ABSTRACT
Nest tree availability is a critical reproductive requirement for woodpeckers. To inform off-reserve management strategies for Okinawa woodpecker (OW) Dendrocopos noguchii (Syn. Sapheopipo noguchii) nest trees, we assessed the characteristics of 213 nest trees as well as the detailed forest age of the nest sites by using aerial photographs and the local forest registers. The woodpecker used 17 tree species and one tree fern. The dominant tree species used were Castanopsis sieboldii (34% of total nest trees), Melia azedarach (11%), Alnus japonica (24%), and Pinus luchuensis (8%). Castanopsis sieboldii were used most often in the forests ≥ 60 years old, while A. japonica, P. luchuensis, and M. azedarach were used most often in the younger forests. The relatively high frequency of nests in A. japonica and P. luchuensis snags was likely affected by tree die-offs due to outbreaks of the introduced leaf beetle Plagiosterna formosana and pine wilt disease caused by the introduced Pine wood nematode Bursaphelenchus xylophilus, respectively. However, these die-offs were temporary, and snags created by exotic pest outbreaks may not be long-lasting resources in the warm, humid climate. To ensure the stable supply of available nest trees for the OW, we recommend preserving areas of forest ≥ 60 years old, retaining a few C. sieboldii trees when logging, and also retaining most regenerating M. azedarach trees during thinning operations in young forests.
Introduction
Bird species endemic to islands are vulnerable to extinction (Johnson and Stattersfield Citation1990; Biber Citation2002). Clarifying the habitat requirements of threatened birds is one of the first steps to successfully conserving them. The Okinawa woodpecker (Dendrocopos noguchii [Syn. Sapheopipo noguchii]; hereafter OW) is endemic to the Yambaru area in the northern Okinawa Island, Japan (). The OW is one of the rarest extant woodpecker species (Winkler et al. Citation2005) and is categorized as Critically Endangered by the Red Lists of the International Union for Conservation of Nature and Natural Resources (IUCN) (BirdLife International Citation2018), Ministry of the Environment of Japan (Ministry of the Environment of Japan Citation2019), and Okinawa Prefecture (Nature Conservation Division, Department of Environmental Affairs, Okinawa Prefectural Government Citation2017).
Figure 1. (a) A male Okinawa woodpecker perched at the entrance of a nest hole in Melia azedarach. Arrows indicate bumps due to internal decay. (b) A nest in an Alnus japonica presumed to have died after being topped to clear electric lines. (c) The leaf beetle Plagiosterna formosana which was first confirmed on Okinawa Island in 2010. (d) An A. japonica snag that fell after an outbreak of P. formosana.

The most effective way to ensure the conservation of endangered species is to establish sufficient protected areas. However, establishing sufficient reserves can be difficult and most of the world’s forests are not in reserves (Lindenmayer et al. Citation2006; Rayner et al. Citation2014). Therefore, “off-reserve management strategies” that account for multiple land uses are required to achieve endangered species’ conservation goals (Lindenmayer et al. Citation2006).
In light of Yambaru’s biodiversity conservation value, the Japanese Ministry of the Environment established Yambaru National Park as Japan’s 33rd national park in 2016. In addition, the Japanese Government has nominated “Amami-Oshima, Tokunoshima, Northern Part of Okinawa Island, and Iriomote Island” (including Yambaru) as a candidate of UNESCO World Natural Heritage Site (Government of Japan Citation2019). The Government of Japan has established a “buffer zone” around the nominated World Natural Heritage Site to allow for balanced use while prioritizing biodiversity conservation. In addition, a surrounding “conservation area” has been established around the “buffer zone” for a comprehensive management plan (Government of Japan Citation2019). Improving forest management strategies to sustain both native biodiversity and local forestry remains a high priority in the region.
The impacts of past and current forestry operations must first be understood before sustainable forestry practices can be developed within endangered species habitat. Yambaru’s forests serves as important habitat for several globally endangered species such as the OW, Okinawa rail Hypotaenidia okinawae, and Okinawa spiny rat Tokudaia mueninki (Government of Japan Citation2019). Yambaru also has a long history of timber production that dates back to at least the 18th century (Nakama et al. Citation2013). In particular, substantial logging occurred between 1945 and the 1970s to meet increased timber demand related to postwar reconstruction (Takashima et al. Citation2008; Saito Citation2011). In addition, most of the coastal forests were converted to agricultural fields after the end of World War II (Takashima et al. Citation2008). As a result, most of Yambaru’s forests are secondary growth, and old-growth forests are limited (Nakasuga Citation1995; Nakama Citation2010; Saito Citation2011). As a habitat for endemic animals, Yambaru forest was likely at its most degraded in the late 20th century (Ishida Citation1989; Ito et al. Citation2000). Regarding OW habitat, previous reports suggest that Castanopsis sieboldii is the main tree species used by OWs and that suitable C. sieboldii are limited to old-growth forest (Short Citation1973; Ogasawara and Ikehara Citation1977; Tamaki and Nakamura Citation1988; Azama and Shimabukuro Citation1993; Ito et al. Citation2000). Azama and Shimabukuro (Citation1993) suggested that forests over 40 years old were important OW nesting habitat and proposed that these forests should be designated as permanent protected areas. However, the majority of Yambaru’s forested area is now more than 40 years old due to the decrease in logging in recent years; in 2008, 84% of the forests in Yambaru were over 40 years old (Department of Agriculture, Forestry and Fisheries, Okinawa prefecture Citation2019). Improvements in food availability have also led to the abandonment of large areas of formerly cultivated land that have since turned to secondary forest (Takashima et al. Citation2008; Saito Citation2011). In recent years, OW nests have been observed in secondary forests <40 years old and pine forests around the old growth forests (Kinjo Citation1997; Kotaka Citation2009, Citation2013, Citation2014).
