Abstract
Global warming is predicted to aggravate the risk of unstable crop production. It is of great concern that damage to rice spikelet sterility and grain quality will increase, resulting in yield and economic losses. To secure the global food supply and farmers’ income, the development of rice cultivars with heat resilience is a pressing concern. Regarding spikelet sterility, rice cultivars with heat tolerance at different growth stages have been identified in recent years. The early-morning flowering (EMF) trait is effective in heat escape because it shifts the time of day of flowering to earlier in the morning when it is cooler. Although varietal differences are very small, there are some genetic resources for EMF in wild rice accessions. Regarding heat-induced grain chalkiness, heat-tolerant japonica cultivars for mitigating white-back type of chalky grains (WBCG) were found. Quantitative trait loci for heat tolerance at flowering, EMF, and for WBCG in grain quality have been mapped on the rice chromosomes. Further genetic efforts have been successfully connected to the development of near-isogenic lines for each trait with tagged molecular markers. These breeding materials are quite unique and useful in facilitating marker-assisted breeding toward the development of heat-resilient rice in terms of spikelet sterility and grain quality.
Introduction
Global warming has been increasing progressively. In the past three decades, Earth’s surface temperature has been becoming warmer than any preceding decade since 1850, and the worst scenario predicted is that global mean surface temperature may rise 4.8 °C by the end of this century relative to the period 1986–2005 (IPCC, Citation2013). This progressive increase in temperatures is not an exception in Japan. Annual mean temperature has risen at a rate of about 0.85 °C in the past 100 years globally (IPCC, Citation2013), while at a higher rate of about 1.14 °C in Japan (Japan Meteorological Agency, Citation2013). It is obvious that the frequency of days of maximum daytime temperature above 35 °C tends to increase after the 1990s in Japan (Japan Meteorological Agency, Citation2013).
Crop growth is seriously affected by the changing climate; thus, global warming has become a major constraint for crop production. High-temperature stress is one of the most serious threats to crop production worldwide (Boyer, Citation1982). The drastic changes in temperature in recent years have caused more frequent occurrence of extreme weather events such as heat waves and drought. Temperate and subtropical agricultural areas might bear substantial crop yield losses because of extreme temperature episodes (Teixeira et al., 2013). The effect of extreme temperature events on crop production is likely to become more frequent in the near future (Tebaldi et al., 2006).
In rice, heat stress at flowering and grain-filling stages seriously affects spikelet fertility and grain quality. Rice is most susceptible to heat stress at flowering, and previous chamber experiments showed that temperatures above 32–36 °C cause high spikelet sterility (Jagadish et al., 2008; Matsui et al., 1997a, 1997b, 2001; Maruyama et al., 2013; Prasad et al., 2006; Satake & Yoshida, Citation1978; Weerakoon et al., 2008). Heat-induced spikelet sterility (HISS) at flowering is associated with reduction in grain yield. Rice grain quality is a determinant factor for market value. Although consumers’ preference is quite diverse in terms of grain shape, amylose content, aroma, etc. (Calingacion et al., 2014), grain chalkiness critically decreases the value in the market because of the breakage of grains during milling (Lyman et al., 2013; Zhao & Fitzgerald, 2013) and decreases cooking and eating quality (Kim et al., 2000; Lisle et al., 2000) in most japonica and indica cultivars. Chalky grains increase when panicles are exposed to high temperatures during grain filling. Chalky grains induced by heat stress are associated with reduction in head rice yield and market values. Among the various types of chalky grains, white-back type of chalky grains (WBCG) are highly correlated with heat stress during grain filling (Wakamatsu et al., 2007).
To secure the global food supply and farmers’ income, the development of heat-resilient rice cultivars is in high demand. In this review, we focus on genetic analyses for HISS at flowering in terms of heat tolerance and escape, and for grain appearance in terms of WBCG. We also describe the mechanisms and varietal differences in these traits, which will be the fundamental information to conduct genetic analyses. The genetic analyses were connected to the detection of quantitative trait loci (QTLs), and then the development of novel near-isogenic lines (NILs) for heat tolerance and escape at flowering stage, and for WBCG, with tagged molecular markers. These breeding materials have a high value for making an impact on formulating a future breeding strategy to mitigate damage by heat stress to spikelet sterility and grain quality in the era of global warming.
Reports on the actual damage by heat stress on spikelet fertility under field conditions
There has been no systematic worldwide monitoring and evaluation of yield losses due to HISS at flowering so far, but heat-vulnerable regions were geographically mapped based on the critical temperatures at flowering stage (Jagadish et al., 2014; Laborte et al., 2012; Wassmann et al., 2009). In fact, some studies on field surveys reported the occurrence of HISS at flowering at the regional level. Osada et al. (1973) and Matsushima et al. (1982) reported a drastic increase in HISS in the hot dry season in Thailand and Sudan, respectively. In Laos and southern India, the combined stresses of heat and intense solar radiation during daytime aggravate the spikelet sterility of local popular cultivars when heading coincides with high temperatures (Ishimaru et al., 2015b). Tian et al. (2009) reported that at least six severe heat events damaged crops in the past 50 years in China. In 2003, approximately 3 million hectares of rice were affected with an estimated loss of about 5.18 million tons of paddy rice because of a heat wave with temperature above 38 °C lasting for more than 20 days in the Yangtze River Valley. The panicle temperature of Chinese hybrid rice exceeded the ambient air temperature by 4.0 °C under humid and low wind conditions, and also caused a severe reduction in spikelet fertility (Tian et al., 2010). In the record hot summer of 2007, the percentages of spikelet sterility rose to 25% when the maximum daily temperature was around 38 °C in the temperate regions of Japan (Hasegawa et al., 2011). Recently, because of the occasional extreme temperature events at flowering stage, reports on serious damage by heat stress have increased in both temperate and tropical regions.
