934 (Bivalvia : Hyriidae) from south-eastern Queensland. Australian Journal of Zoology 67, 1–8.
- Klunzinger, M.W., Beatty, S.J., Morgan, D.L., Thomson, G.J. & Lymbery, A.J. (2012) Glochidia ecology in wild fish populations and laboratory determination of competent host fishes for an endemic freshwater mussel of south-western Australia. Australian Journal of Zoology 60, 26–36.
- Klunzinger, M.W., Beatty, S.J., Morgan, D.L., Thomson, G.J. & Lymbery, A.J. (2013) Morphological and morphometrical description of the glochidia of Westralunio carteri Iredale, 1934 (Bivalvia: Unionoida: Hyriidae). Molluscan Research 33, 104–109.
- Klunzinger, M.W., Whisson, C., Zieritz, A., Benson, J.A., Stewart, B.A. & Kirkendale, L. (2022) Integrated taxonomy reveals new threatened freshwater mussels (Bivalvia: Hyriidae: Westralunio) from southwestern Australia. Scientific Reports 12, 20385.
- Lopes-Lima, M., Bolotov, I.N., Do, V.T., Aldridge, D.C., Fonseca, M.M., Gan, H.M., Gofarov, M.Y., Kondakov, A.V., Prié, V., Sousa, R., Varandas, S., Vikhrev, I.V., Teixeira, A., Wu, T., Wu, X., Zieritz, A., Froufe, E. & Bogan, A.E. (2018) Expansion and systematics redefinition of the most threatened freshwater mussel family, the Margaritiferidae. Molecular Phylogenetics and Evolution 127, 98–118.
- Mansur, M.C.D. (1999) Gloquídio de Diplodon martensi (Ihering) (Mollusca, Bivalvia, Hyriidae) e seu ciclo parasitário. Revista Brasileira de Zootecnia 16(Suppl. 2), 185–194.
- Mansur, M.C.D. & Silva, M.G.O. (1999) Description of glochidia of five species of freshwater mussels (Hyriidae: Unionoidea) from South America. Malacologia 41, 475–483.
- Marshall, B.A., Fenwick, M.C. & Ritchie, P.A. (2014) New Zealand Recent Hyriidae (Mollusca: Bivalvia: Unionida). Molluscan Research 34, 181–200.
- McMichael, D.F. & Hiscock, I.D. (1958) A monograph of the freshwater mussels (Mollusca: Pelecypoda) of the Australian Region. Australian Journal of Marine and Freshwater Research 9, 372–507.
- Melchior, M., Collier, K.J. & Clearwater, S.J. (2021a) First record of complex release strategies and morphometry of glochidia in sympatric Echyridella species (Bivalvia: Unionida: Hyriidae). Hydrobiologia 848, 3115–3126.
- Melchior, M., Squires, N.J., Clearwater, S.J. & Collier, K.J. (2021b) Discovery of a host fish species for the threatened New Zealand freshwater mussel Echyridella aucklandica (Bivalvia: Unionida: Hyriidae). New Zealand Journal of Marine and Freshwater Research 57, 152–159. doi:10.1080/00288330.2021.1963290.
- Miyahira, I.C., Barbosa dos Santos, S. & Mansur, M.C.D. (2017) Freshwater mussels from South America: state of the art of Unionida, specially Rhipidodontini. Biota Neotropica 17, e20170341.
- Miyahira, I.C., Mansur, M.C.D. & dos Santos, S.B. (2019) Redescription of Diplodon ellipticus Spix in Wagner, 1827, Diplodon multistriatus (Lea, 1831), and Rhipidodonta garbei (Ihering, 1910) (Bivalvia: Hyriidae) from coastal rivers of eastern and northeastern Brazil. Archiv für Molluskenkunde 148, 9–34.
- Parodiz, J.J. & Bonetto, A.A. (1963) Taxonomy and zoogeographic relationships of the South American Naiades. International Journal of Malacology 1, 179–213.
- Pfeiffer, J.M. III & Graf, D.L. (2020) Evolution of bilaterally asymmetrical larvae in freshwater mussels (Bivalvia: Unionoida: Unionidae). Zoological Journal of the Linnean Society 175, 307–318.
- Pimpão, D.M., Mansur, M.C.D., Aydos Bergonci, P.E. & Beasley, C.R. (2012) Comparative morphometry and morphology of glochidial shells of Amazonian Hyriidae (Mollusca: Bivalvia: Unionida). American Malacological Bulletin 30, 73–84.
- Ponder, W.F., Hallan, A., Shea, M.E., Clark, S.A., Richards, K., Klunzinger, M.W. & Kessner, V. (2022) Australian Freshwater Molluscs. Version 3. https://keys.lucidcentral.org/keys/v3/freshwater_molluscs/.
- Ponder, W.F., Lindberg, D.R. & Ponder, J.M. (2020) Biology and Evolution of the Mollusca. Volume 2. CRC Press, Boca Raton, FL, USA. pp. 169–170.
- Santos-Neto, G.C., Beasley, C.R., Schneider, H., Pimpão, D.M., Hoeh, W.R., de Simone, L.R.L. & Tagliaro, C.H. (2016) Genetic relationships among freshwater mussel species from fifteen Amazonian rivers and inferences on the evolution of the Hyriidae (Mollusca: Bivalvia: Unionida). Molecular Phylogenetics and Evolution 100, 148–159.
- Surber, T. (1912) Identification of glochidia of freshwater mussels. Bureau of Fisheries Document No. 177. Government Printing Office, Washington, DC.
- Tapparone Canefri, C. (1883) Fauna malacologica della Nuova Guinea e delle isole adiacenti. Annali del Museo civico di storia naturale di Genova 19, 6–313.
- Vale, R.S., Beasley, C.R., Tagliaro, C.H. & Mansur, M.C.D. (2005) The glochidium and marsupium of Castalia ambigua ambigua Lamarck, 1819, from northern Brazil. American Malacological Bulletin 20, 43–48.
- Walker, K.F. (1981) Ecology of freshwater mussels in the River Murray. Australian Water Resources Council Technical Paper No. 63. Australian Water Resources Council, Canberra.
- Walker, K.F., Jones, H.A. & Klunzinger, M.W. (2014) Bivalves in a bottleneck: taxonomy, phylogeography and conservation of freshwater mussels (Bivalvia: Unionoida) in Australasia. Hydrobiologia 735, 61–79.
- Widarto, T.H. (1993) Aspects of the biology of Velesunio ambiguus Philippi from a tropical freshwater environment, Ross River, Townsville, Australia. M.Sc. Thesis, James Cook University of North Queensland, Townsville.
- Zieritz, A., Sartori, A.F. & Klunzinger, M.W. (2013) Morphological evidence shows that not all Velesunioninae have smooth umbos. Journal of Molluscan Studies 79, 277–282.