Prior studies have addressed how to maintain or create suitable nest trees for woodpeckers, but our understanding is limited to results from temperate and boreal forests in North America and Europe (Cooke and Hannon Citation2012; Kilgo and Vukovich Citation2014; Bell et al. Citation2015). Research in Asia is lacking, especially from the subtropical and temperate zones (Cockle et al. Citation2011, Citation2012). The availability of nest trees is a critical reproductive requirement for woodpeckers (Winkler and Christie Citation2010), but management strategies may differ between regions with different forest types. Woodpecker nests tend to be in large living trees with decayed heartwood and snags (Jackson and Jackson Citation2004; Virkkala Citation2006), both of which tend to be more abundant in old-growth forests (Stokland et al. Citation2012).
We conducted OW nest tree surveys from 2005 to 2019 to assess what types of trees serve as OW reproductive habitats in forests of various ages. Specifically, we sought to compare nest trees species and characteristics between different forest ages to inform sustainable forestry practices in off-reserve forests and improve the conservation of the OW. Since two exotic forest pest outbreaks occurred during the study period (Kotaka Citation2009, Citation2013), we also discuss their effects on nest tree use by OW. To the best of our knowledge, this is the first study to describe the influences of exotic forest pests on the nest tree use of woodpeckers inhabiting an island forest ecosystem.
Materials and methods
Study site
We conducted our survey in the forests of northern Okinawa Island, Japan. This forest consists mainly of broadleaved trees (C. sieboldii and Schima wallichii are dominant) and natural or planted Pinus luchuensis (Yamamori Citation1979; Department of Environmental Affairs, Okinawa Prefecture Citation1993; Ito Citation1997; Kubota et al. Citation2005; Takashima et al. Citation2014). Pinus luchuensis is one of Okinawa’s most important timber trees and the majority of P. luchuensis plantations (12,816 ha) were established during the 1950s and the 1960s (Yamamori Citation1979). The maximum altitude in the Yambaru area is 503 m, which is on Mt. Yonahadake in Kunigami Village. Yambaru’s subtropical climate is humid, with a mean annual temperature of 20.9°C, mean annual rainfall of 2,603 mm (based on data from Oku, Kunigami Village at 232 m above sea level), taken from 1990 to 2019 (Japan Meteorological Agency Citation2020).
Methods
OW nest tree survey
We established 12 belt transects to systematically explore the nest trees of Okinawa woodpecker. The transects were 1.4–5.3 km long and 200 m width, on the total 42.6 km long and 817.5 ha, at between 5–400 m elevation in Kunigami Village. We surveyed for active OW nests along each transect at least twice during each breeding season (April to June) from 2005 to 2019. In the surveys, we also recorded the locations where adult OWs were observed, and for those locations, we later entered the forest to search for active nests. If we still could not find the nests, we conducted surveys up to five times. Adult OW calls and drumming can be detected from a distance more than 200 m if there is no wind and no rainfall. So, we selected such favorable weather conditions for the surveys. The begging call of the nestlings is also loud, and that can be heard from a distance more than 100 m during the latter half of the nestling period. We defined nests as active if they contained nestlings or eggs. After we confirmed whether the nestlings fledged or the nest failed, we measured the nest tree’s diameter at breast height (DBH) and recorded its GPS location. We also recorded the nest tree species and tree condition (i.e. live or dead). If the same tree was used in multiple years, only the first record was included in the nest tree characteristics analysis. Only the data systematically surveyed within the 12 belt transects were used for analysis of the annual change in the nest tree use by the OW from 2005 to 2019.