Genetic variation in heat tolerance at flowering and flower opening time
Studies on HISS showed that flowering stage is the most susceptible to high temperatures, followed by booting stage (Satake & Yoshida, Citation1978). High temperatures above 35 °C at flowering stage cause the failure of anther dehiscence, and thus a lesser number of pollen shed on stigma, resulting in incompletion of fertilization (Jagadish et al., 2010; Matsui et al., 1997a, 1997b; Prasad et al., 2006; Zhong et al., 2005). Even if sufficient number of pollen shed on stigma, in some cases pollen germination and pollen tube growth are poor under heat stress (Satake & Yoshida, Citation1978). Thus, aberrant anther dehiscence is considered as the primary cause and disturbed pollen development after shedding as a secondary cause for HISS at flowering. Heat exposure to flowering spikelets for 1 h is sufficient to induce sterility (Jagadish et al., 2007), whereas heat stress 1 h after flowering does not lead to spikelet sterility (Ishimaru et al., 2010; Satake & Yoshida, Citation1978), possibly because of the completion of fertilization. Genotypes with better anther dehiscence and flower opening at a cooler time of the day (i.e. early-morning flowering) are proposed to be desirable for heat tolerance and heat escape, respectively (Satake & Yoshida, Citation1978).
To date, screening for heat tolerance has been conducted in the environmentally controlled chambers and open fields in heat-vulnerable regions. In the chamber experiments, heat tolerance at flowering is often tested at 37.5–38.0 °C (relative humidity around 60–70%) to have a great contrast in spikelet fertility between susceptible and tolerant genotypes (Kobayasi et al., 2011; Mackill et al., 1982; Matsui & Omasa, Citation2002; Matsui et al., 2001; Satake & Yoshida, Citation1978; Shi et al., Citation2015). N22, an Indian aus-type landrace, was identified as one of the most heat-tolerant genotypes in both chamber and open field experiments (Jagadish et al., 2010; Mackill et al., 1982; Manigbas et al., 2014; Poli et al., 2013; Ye et al., 2012), while Moroberekan, an African japonica upland cultivar, is known as one of the most heat-susceptible cultivars (Jagadish et al., 2008). These cultivars can be used as check cultivars in heat tolerance tests (Shi et al., Citation2015). In the japonica cultivars, Akita-komachi and Nipponbare (Maruyama et al., 2013; Matsui et al., 2001), Hitomebore (Maruyama et al., 2013), and Todorokiwase (Tenorio et al., 2013) are classified as considerably heat-tolerant genotypes. In indica cultivars, IR24 and IR36 (Maruyama et al., 2013), Ciherang, ADT36, and BG90-2 (Shi et al., Citation2015), and Dular (Tenorio et al., 2013) are known as heat-tolerant genotypes. It is notable that Giza178, an Egyptian cultivar developed from a japonica-indica cross, has considerable heat tolerance at booting stage as well as flowering stage (Tenorio et al., 2013). There is wide genetic variation in heat tolerance at flowering stage among cultivars. These heat-tolerant accessions at booting and flowering stage are considered to be useful genotypes for the breeding program to improve heat resilience in terms of spikelet sterility.
There is very small genetic variation in flower opening time (FOT) among cultivars. In fact, Hirabayashi et al. (2015) revealed that the differences in peak FOT of 23 popular cultivars in the tropics and subtropics were only within 1.5 h. However, the wide variation in FOT among wild rice accessions is known (Sheehy et al., 2007). For example, some accessions of Oryza eichingeri and O. officinalis show early-morning flowering (EMF) phenotypes, an accession of O. australiensis shows late-afternoon flowering phenotype, and an accession of O. alta shows midnight flowering phenotype (Sheehy et al., 2007). The shift in FOT to a time at cooler temperature was demonstrated to be effective for escaping from heat stress at flower opening (Hirabayashi et al., 2015; Ishimaru et al., 2010, 2012). The variation in FOT among wild rice accessions could be useful genetic resources to adjust FOT of rice cultivars to the appropriate time of day to retain high fertility under heat stress.
Genetic analyses for HISS at flowering
Using heat-tolerant genotypes at flowering, breeding populations have been developed for genetic studies. Studies on QTL mapping for heat tolerance have been conducted at booting and flowering stages using various populations. Almost 60 QTLs associated with heat tolerance at flowering stage have been identified so far. The QTLs are distributed on 11 chromosomes (except chromosome 7). Some of the QTLs identified in different populations are overlapped or closely linked (Table ). Among the identified QTLs, a QTL for spikelet fertility under high temperature, qHTSF4.1 from N22, was identified in different genetic backgrounds in different studies (Jagadish et al., 2010; Xiao et al., 2011; Ye et al., 2012, 2015a). To fine map and validate the effect of heat tolerance QTL qHTSF4.1, PCR-based SNP markers were developed and used to genotype BC2F2, BC3F2, BC3F3, and BC5F2 populations from the cross combination of IR64/N22. The QTL interval was narrowed down to about 1.2 Mb (Ye et al., 2015b). A near-isogenic line (NIL) carrying qHTSF4.1 in the IR64 background was developed. More than 99% of the IR64 genome was recovered in BC5F2 plants, but only a very small fragment from N22 was retained in the region of qHTSF4.1. The heat tolerance of the NIL carrying qHTSF4.1 was increased consistently in all of the backcross populations. In BC3F3, spikelet fertility of plants with qHTSF4.1 (34.7 ± 14.2%) was significantly higher than in those without the QTL (22.5 ± 7.9%) and recurrent parent IR64 (15.1 ± 6.3%), while, in BC5F2, spikelet fertility of plants with qHTSF4.1 (44.6 ± 13.1%) was significantly higher than in plants without the QTL (27.1 ± 9.6%) and recurrent parent IR64 (19.4 ± 8.4%) (Ye et al., 2015b). N22 has many undesirable agronomic characteristics (Manigbas et al., 2014), but the results imply the importance of making best use of the landrace as a genetic resource for future breeding challenges. In the genetic analyses using Giza178 as a donor parent, some other QTLs for heat tolerance at flowering were detected (Ye et al., 2015a), and further efforts are being made to develop the series of NILs carrying the QTLs at different loci for heat tolerance at flowering.