Forest age analysis
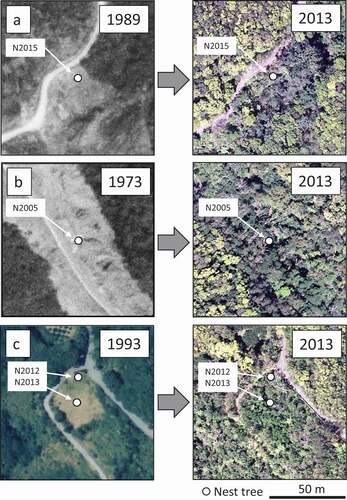
Nest tree locations, aerial photographs (US Air Force 1944, 1946, 1962; Geospatial Information Authority of Japan 1973, 1977, 1989, 2002; Nakanihon Air Service 2011; NTT Geospace 2013), the local forest register, and forest geographic information system (GIS) maps created by Okinawa Prefecture and National Forest were integrated using QGIS 3.12 software (QGIS Development Team Citation2020) to determine the forest age of each nest site. When there was no contradiction between forest age estimates from aerial photographs and that recorded in the local forest register, we used the forest age recorded in the local forest register (). When there was a contradiction, we measured forest age from the central year between when aerial photographs were first confirmed logging and the previous year when aerial photographs were taken. For sites in pine plantations, forest age was measured as years since planting according to the forest register. For sites logged due to construction (e.g. of roads), forest age was measured as the number of years since completion of construction (). When detailed construction information was not available and in the case of abandoned cultivated land (), forest age was calculated in the same manner as when the forest register and aerial photographs were contradictory. Forests ≥ 60 years old were grouped into one category. This is because the oldest aerial photographs were taken in 1944, and the most of nest sites with forests ≥ 60 years old could not be confirmed with the photographs. Other forests were divided into two age classes: 20–39-year-old and 40–59-year-old forests. When nest trees were presumed to have died due to topping or girdling, the cause was recorded (Kotaka Citation2018). In this analysis, we included data on nest trees that were based on information from local residents and naturalists, as well as from the OW “Plan for Protection and Recovery Program” carried out by the Ministry of the Environment from 1999 to 2019 other than the data from the systematic belt transect surveys. We compared the relative frequency of nest tree species used per forest age class and the proportion of dead nest trees used per nest tree species and per forest age class using Fisher’s exact tests. Statistical analysis was conducted using R 4.0.2 (R Core Team Citation2020).
Annual nest tree usage patterns
We analyzed the annual change of the active nests density and nest tree use of the four primary tree species (see Results) in the 12 belt transects from 2005 to 2019. In this analysis, we included the reused nest trees to clarify how much each tree species contributes to the reproduction during each breeding season. Based on the data provided by Okinawa Prefecture (Department of Agriculture, Forestry and Fisheries, Okinawa prefecture Citation2020 including their unpublished data in FY2019), the amount of P. luchuensis trees damaged by pine wilt disease in Kunigami Village from FY1999 to FY2019 was calculated. An exotic leaf beetle Plagiosterna formosana was first recorded in Okinawa Island in 2010 (Kotaka Citation2013), and Alnus japonica trees that had subsequently died due to the leaf beetle outbreak began to be used for nesting by OW in 2012 (Kotaka Citation2013). Judging from the timing of the increase in mortality of A. japonica, we divided the survey period into two periods (i.e. 2005‒2011 and 2012‒2019), and conducted a Wilcoxon test to compare the frequency of active nests of dead A. japonica trees between the two periods by using R 4.0.2 (R Core Team Citation2020). This analysis was not performed on P. luchuensis because the amount of damaged pine trees has continued to decline since 2001, and we could not divide the survey period into two periods appropriately.
Results
Nest tree characteristics
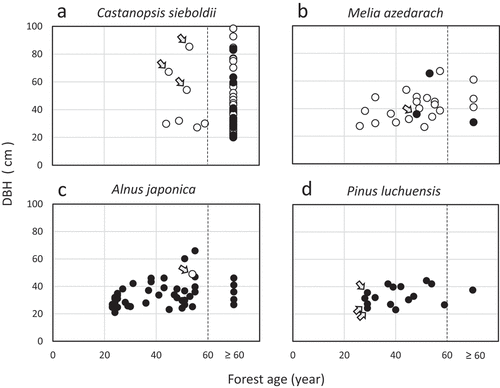
We confirmed 213 active OW nest trees from 1999 to 2019 (). These included 17 tree species and one tree fern (Cyathea lepifera). Two nest trees were too decayed to be identified to species. Castanopsis sieboldii was the most frequently used species for nesting, followed by A. japonica, Melia azedarach, and P. luchuensis (). Of the four primary species most frequently used, the percentage of dead trees in the nest trees was low in C. sieboldii and M. azedarach, and high in A. japonica and P. luchuensis (). There was a significant difference between species in the proportion of dead trees used (Fisher’s exact test: P < 0.01). Among the four major nest tree species, the reuse rate of M. azedarach was the highest (). Mean (±SD) DBH of nest trees was 38.5 ± 14.8 cm (range: 20.0–98.4 cm).
Table 1. Nest tree characteristics: status of nest trees, diameter at breast height (DBH) and rate of reuse
Influences of forest age
The relative percentages of nest tree species used differed significantly by forest age class (; Fisher’s exact test: P < 0.01). In C. sieboldii, most of the nest trees were found in ≥60 years old forests, while those of the other three species were found in <60 years old forests (). Of a total of 16 P. luchuensis nest trees, seven had been planted, eight had regenerated after clear cutting (3) or road construction (5), and one had grown in abandoned farmland. The percentages of dead trees among all nest trees decreased with increasing forest age (Fisher’s exact test: P < 0.01).