Table 1. Overlapped or closely linked QTLs for heat tolerance identified in different populations.
Recent studies showed that heat tolerance at flowering stage in rice is controlled by recessive genes (Fu et al., 2015; Ye et al., 2012, 2015b). Among the QTLs identified for rice heat tolerance at flowering stage, even QTLs with a large effect can explain only around 20% of the variation, and the additive effect of each QTL is low (Table ). Introducing one or a few QTLs into a genetic background may not be sufficient to significantly increase its heat tolerance. More heat tolerance donors and QTLs need to be identified and used in our breeding programs. A thermotolerance gene (TT1) in African rice (O. glaberrima) variety CG14 was cloned (Li et al., 2015). Although TT1 is mainly responsible for heat stress at seedling stage, it also showed some improvement in spikelet fertility at flowering stage (Li et al., 2015). Once more QTLs and genes are validated or cloned, it is possible to breed thermotolerant rice cultivars by marker-assisted selection.
Genetic analyses for EMF trait
The shift in FOT to cooler early morning was proposed to be effective in heat escape at flowering (Satake & Yoshida, Citation1978). Because of small genetic variation in FOT in O. sativa, screening was conducted using accessions of O. glaberrima (Jagadish et al., 2008; Nishiyama & Blanco, Citation1980) and wild rice (Sheehy et al., 2007). CG14, an accession of O. glaberrima, was identified as an EMF cultivar (Jagadish et al., 2008). Two wild rice accessions have been used to map the QTL for EMF. Thanh et al. (2010) made the first attempt using the mapping population of Nipponbare/O. rufipogon. Three QTLs for EMF were detected on chromosomes 4, 5, and 10, indicating that the alleles of O. rufipogon shifted FOT to 30 min earlier than Nipponbare (Thanh et al., 2010). Another attempt was made by Hirabayashi et al. (2015), using O. officinalis as a donor parent. The introgression lines (ILs) of O. officinalis were developed by interspecific hybridization between O. sativa (AA genome) and a wild rice, O. officinalis Wall ex Watt (CC genome) (Masumizu et al., 2007). A total of 398 ILs were screened on the criterion of the start of flower opening before 0700H under the field conditions of Japan. As a result, 27 plants were selected as candidates of EMF lines in the first screening. The phenotyping of EMF traits was conducted from the start until the end of flowering using three candidate lines, EMF20, EMF44, and EMF71. The start of FOT was similar between EMF20 and EMF44, but the end of FOT was much earlier in EMF20 than in EMF44 (Figure ). Thus, EMF20 finished flowering before Koshihikari started flowering, while FOT partially overlapped between EMF44 and Koshihikari (Figure ). EMF71 obviously started flowering earlier than Koshihikari, but the end of FOT was the same as that of Koshihikari (Figure ). In terms of heat escape at flowering, the end of FOT, rather than the start of FOT, is critical. As a conclusion, EMF20 was determined as the best donor among ILs of O. officinalis to be crossed with O. sativa. A mapping population derived from a cross between Nanjing 11 and EMF20 was developed (Hirabayashi et al., 2015). In both the F2 and F3 populations, the EMF20 alleles of QTLs on chromosomes 3 and 8 advanced FOT to early in the morning; these two QTLs were designated as qEMF3 and qEMF8. An NIL carrying qEMF3 was developed in the genetic background of Nanjing 11 and then qEMF3 was transferred to the genetic background of IR64. NILs carrying qEMF3 had earlier FOT by 1.5–2.0 h than recurrent parents (Hirabayashi et al., 2015). It is remarkable that the EMF phenotype was observed in both temperate and tropical climates, and in two genetic backgrounds of indica cultivars, whereas none of the popular cultivars in the tropics and subtropics had the EMF trait (Hirabayashi et al., 2015). Shi et al. (Citation2015) clarified the varietal differences in heat tolerance among popular cultivars in the tropics and subtropics. The heat tolerance test with additional entries of popular cultivars has revealed variation in heat tolerance among the tested genotypes (Table ). The results from the observation of FOT and the heat tolerance test imply that the heat resilience of cultivars with heat susceptibility and late FOT, such as Caiapo and Pusa Basmati, must be improved. qEMF3 does not affect heat tolerance (Table ), suggesting that the pyramiding of QTLs for heat tolerance (i.e. qHTSF4.1) and EMF (i.e. qEMF3) could be effective in conferring robust heat resilience at flowering. Broad application of qEMF3 to breeding programs is expected. The transfer of qEMF3 to heat-susceptible and late FOT cultivars and the pyramiding of QTLs for heat tolerance and EMF through marker-assisted selection are ongoing projects.
Figure 1. Flowering pattern of Kosihikari and alien introgression lines of O. officinalis. Opened spikelet number per panicle was counted every 20 min. Closed rhomboids, gray squares, gray triangles, and open circles indicate opened spikelet number per panicle of EMF20, EMF44, EMF71, and Koshihikari, respectively.
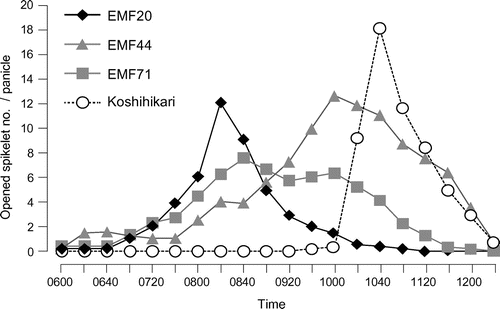
Table 2. Spikelet sterility (%) of Nanjing11, Nanjing11 + qEMF3, and popular cultivars in tropics in control and heat treatment.
Reports on the actual damage by heat stress on WBCG under field conditions
The chalky grains induced by heat stress during grain filling are a big issue in Japan. Field surveys on the various types of chalky grains were conducted in the hot summer seasons (Kawatsu et al., 2007; Morita, Citation2008; Terashima et al., 2001), and a detailed analysis indicated that average daily mean temperatures above 27 °C during the first 20 days after heading were the critical threshold to induce chalky grains in Japanese cultivars (Wakamatsu et al., 2007). Trends are observed that the percentages of translucent rice grains have decreased in the warm-temperate regions of Japan (Okada et al., 2009). The hottest average temperature was recorded in the summer of 2010 in Japan, and heat stress during grain filling seriously affected grain quality (Kondo et al., 2012; Nakagawa et al., 2012). Especially, a high frequency of WBCG was observed in that year (Nakagawa et al., 2012). Intense solar radiation worsens the frequency of WBCG under heat stress (Nakagawa et al., 2012; Tanaka et al., 2010; Wakamatsu et al., 2009).
Genetic variation in heat tolerance to WBCG
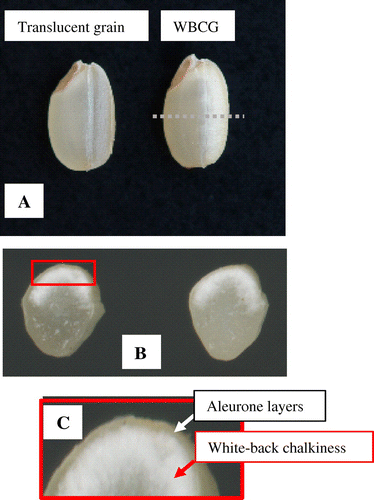
White-back grains show chalky phenotype in only a few layers of starchy endosperm cells adjacent to dorsal aleurone cells (Figure ). In the chalky part, filling of starch granules is aberrant, resulting in loosely packed amyloplasts with gaps between them (Ishimaru et al., 2009; Yamakawa et al., 2007; Zakaria et al., 2002).
Figure 2. Phenotype of WBCG. A: Overview of whole grain, B: Median transversal section of WBCG, C: Magnified view at chalky part. The outermost position indicated by white arrow is aleurone layers at dorsal side of the grain. The phenotype of starchy endosperm adjacent to dorsal aleurone layers is called as white-back chalkiness (red arrow).
Great efforts have been made to understand varietal differences in WBCG to find susceptible and tolerant genotypes. To screen the genotypes, large-scale facilities that can stably increase average temperatures above 27 °C are necessary. Greenhouse (Iida et al., 2002; Kobayashi et al., 2007), hot-water irrigation (Ishizaki, Citation2006), temperature gradient chambers (Nagahata & Yamamoto, Citation2005; Tsukaguchi et al., 2012), and multi-environment testing having wide variation in temperatures (Wada et al., 2015) were used to ensure sufficient heat stress for WBCG during grain filling. Staggered sowing is also an effective approach for making the heading date of entries with the sensitive stage for WBCG coincide during the hottest summer season (Nishimura et al., Citation2000; Wakamatsu et al., 2009). Japanese cultivars, Hatsuboshi (Iida et al., 2002; Wakamatsu et al., 2007) and Hinohikari (Wakamatsu et al., 2007, 2008), are known as susceptible cultivars, while Hana-echizen (Kobayashi et al., 2007), Koshijiwase (Iida et al., 2002; Tabata et al., 2007), Fusaotome (Tanaka et al., 2010; Wakamatsu et al., 2008), Kokoromachi (Shirasawa et al., 2013), Chikushi52 (Wada et al., 2015), and Genkitsukushi (Hamaji et al., 2012) are known as tolerant cultivars. indica cultivars, Habataki (Murata et al., 2014), Kasalath (Ebitani et al., 2008), and Takanari (Tsukaguchi and Iida, Citation2008), are used as donors of tolerance of WBCG, suggesting that some indica genotypes can be potential donors for improving grain appearance in japonica cultivars under heat stress. Some check cultivars for WBCG have been determined (Iida et al., 2002; Ishizaki, Citation2006; Wakamatsu et al., 2007), and the tolerant cultivars are used for breeding programs in Japan.
Genetic analysis for WBCG
Several QTLs associated with tolerance of WBCG have been reported (Table ). Tabata et al. (2007) detected four QTLs on chromosomes 1 (two QTLs), 2, and 8 using RILs derived from a cross between Koshijiwase and Chiyonishiki. Ebitani et al. (2008) detected nine QTLs on chromosomes 2, 5, 6, 8, 9, 10, 11, and 12 using CSSLs of indica cultivar Kasalath in the Koshihikari genetic background. In their study, four QTLs of the Kasalath allele decreased WBCG, and the remaining five QTLs of the Kasalath allele increased WBCG. Kobayashi et al. (2007) detected three QTLs on chromosomes 3, 4, and 6 using F2 and F3 populations derived from a cross between Hana-echizen and Niigatawase. A NIL carrying a QTL on chromosome 6 (qWB6) introduced from Hana-echizen into the Niigatawase background was developed (Kobayashi et al., 2013). This NIL carrying qWB6 significantly decreased WBCG at 27.3–31.1 °C during grain filling. In addition to qWB6, Kobayashi et al. (2013) detected a QTL on chromosome 9 (qWB9) at which the Niigatawase allele decreased WBCG. This result is interesting because Niigatawase itself is susceptible to high temperature. Shirasawa et al. (2013) detected three QTLs on chromosomes 1, 6, and 11 using RILs derived from a cross between Kokoromachi and Tohoku 168. The Kokoromachi allele decreased WBCG at the QTLs on chromosomes 1 and 6. A NIL was developed in which the QTL region of chromosome 6 was introduced into the background of Tohoku 168. The NIL showed significantly lower WBCG than Tohoku 168. The loci of qWB6 (Kobayashi et al., 2007) and the QTL on chromosome 6 (Shirasawa et al., 2013) were very close, but the allelism of these QTLs is not yet clarified because of the lack of a common DNA marker shared by the two studies. In this QTL region, a waxy gene, which dominantly affects amylose content, is located. Further studies must be conducted to clarify the genetic involvement of the waxy gene in WBCG. Wada et al. (2015) detected three QTLs for WBCG on chromosomes 1, 3, and 8 using RILs derived from a cross between Tsukushiroman and Chikushi 52. Two NILs carrying a QTL for heat tolerance on chromosome 8 from Chikushi 52 showed significantly lower WBCG than Tsukushiroman at 27.5 and 29.1 °C (Wada et al., 2015). Murata et al. (2014) detected a QTL on chromosome 7 (Apq1; Appearance quality of brown rice 1) using CSSLs of indica cultivar Habataki in the Koshihikari genetic background. The Apq1-NIL showed significantly higher percentages of translucent grains than Koshihikari, resulting from the reduction in WBCG at 28.2 °C. It was found that the NIL carrying a QTL for seed dormancy, Sdr4 from Kasalath, which reduces the occurrence of pre-harvest sprouting trait (Sugimoto et al., 2010), significantly lowered the occurrence of WBCG in the genetic background of Koshihikari (Kobayashi et al., 2015).