Table 2. Forest age class of the nest sites and nest tree composition
The minimum forest ages of nest sites were 44 years for C. sieboldii, 23 years for A. japonica, 26 years for M. azedarach, and 28 years for P. luchuensis (). Three C. sieboldii nest trees were determined from the aerial photographs to be retention trees when the forest stand was logged (). Five nest trees were artificially killed: one M. azedarach was girdled (), while one A. japonica () and three P. luchuensis were topped ().
Figure 3. Relationship between estimated nest site forest age and nest tree diameter at breast height. White circles indicate live trees and black circles indicate dead trees. The arrows in Castanopsis sieboldii indicate trees estimated to have been left uncut during logging (a). The arrow in Melia azedarach indicates a girdled tree (b). The arrows in Alnus japonica (c) and Pinus luchuensis (d) indicate topped trees. Forest ages of the nest sites ≥ 60 years old are combined into one category
Influences of exotic forest pests
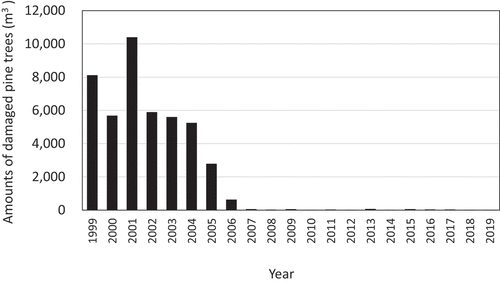
The amount of damaged pine trees in Kunigami Village was relatively large from 1999 to 2006, peaking at 10,383 m3 in 2001, and exceeding 5,000 m3 until 2004 (). However, it has decreased since then and has remained low at 2,770 m3 in 2005, 614 m3 in 2006, and 2–53 m3 from 2007 to 2019.
Figure 4. Annual changes in the volume (m3) of Pinus luchuensis trees damaged by pine wilt disease from 1999 to 2019 (on the total of each fiscal year)
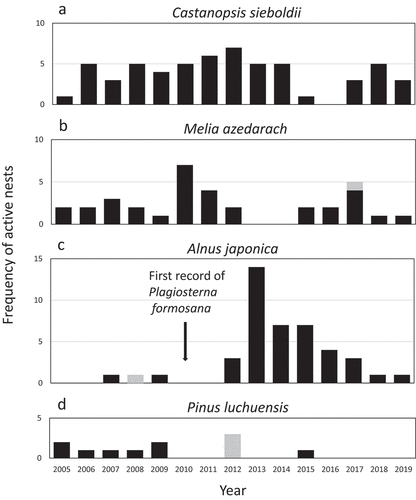
We found a total of 186 active nests in 163 nest trees in the 12 belt transects from 2005 to 2019 (). In 2013, the nesting density of OW was the highest (3.11/100 ha) and was more than twice as high as the mean nesting density (1.52/100 ha) from 2005 to 2019 (). Annual change in the frequency of active nests differed between the four primary nest tree species from 2005 to 2019 (). Castanopsis sieboldii and M. azedarach were used for almost the entire study period (). The usage of dead A. japonica trees peaked in 2013 (), and differed significantly between the first seven years and the following eight years (W = 3, P < 0.01). The usage of dead P. luchuensis trees was continuously observed until 2009, but became rare after 2010 ().
Figure 5. Annual changes in the average density of active nests from 2005 to 2019. Error bars indicate standard deviation
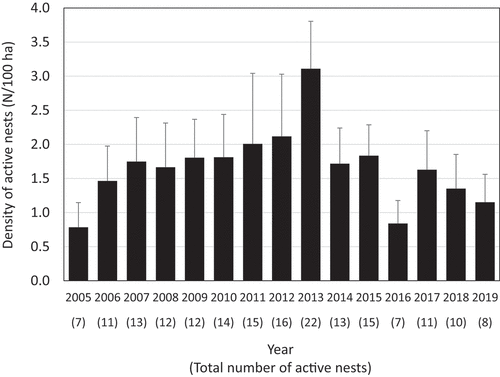
Figure 6. Annual changes in the number of active nests in Castanopsis sieboldii (a), Melia azedarach (b), Alnus japonica (c), and Pinus luchuensis (d). Gray bar: Nest trees that were artificially killed (i.e. topped or girdled). Alnus japonica trees that died after an outbreak of the exotic leaf beetle, Plagiosterna formosana which was first recorded in Okinawa in 2010
Discussion
Nest tree characteristics
The four major tree species among 213 nest trees confirmed between 1999 and 2019 were C. sieboldii, A. japonica, M. azedarach, and P. luchuensis. The minimum DBH of the nest trees was 20 cm. The composition ratio of living and dead trees differed among the nest tree species; the proportion of living trees was high for C. sieboldii and M. azedarach, while most A. japonica and P. luchuensis nest trees were dead. Several studies have reported that woodpeckers prefer nesting in trees with decayed heartwood (Conner et al. Citation1976; Jackson and Jackson Citation2004). Therefore, it was thought that C. sieboldii and M. azedarach, in which the live trees were often used for nesting, were also prone to heartwood decay. In fact, a study using a resistograph (Rinn et al. Citation1996) reported that OW also nests in live C. sieboldii with decayed heartwood (Kotaka et al. Citation2006).