Table 3. QTLs associated with tolerance of WBCG.
Some studies described the candidate genes for WBCG with a gene expression approach. Yamakawa et al. (2008) highlighted GBSSI (Wx) and PPDKB as candidate genes for WBCG among grain-filling-related genes because QTLs associated with WBCG were detected near the genes and their transcriptional responses to high temperature were highly downregulated. Murata et al. (2014) described that SuSy3 in the candidate region of Apq1 shows higher expression in ovary and endosperm at the filling stage according to RiceExpro databases (http://ricexpro.dna.affrc.go.jp/). In addition, the expression level of α-amylase genes was closely related to grain quality under heat stress (Hakata et al., 2012). The expression level of Amy1A and Amy1C in kernels of Sdr4-NIL, which had lower WBGC, was significantly lower (Kobayashi et al., 2015). Recently, the involvement of storage protein biosynthesis in the formation of the chalky phenotype was implied (Sreenivasulu et al., 2015). Laser microdissection-based expression analyses were developed to investigate the gene profile in targeted fine tissues of developing endosperm (Ishimaru et al., 2007, 2015a), making it possible to understand the gene profile in the endosperm tissues corresponding to the region of WBCG. Gene profiling by comprehensive expression analyses could be useful to narrow down the candidate genes in high-resolution linkage mapping for WBCG.
Future prospects
Although many QTLs for heat tolerance at flowering, EMF, and for WBCG during grain filling have been detected, very few cases exist in which subsequent breeding efforts helped to successfully develop novel NILs. The NILs introduced in this review are quite unique breeding materials with tagged molecular markers; thereby, QTLs can be transferred to different genetic backgrounds through marker-assisted breeding to facilitate local breeding programs. The cloning of causal genes is an ongoing project although large-scale environmentally controlled growth chambers or phenotyping systems are required for validating and characterizing the QTLs for all three traits. Heat tolerance at flowering, EMF, and WBCG are difficult traits for which to conduct precise phenotyping in large mapping populations. The cloning of causal gene will unveil the complex genetic control of each trait under heat stress. The degree of damage by heat stress is determined by the complexity of climatic conditions (i.e. microclimates). So far, it can be stated that genetic analyses of spikelet sterility and grain quality under heat stress have been conducted under limited environmental conditions, without taking the multiple effects of microclimates into consideration. In this regard, the currently developed NILs may not be sufficient to tackle the expected and unpredictable future climates. The untapped genetic resources such as landrace and wild rice accessions must be further utilized for the breeding program. Using the wide diversity of rice germplasms, we will be able to explore the novel QTLs and alleles that are expected to have different effects from the identified QTLs. Further efforts are required for the development of heat-resilient rice to cope with the challenges of climate change.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
Our thanks go to Dr. N. Sreenivasulu and Dr. K. K. Jena (IRRI) for critical reading of the manuscript and to B. Hardy (IRRI) for English editing.
Funding
This study was financially supported by the Japanese government (IRRI-Japan Collaborative Research Project) with T. I.; the Cereal Systems Initiative for South Asia (CSISA) project (through the Bill & Melinda Gates Foundation) with C. Y.; the National Agricultural and Food Research Organization (Japan) with H. H.; KAKENHI [grant number 15K18625] with K. S.; the Ministry of Agriculture, Forestry and Fisheries of Japan [Development of mitigation and adaptation techniques to global warming in the sectors of agriculture, forestry, and fisheries 1202] with A. K.
References
- Boyer, J. S. (1982). Crop productivity and environment. Science, 218, 443–448.10.1126/science.218.4571.443
- Calingacion, M., Laborte, A., Nelson, A., Resurreccion, A., Concepcion, J. C., Daygon, V. D., Mumm, R., et al. (2014). Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLoS ONE, 9, e85106.
- Cao, L., Zhao, J., Zhan, X., Li, D., He, L., & Cheng, S. (2003). Mapping QTLs for heat tolerance and correlation between heat tolerance and photosynthetic rate in rice. Chinese Journal of Rice Science, 17, 223–227*.
- Cao, Z., Xie, H., Nie, Y., Mao, L., Li, Y., & Cai, Y. (2015). Mapping a QTL (qHTH5) for heat tolerance at the heading stage on rice chromosome5 and its genetic effect analysis. Chinese Journal of Rice Science, 29, 119–125*.
- Chen, Q., Yu, S., Li, C., & Mou, T. (2008). Identification of QTLs for heat tolerance at flowering stage in rice. Scientia Agricultura Sinica Journal, 41, 315–321.