Forest age, nest tree characteristics and causes of tree death
Castanopsis sieboldii was the most-used nest tree species overall, and no C. sieboldii were used in forests < 40 years old. This result is consistent with Azama and Shimabukuro (Citation1993), who concluded that ≥ 40-year-old forests serve as important OW breeding habitat. C. sieboldii was frequently used by OW partly because it is one of the dominant tree species in Yambaru’s secondary forests and very abundant (Department of Environmental Affairs, Okinawa Prefecture Citation1993; Kubota et al. Citation2005; Fujii et al. Citation2009). However, C. sieboldii is a dominant tree species even in younger forests (Department of Environmental Affairs, Okinawa Prefecture Citation1993; Kubota et al. Citation2005; Oshima and Takashima Citation2020) and although it has grown to a size that allows nesting, it has been used less frequently in 40–60-year-old forests. Schima wallichii is also an abundant tree species in broad-leaved forests in the Yambaru area (Department of Environmental Affairs, Okinawa Prefecture Citation1993; Kubota et al. Citation2005), but it was used less frequently across all age classes. In a recent study investigating the cavity formation rate in the Yambaru area, it was found that the frequency of natural cavities was much lower in S. wallichii than that in C. sieboldii, and that the cavity formation rate of C. sieboldii was positively related to the tree size (Takashima et al. unpublished data). Evidently, not only the abundance of large diameter trees, but also the decay condition, are important for the suitability of OW nest trees.
Melia azedarach were used as nest trees in forests up to 18 years younger than the youngest C. sieboldii used. Melia azedarach is a fast-growing pioneer tree species (Fukuyama Citation1996), and a high proportion of these trees used were alive. In the vicinity of the nest cavities of all 21 live M. azedarach, we observed bumps of previously broken branches, which is a sign of internal decay (). The relatively high reuse rate of M. azedarach may be due to the fact that this tree species provides nest trees in younger forests, which are generally lacking available trees for woodpeckers (Jackson and Jackson Citation2004; Cockle et al. Citation2011).
In this study, dead trees were most commonly used in younger forests. In general, dead trees are a habitat element that characterizes old growth forests (Stokland et al. Citation2012). In this study, dead A. japonica and P. luchuensis were uncharacteristically abundant in younger forests likely due to die-offs caused by two exotic forest pests, P. formosana and pine wilt disease (Kotaka Citation2009, Citation2013). Plagiosterna formosana, a beetle that feeds on A. japonica leaves (), was recorded for the first time on Okinawa Island in 2010, and many A. japonica died due to the subsequent outbreak of this pest. The increase in dead tree resources after the outbreak of exotic insects P. formosana may have contributed to the increase in OW nest density seen in 2012 and 2013. In 2013, the mortality rate of A. japonica ≥15 cm DBH surrounding the nest trees was 97% (Kotaka Citation2013). The decrease in the number of A. japonica used by OWs after 2013 is likely due to the decomposition of trees that died almost all at once after the outbreak of P. formosana ().
Damage to P. luchuensis by pine wilt disease, an exotic tree disease from the United States, was first confirmed in Okinawa in the 1970s (Kuniyoshi Citation1974) and spread to the Yambaru area in the late 1990s. The cause of this disease is infection by the pine wood nematode, B. xylophilus (Kiyohara and Tokushige Citation1971; Mamiya Citation1983). Even though the amounts of damaged pine trees decreased considerably after 2007 (), the usage of dead P. luchuensis trees by the OW was continuously observed until 2009 (). This may indicate that dead pine trees could be used as the nest trees for several years. The high usage of A. japonica and P. luchuensis by OW was likely caused by the temporary increase in the number of available dead trees due to these two tree diseases.
Management implications
Based on our results, we suggest that a stable supply of suitable OW nest trees could be maintained by conserving stands with large living C. sieboldii trees, leaving some living C. sieboldii trees in logged stands, and actively retaining living M. azedarach in younger stands during thinning. Forest stands ≥ 60 years old with available trees of C. sieboldii for OW nesting are worth to conserve at the stand level. We don’t have enough information about forests over 60 years old in 2005, but those forests including primeval forest should be conserved for biodiversity conservation.
We identified three cases where C. sieboldii trees retained through past logging were used for nesting by OW. This suggests that retaining a few C. sieboldii, a species which takes a long time to reach a suitable size and level of decay, per ha during logging could be an effective method for maintaining OW nesting habitat in areas used for forestry. Melia azedarach is a pioneer species that reaches a suitable size quicker than C. sieboldii (Fukuyama Citation1996; Takashima and Inafuku Citation2017; Oshima and Takashima Citation2020). Actively retaining some regenerating M. azedarach in stands following clear-cutting would help provide OW nest trees in young forests.
Dead A. japonica and P. luchuensis were common nest trees in young forests during our study period. However, these snags were considered to be ephemeral resources to be used for a few years following exotic forest pest outbreaks. Some policies in temperate and boreal forest have been started to include guidelines to retain some dead trees for woodpeckers (Bull and Holthausen Citation1993; Nappi et al. Citation2003; Hutto Citation2006). However, several studies have reported that dead trees have low strength and a high risk of collapse due to decomposition and wind, which may lead to lower woodpecker breeding success than that in live trees (Kilham Citation1971; Kotaka Citation2009). Moreover, in humid subtropical and tropical forest ecosystems, decomposition rates are high, and the lifespan of dead trees is short (Cockle et al. Citation2011, Citation2017). Okinawa Island is also in a typhoon-prone area, which may reduce the longevity of snags.