- Cheng, L., Wang, J. M., Uzokwe, V., Meng, L. J., Wang, Y., Sun, Y., Zhu, L. H., Xu, J. L., & Li, Z. K. (2012). Genetic analysis of cold tolerance at seedling stage and heat tolerance at anthesis in rice. Journal of Integrative Agriculture, 11, 359–367*.
- Ebitani, T., Yamamoto, Y., Yano, M., & Funane, M. (2008). Identification of quantitative trait loci for grain appearance using chromosome segment substitution lines in rice. Breeding Research, 10, 91–99**.
- Fu, F., Zhang, C., Yang, Y., Xiong, J., Yang, X., Zhang, X., Jin, Q., & Tao, L. (2015). Male parent plays more important role in heat tolerance in three-line hybrid rice. Rice Science, 22, 116–122.
- Hakata, M., Kuroda, M., Miyashita, T., Yamaguchi, T., Kojima, M., Sakakibara, H., Mitsui, T., & Yamakawa, H. (2012). Suppression of α-amylase genes improves quality of rice grain ripened under high temperature. Plant Biotechnology Journal, 10, 1110–1117.
- Hamaji, Y., Miyazaki, M., Tsubone, M., Oono, Y., & Odahara, K. (2012). Effects of high air temperature in the summer of 2010 on the kernel quality of the heat-tolerant rice cultivar ‘Genkitsukushi’. Japanese Journal of Crop Science, 81, 332–338**.
- Hasegawa, T., Ishimaru, T., Kondo, M., Kuwagata, T., Yoshimoto, M., & Fukuoka, M. (2011). Spikelet sterility of rice observed in the record hot summer of 2007 and the factors associated with its variation. Journal of Agricultural Meteorology, 67, 225–232.
- Hirabayashi, H., Sasaki, K., Kambe, T., Gannaban, R. B., Miras, M. A., Mendioro, M. S., Simon, E. V., et al. (2015). qEMF3, a novel QTL for the earlymorning flowering trait from wild rice, Oryza officinalis, to mitigate heat stress damage at flowering in rice. Journal of Experimental Botany, 66, 1227–1236.
- Hori, K., Kataoka, T., Miura, K., Yamaguchi, M., Saka, N., Nakahara, T., Sunohara, Y., Ebana, K., & Yano, M. (2012). Variation in heading date conceals quantitative trait loci for other traits of importance in breeding selection of rice. Breeding Science, 62, 223–234.
- Iida, Y., Yokota, K., Kirihara, T., & Suga, R. (2002). Comparison of the occurrence of kernel damage in rice plants grown in a heated greenhouse and in a paddy field of high temperature year. Japanese Journal of Crop Science, 71, 174–177**.
- IPCC. (2013). Climate change 2013: The physical science basis. Fifth Assessment Report, Working Group I, Summary for Policymakers, Cambridge, Cambridge University Press.
- Ishimaru, T., Nakazono, M., Masumura, T., Abiko, M., San-oh, Y., Nishizawa, N. K., & Kondo, M. (2007). A method for obtaining high integrity RNA from developing aleurone cells and starchy endosperm in rice (Oryza sativa L.) by laser microdissection. Plant Science, 173, 321–326.
- Ishimaru, T., Horigane, A. K., Ida, M., Iwasawa, N., San-oh, Y. A., Nakazono, M., Nishizawa, N. K., Masumura, T., Kondo, M., & Yoshida, M. (2009). Formation of grain chalkiness and changes in water distribution in developing rice caryopses grown under high-temperature stress. Journal of Cereal Science, 50, 166–174.
- Ishimaru, T., Hirabayashi, H., Ida, M., Takai, T., San-Oh, Y. A., Yoshinaga, S., Ando, I., Ogawa, T., & Kondo, M. (2010). A genetic resource for early-morning flowering trait of wild rice Oryza officinalis to mitigate hightemperature-induced spikelet sterility at anthesis. Annals of Botany, 106, 515–520.
- Ishimaru, T., Hirabayashi, H., Kuwagata, T., Ogawa, T., & Kondo, M. (2012). The early-morning flowering trait of rice reduces spikelet sterility under windy and elevated temperature condition at anthesis. Plant Production Science, 15, 19–22.
- Ishimaru, T., Ida, M., Hirose, S., Shimamura, S., Masumura, T., Nishizawa, N. K., Nakazono, M., & Kondo, M. (2015a). Laser microdissection-based gene expression analysis in the aleurone layer and starchy endosperm of developing rice caryopses in the early storage phase. Rice, 8, 22.
- Ishimaru, T., Seefong, X., Nallathambi, J., Sathishraj, R., Yoshimoto, M., Phoudalay, L., Samson, B., et al. (2015b). Quantifying rice spikelet sterility in potential heat-vulnerable regions: Field surveys in Laos and southern India. Field Crops Research.. In Press. doi: http://dx.doi.org/10.1016/j.fcr.2015.08.006
- Ishizaki, K. (2006). Evaluation of various screening systems for high grain quality in rice cultivars under high-temperature grain-filling conditions, and the selection of their standard cultivars. Japanese Journal of Crop Science, 75, 502–506**.10.1626/jcs.75.502
- Jagadish, S. V. K., Craufurd, P. Q., & Wheeler, T. R. (2007). High temperature stress and spikelet fertility in rice (Oryza sativa L.). Journal of Experimental Botany, 58, 1627–1635.
- Jagadish, S. V. K., Craufurd, P. Q., & Wheeler, T. R. (2008). Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Science, 48, 1140–1146.
- Jagadish, S. V. K., Muthurajan, R., Oane, R., Wheeler, T. R., Heuer, S., Bennett, J., & Craufurd, P. Q. (2010). Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). Journal of Experimental Botany, 61, 143–156.