We confirmed several cases in which artificially created snags were used as nests by OW. These cases suggest that snag creations could be an effective means of increasing the number of nest trees available. Alnus japonica is an exotic tree species that is presumed to have been introduced from Taiwan to Okinawa Island approximately 100 years ago for human use (Takara and Amano Citation1977). Since A. japonica is not native, it is a good candidate for experimental artificial snag creation. This is a proposal to use existing exotic trees and not a proposal to increase the number of exotic species in order to increase the number of dead trees. Dead P. luchuensis may become a breeding substrate for the vector insect, Monochamus alternatus (Mamiya and Enda Citation1972; Morimoto and Iwasaki Citation1972), and killing P. luchuensis may promote the spread of pine wilt disease. In addition, P. luchuensis is endemic to the Ryukyu Islands, and serves as a food resource for the Endangered Diplothrix legata (Kudaka and Kudaka Citation2017). For these reasons, P. luchuensis should not be killed to create OW nesting habitat even in pine plantations. The creation of dead trees is only the next best thing, and the best way is to restore and preserve the natural old forest.
Acknowledgments
We thank M. Kudaka, N. Kudaka, K. Oshiro, A. Takashima, K. Nataka, R. Azuma, M. Kinjo, K. Takehara, K. Ozaki, K. Ishida, Y Sawashi, M. Toyama, T. Kudo, residents of Kunigami Village, the Okinawa Prefectural Government, and the Yambaru Ranger Office of the Ministry of the Environment of Japan for supporting our research. We deeply appreciate the editors and reviewers, whose various comments helped us to improve this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Azama Y, Shimabukuro M 1993. Survey on the habitat of Okinawa woodpeckers in the northern part of Okinawa Island (Kunigami Village, Ogimi Village, Higashi Village). Habitat surveys for endangered birds VI:41–58. Okinawa Prefectural Government, Naha (Japan. Japanese)
- Bell D, Hjältén J, Nilsson C, Jørgensen D, Johansson T. 2015. Forest restoration to attract a putative umbrella species, the white backed woodpecker, benefited saproxylic beetles. Ecosphere 6(12):1–14. https://doi.org/10.1890/ES14-00551.1
- Biber E. 2002. Patterns of endemic extinctions among island bird species. Ecography. 25:661–676. doi:10.1034/j.1600-0587.2002.t01-1-250603.x.
- BirdLife International 2018 Dendrocopos noguchii (amended version of 2016 assessment). The IUCN Red List of Threatened Species 2018. [accessed 2020 Jul 30] https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22681531A125513230.en
- Bull EL, Holthausen RS. 1993. Habitat use and management of pileated woodpeckers in northeastern Oregon. J Wildl Manag. 57:335–345. doi:10.2307/3809431.
- Cockle KL, Martin K, Bodrati A. 2017. Persistence and loss of tree cavities used by birds in the subtropical Atlantic Forest. For Ecol Man. 384:200–207. doi:10.1016/j.foreco.2016.10.052.
- Cockle KL, Martin K, Robledo G. 2012. Linking fungi, trees, and hole-using birds in a neotropical tree-cavity network: pathways of cavity production and implications for conservation. For Eco Man. 264:210–219. doi:10.1016/j.foreco.2011.10.015.
- Cockle KL, Martin K, Wesołowski T. 2011. Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Front Ecol Environ. 9:377–382. doi:10.1890/110013.
- Conner RN, Jr OK M, Adkisson CS. 1976. Woodpecker dependence on trees infected by fungal heart rots. Wilson Bull. 88:575–581.
- Cooke HA, Hannon SJ. 2012. Nest-site selection by old boreal forest cavity excavators as a basis for structural retention guidelines in spatially-aggregated harvests. For Ecol Man. 269:37–51. doi:10.1016/j.foreco.2011.12.042.
- Department of Agriculture, Forestry and Fisheries, Okinawa prefecture. 2019. Promotion of Yambaru-style forestry (revised version). Okinawa prefecture Web. [accessed 2020 Sept 3] https://www.pref.okinawa.jp/site/norin/shinrin/kikaku/documents/sesakuhoushin_r0109syuusei.pdf. Japanese.
- Department of Agriculture, Forestry and Fisheries, Okinawa prefecture. 2020. Reiwa first year Okinawa type forest environment conservation business control strategy study consignment business report. Okinawa prefecture Web. [accessed 2021 Mar 5] https://www.pref.okinawa.lg.jp/site/norin/shinrin/keiei/shinrinkankyohozen/r1report.html. Japanese.
- Department of Environmental Affairs, Okinawa Prefecture. 1993. Habitat surveys for endangered birds VI. Okinawa Prefectural Government, Naha (Japan. Japanese)
- Fujii S, Kubota Y, Enoki T. 2009. Resilience of stand structure and tree species diversity in subtropical forest degraded by clear logging. J For Res. 14:373–387. doi:10.1007/s10310-009-0151-7.
- Fukuyama N. 1996. Grow of Sendan (Melia azedarch) in the young stand. Nchirinkyushi. 49:83–84. Japanese.
- Government of Japan. 2019. Nomination of Amami-Oshima Island, Tokunoshima Island, Northern Part of Okinawa Island, and Iriomote Island for inscription on the World Heritage List. Government of Japan. [accessed 2020 Oct 20]. https://www.env.go.jp/press/files/jp/110737.pdf
- Hutto RL. 2006. Toward meaningful snag‐management guidelines for postfire salvage logging in North American conifer forests. Conserv Biol. 20:984–993. doi:10.1111/j.1523-1739.2006.00494.x.
- Ishida K. 1989. The protection of and research strategy for the populations of Dendrocopos leucotos owstoni and Sapheopipo noguchii. Strix. 8:249–260. Japanese.
- Ito Y. 1997. Diversity of forest tree species in Yanbaru, the northern part of Okinawa Island. Plant Ecol. 133:125–133. doi:10.1023/A:1009748016272.
- Ito Y, Miyagi K, Ota H. 2000. Imminent extinction crisis among the endemic species of the forests of Yanbaru, Okinawa, Japan. Oryx. 34:305–316. doi:10.1046/j.1365-3008.2000.00136.x.
- Jackson JA, Jackson BJ. 2004. Ecological relationships between fungi and woodpecker cavity sites. Condor 106:37–49. doi:10.1007/s10531-004-0657-4.
- Japan Meteorological Agency. 2020. Various climate data and materials. [accessed 2020 Jul 30]. https://www.jma.go.jp/jma/menu/menureport.html. Japanese.
- Johnson TH, Stattersfield AJ. 1990. A global review of island endemic birds. Ibis. 132:167–180. doi:10.1111/j.1474-919X.1990.tb01036.x.
- Kilgo JC, Vukovich MA. 2014. Can snag creation benefit a primary cavity nester response to an experimental pulse in snag abundance. Biol Conserv. 171:21–28. doi:10.1016/j.biocon.2014.01.003.
- Kilham L. 1971. Reproductive behavior of Yellow-bellied Sapsuckers. I. Preference for nesting in Fomes-infected aspens and nest hole interrelations with flying squirrels, raccoons, and other animals. Wil Bull. 83:159–171.
- Kinjo M. 1997. Okinawa Woodpecker. In: Hidaka T, Higuchi H, Morioka H, Yamagishi S, editors. Encyclopedia of Animals in Japan, Volume 4, Birds II. Tokyo: Heibonsha. Japanese; p. 61–62.
- Kiyohara T, Tokushige Y. 1971. Inoculation experiments of a nematode, Bursaphelenchus sp., onto pine trees. J Jap For Soc. 53:210–218. Japanese.
- Kotaka N. 2009. Breeding failure of Okinawa Woodpeckers nesting in dead pine tree in pin wilt disease infested area. Kyushu J For Res. 62:98–99. Japanese.
- Kotaka N. 2013. Nesting on Alnus japonica snags by Dendrocopos noguchii: infulence of the intrusion and outbreak of Plagiosterna formosana in Okinawa Island. Kyushu J For Res. 66:77–80. Japanese.
- Kotaka N. 2014. Okinawa woodpecker. In: Aves E, editor. Red data book 2014. Vol. 2, GYOSEI Corpration (Tokyo): Ministry of the Environment; p. 64–65. Japanese.
- Kotaka N. 2018. A rare woodpecker species on a southern island: nest-web among the nest cavity of the Okinawa Woodpecker. In: Takagi M, Mizuta T, editors. Ornithology on islands – natural history studies of bird in the Nansei Archipelago. Tokyo: Kaiyusha; 271–285. Japanese.
- Kotaka N, Sato H, Toyama M, Enoki T, Yamashita K, Nagao H. 2006. Variation of the tree hardness inside the Okinawa Woodpecker nest-tree. Kyushu J For Res. 59:194–196. Japanese.
- Kubota Y, Katsuda K, Kikuzawa K. 2005. Secondary succession and effects of clear-logging on diversity in the subtropical forests on Okinawa Island, southern Japan. Biodivers Conserv. 14:879–901.
- Kudaka N, Kudaka M. 2017. Food habits and habitat of the Ryukyu long-furred rat in Yambaru forest, northern Okinawa Island. J Mammal Soc Jpn. 57:195–202. Japanese.
- Kuniyoshi K. 1974. Occurrence of pine infestations in Okinawa Prefecture caused by pine wood nematodes. Shinrin Boeki (Forest Pests). 23:40–42.
- Lindenmayer DB, Franklin JF, Fischer J. 2006. General management principles and a checklist of strategies to guide forest biodiversity conservation. Biol Conserv. 131(3):433–445. doi:10.1016/j.biocon.2006.02.019.
- Mamiya Y. 1983. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu Rev Phytopathol. 21:201–220. doi:10.1146/annurev.py.21.090183.001221.