- Jagadish, S. V. K., Murty, M. V. R., & Quick, W. P. (2014). Rice response to rising temperatures-challenges, perspective and future directions. Plant, Cell & Environment, 38, 1686–1698.
- Japan Meteorological Agency. (2013). Climate change monitoring report, Tokyo. http://www.jma.go.jp/jma/en/NMHS/indexe_ccmr.html
- Kawatsu, S., Homma, K., Horie, T., & Shiraiwa, T. (2007). Change of weather condition and its effect on rice production during the past 40 years in Japan. Japanese Journal of Crop Science, 76, 423–432**.
- Kim, S. S., Lee, S. E., Kim, O. W., & Kim, D. C. (2000). Physicochemical characteristics of chalky kernels and their effects on sensory quality of cooked rice. Cereal Chemistry, 77, 376–379.
- Kobayashi, A., Bao, G., Ye, S., & Tomita, K. (2007). Detection of quantitative trait loci for white-back and basal-white kernels under high temperature stress in Japonica rice varieties. Breeding Science, 57, 107–116.
- Kobayashi, A., Sonoda, J., Sugimoto, K., Kondo, M., Iwasawa, N., Hayashi, T., Tomita, K., Yano, M., & Shimizu, T. (2013). Detection and verification of QTLs associated with heat-induced quality decline of rice (Oryza sativa L.) using recombinant inbred lines and near-isogenic lines. Breeding Science, 63, 339–349.
- Kobayashi, A., Sugimoto, K., Hayashi, T., Kondo, M., Sonoda, J., Tsukaguchi, T., Wada, T., et al. (2015). Development of a near isogenic line of ‘Koshihikari’ introgressed with seed dormancy gene and evaluation of its tolerance to heat-induced quality decline. Breeding Research.In Press.**
- Kobayasi, K., Matsui, T., Murata, Y., & Yamamoto, M. (2011). Percentage of dehisced thecae and length of dehiscence control pollination stability of rice cultivars at high temperature. Plant Production Science, 14, 89–95.
- Kondo, M., Iwasawa, N., Yoshida, H., Nakagawa, H., Ohno, H., Nakazono, K., Usui, Y., et al. (2012). Factors influencing the appearance quality in rice under high temperature in 2010. Japanese Journal of Crop Science, 81, 120–121***.
- Laborte, A., Nelson, A., Jagadish, S. V. K., Aunario, J., Sparks, A., Ye, C., & Redoña, E. (2012). Rice feels the heat. Rice Today July September., 30–31.
- Li, X., Chao, D., Wu, Y., Huang, X., Chen, K., Cui, L. G., Su, L., et al. (2015). Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nature Genetics, 47, 1–10.
- Lisle, A. J., Martin, M., & Fitzgerald, M. A. (2000). Chalky and translucent rice grains differs in starch composition and structure and cooking properties. Cereal Chemistry, 77, 627–632.
- Lyman, N. B., Jagadish, S. V. K., Nalley, L. L., Dixon, B. L., & Siebenmorgen, T. (2013). Neglecting rice milling yield and quality underestimates economic losses from hightemperature stress. PLoS ONE, 8, e72157.
- Mackill, D. J., Coffman, W. R., & Rutger, J. N. (1982). Pollen shedding and combining ability for high temperature tolerance in rice. Crop Science, 22, 730–733.
- Manigbas, N. L., Lambio, L. A. F., Madrid, L. B., & Cardenas, C. C. (2014). Germplasm innovation of heat tolerance in rice for irrigated lowland conditions in the Philippines. Rice Science, 21, 162–169.
- Maruyama, A., Weerakoon, W. M. W., Wakiyama, Y., & Ohba, K. (2013). Effects of increasing temperatures on spikelet fertility in different rice cultivars based on temperature gradient chamber experiments. Journal of Agronomy and Crop Science, 199, 416–423.
- Masumizu, C., Kodama, H., Kitanosono, R., Ide, K., Inazaki, A., Kaji, R., & Ogawa, T. (2007). Variation of agronomic characters of introgression lines carrying wild rice, Oryza Officinalis (C genome), traits in cultivated rice (O. sativa, A genome) background. Bulletin 'of' Faculty of Agriculture miyazaki university, 53, 33–42**.
- Matsui, T., Namuco, O., Ziska, L., & Horie, T. (1997a). Effect of high temperature and 'CO2' concentration on spikelet sterility in Indica rice. Field Crops Research, 51, 213–219.
- Matsui, T., Omasa, K., & Horie, T. (1997b). High temperature induced spikelet sterility of japonica rice at flowering in relation to air humidity and wind velocity conditions. Japanese Journal of Crop Science, 66, 449–455.
- Matsui, T., Omasa, K., & Horie, T. (2001). The differences in sterility due to high temperature during the flowering period among japonica rice varieties. Plant Production Science, 4, 90–93.
- Matsui, T., & Omasa, K. (2002). Rice cultivars tolerant to high temperature at flowering: Anther characteristics. Annals of Botany, 89, 683–687.10.1093/aob/mcf112
- Matsushima, S., Ikewada, H., Maeda, A., Honma, S., & Niki, H. (1982). Studies on rice cultivation in the tropics. 1. Yielding and ripening responses of the rice plant to the extremely hot and dry climate in Sudan. Japanese Journal of Tropical Agriculture, 26, 19–25.
- Morita, S. (2008). Prospect for developing measures to prevent high-temperature damage to rice grain ripening. Japanese Journal of Crop Science, 77, 1–12**.10.1626/jcs.77.1
- Murata, K., Iyama, Y., Yamaguchi, T., Ozaki, H., Kidani, Y., & Ebitani, T. (2014). Identification of a novel gene (Apq1) from the indica rice cultivar ‘Habataki’ that improves the quality of grains produced under high temperature stress. Breeding Science, 64, 273–281.