- Mamiya Y, Enda N. 1972. Transmission of Bursaphelenchus lignicolus (Nematoda: aphelenchoididae) by Monochamus alternatus (Coleoptera: cerambycidae). Nematologica 18:159–162. doi:10.1163/187529272X00395.
- Ministry of the Environment of Japan. 2019. Red list 2019, Ministry of the Environment. [accessed 2020 Jul 22]. http://www.env.go.jp/press/files/jp/110615.pdf. Japanese.
- Morimoto K, Iwasaki A. 1972. Role of Monochamus alternatus (Coleoptera: cerambycidae) as a vector of Bursaphelenchus lignicolus (Nematoda: aphelenchoididae). J Japn For Soc. 54:177–183. Japanese.
- Nakama Y. 2010. Reminiscences on the forestry life in Kunigami village, the northern part of main island of Okinawa. Sci Bull Fac Agric Univ Ryukyus. 57:41–57. Japanese.
- Nakama Y, John MP, Bixia C. 2013. Modern Japanese & English Translations and Content Analysis of ‘The Scope of the Bureau of Forest Administration’ from the ‘Eight Volumes on Forest Administration. Sci Bull Fac Agric Univ Ryukyus. 60:45–58.
- Nakasuga T. 1995. Historical changes of forestry in Okinawa Islands. Nara: Hirugi-sha. Japanese.
- Nappi A, Drapeau P, Giroux JF, Savard JPL. 2003. Snag use by foraging black-backed woodpeckers (Picoides arcticus) in a recently burned eastern boreal forest. Auk 120:505–511. doi:10.1093/auk/120.2.505.
- Nature Conservation Division, Department of Environmental Affairs, Okinawa Prefectural Government. 2017. Threatened Wildlife in Okinawa. Third ed. Japanese: (Animals) -Red Data Okinawa.
- Ogasawara K, Ikehara S. 1977. Ecological and behavioral observations of Okinawa Woodpecker Sapheopipo noguchii, with notes on conservation. J Yamashina Inst Ornithol. 9:143–158. doi:10.3312/jyio1952.9.143.
- Oshima Y, Takashima A. 2020. Succession from 30 to 40-year-old secondary forest in the Yambaru area of Okinawa Island. Kyushu J For Res. 73:27–32. Japanese.
- QGIS Development Team. 2020. QGIS Geographic Information System. Open Source Geospatial Foundation Project
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna (Austria):R Foundation for Statistical Computing.
- Rayner L, Lindenmayer DB, Wood JT, Gibbons P, Manning AD. 2014. Are protected areas maintaining bird diversity. Ecography. 37(1):43–53. doi:10.1111/j.1600-0587.2013.00388.x.
- Rinn F, Schweingruber FH, Schär E. 1996. Resistograph and X-ray density charts of wood. Comparative drill resistance profiles and X-ray density charts of different wood species. Holzforschung Int J Bio Chem Physics Tech Wood. 50:303–311.
- Saito K. 2011. Forest age distribution in Kunigami-village, Okinawa, based on forest register data. Pap Env Inform Sci. 25:245–250. Japanese.
- Short LL. 1973. Habits, relationships, and conservation of the Okinawa woodpecker. Wilson Bull. 85:5–20.
- Stokland JN, Siitonen J, Jonsson BG. 2012. Biodiversity in dead wood. Cambridge: Cambridge university press..
- Takara T, Amano T. 1977. Okinawa animal and plant research history. Naha (Japan): Naha press. Japanese.
- Takashima A, Inafuku S. 2017. Current stand dynamics of 65- to 70-year-old secondary forest in the Yambaru area of Okinawa Island. Kyushu J For Res. 70:17–20. Japanese.
- Takashima A, Kajisa T, Saito K. 2008. The changes of forest use and development in the northern part of Okinawa Island based on aerial photographs interpretation. Kyushu J For Res. 61:57–60. Japanese.
- Takashima A, Oshima J, Kudaka M, Saito K. 2014. Stand structure of the subtropical natural forest in the Yambaru area of Okinawa Island: a comparison of a 60-year-old secondary forest and a mature non-clear-cut forest. Jpn J For Plann. 48:27–34. Japanese.
- Tamaki C, Nakamura T. 1988. Special natural monument of Japan, Okinawa Woodpecker – ecology and Habitat – aki-Shobo. Japanese.
- Virkkala R. 2006. Why study woodpeckers? The significance of woodpeckers in forest ecosystems. Ann Zool Fennici. 43:82–85.
- Winkler H, Christie DA. 2010. Woodpeckers. Sussex: A&C Black.
- Winkler H, Kotaka N, Gamauf A, Nittinger F, Haring E. 2005. On the phylogenetic position of the Okinawa woodpecker (Sapheopipo noguchii). J Ornithol. 146:103–110. doi:10.1007/s10336-004-0063-4.
- Yamamori N. 1979. Studies on the characteristics of water and silvicultural techniques for avoiding drought damages of Pinus luchuensis stands. Sci Bull Fac Agric Univ Ryukyus. 26:573–716. Japanese.