- Nagahata, H., & Yamamoto, K. (2005). Evaluation of high temperature ripening ability using the temperature gradient chamber. Breeding Research, 7, 95–101**.10.1270/jsbbr.7.95
- Nakagawa, H., Yoshida, H., Ohno, H., Nakazono, K., Kondo, M., Iwasawa, N., Usui, Y., et al. (2012). What deteriorated the appearance of Koshihikari rice produced in and around Hokuriku region in 2010? Japanese Journal of Crop Science, 81, 126–127***.
- Nishimura, M., Kaji, R., & Ogawa, T. (2000). Varietal differences in the occurrence of coarse grain due to the high temperatures stress given during the ripening period of rice plants. Breeding Research, 2, 17–22**.10.1270/jsbbr.2.17
- Nishiyama, I., & Blanco, L. (1980). Avoidance of high temperature sterility by flower opening in the early morning. Japan Agricultural Research Quarterly, 14, 116–117.
- Okada, M., Iizumi, T., Hayashi, Y., & Yokosawa, M. (2009). A climatological analysis on the recent declining trend of rice quality in Japan. Journal of Agricultural Meteorology, 5, 327–337.
- Osada, A., Sasiprapa, V., Rahong, M., Dhammanuvong, S., & Chakrabandhu, H. (1973). Abnormal occurrence of empty grains of indica rice plants in the dry, hot season in Thailand. Journal of Crop Science, 42, 103–109.
- Poli, Y., Basava, R. K., Panigrahy, M., Vinukonda, V. P., Dokula, N. R., Voleti, S. R., Desiraju, S., & Neelamraju, S. (2013). Characterization of a Nagina22 rice mutant for heat tolerance and mapping of yield traits. Rice, 6, 36.
- Prasad, P., Boote, K., Allen, L., Sheehy, J., & Thomas, J. (2006). Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Research, 95, 398–411.
- Satake, T., & yoshida, S. (1978). High temperature induced sterility in Indica rice at flowering. Japanese Journal of Crop Science, 47, 6–17.10.1626/jcs.47.6
- Sheehy, J. E., Mabilangan, A. E., Dionora, M. J. A., & Pablico, P. P. (2007). Time of day of flowering in wild species of genus Oryza. International Rice Research 'Notes', 32, 12–13.
- Shi, W., et al. (2015). Popular rice (Oryza sativa L.) cultivars show contrasting responses to heat stress at gametogenesis and anthesis. Crop Science, 55, 589–596.10.2135/cropsci2014.01.0054
- Shirasawa, K., Sekii, T., Ogihara, Y., Yamada, T., Shirasawa, S., Kishitani, S., Sasaki, K., Nisimura, M., Nagano, K., & Nishio, T. (2013). Identification of the chromosomal region responsible for high-temperature stress tolerance during the grain-filling period in rice. Molecular Breeding, 32, 223–232.
- Sreenivasulu, N., Butardo, V. M., Misra, G., Cuevas, R. P., Anacleto, R., & Kishor, P. B. K. (2015). Designing climate-resilient rice with ideal grain quality suited for high-temperatures. Journal of Experimental Botany, 66, 1737–1748.
- Sugimoto, K., Takeuchi, Y., Ebana, K., Miyao, A., Hirochika, H., Hara, N., Ishiyama, K., et al. (2010). Molecular cloning of Sdr4, a regulator involved in seed dormancy and domestication of rice. Proceedings of the National Academy of Sciences, 107, 5792–5797.
- Tabata, M., Hirabayashi, H., Takeuchi, Y., Ando, I., Iida, Y., & Ohsawa, R. (2007). Mapping of quantitative trait loci for the occurrence of white-back kernels associated with high temperatures during the ripening period of rice (Oryza sativa L.). Breeding Science, 57, 47–52.
- Tanaka, K., Miyazaki, M., Uchikawa, O., & Araki, M. (2010). Effects of the nitrogen nutrient condition and nitrogen application on kernel quality of rice. Japanese Journal of Crop Science, 79, 450–459**.
- Tebaldi, C., Hayhoe, K., Arblaster, J., & Meehl, G. (2006). Going to the extremes-An intercomparison of model-simulated historical and future changes in extreme events. Climate Change, 79, 185–211.
- Teixeira, E. I., Fischer, G., van Velthuizen, H., Walter, C., & Ewert, F. (2013). Global hot-spots of heat stress on agricultural crops due to climate change. Agricultural and Forest Meteorology, 170, 206–215.
- Tenorio, F. A., Ye, C., Redoña, E., Sierra, S., Laza, M., & Argayoso, M. A. (2013). Screening rice genetic resources for heat tolerance. SABRAO Journal of Breeding and Genetics, 45, 341–351.
- Terashima, K., Saito, Y., Sakai, N., Watabe, T., Ogata, T., & Akita, S. (2001). Effects of high air temperature in summer of 1999 on ripening and grain quality of rice. Japanese Journal of Crop Science, 70, 449–458**.
- Thanh, P. T., Phan, P. D. T., Mori, N., Ishikawa, R., & Ishii, T. (2010). QTL analysis for flowering time using backcross population between Oryza sativa Nipponbare and O. rufipogon. Genes & Genetic Systems, 85, 273–279.
- Tian, X., Luo, H., Zhou, H., & Wu, C. (2009). Research on heat stress of rice in China: progress and prospect. Chinese Agricultural Science Bulletin, 25, 166–168*.
- Tian, X., Matsui, T., Li, S., Yoshimoto, M., Kobayasi, K., & Hasegawa, T. (2010). Heat-induced floret sterility of hybrid rice (Oryza sativa L.) cultivars under humid and low wind conditions in the field of Jianghan Basin. Plant Production Science, 13, 243–251.
- Tsukaguchi, T., & Iida, Y. (2008). Effects of assimilate supply and high temperature during grain-filling period on the occurrence of various types of chalky kernels in rice plants (Oryza sativa L.). Plant Production Science, 11, 203–210.10.1626/pps.11.203
- Tsukaguchi, T., Yamamura, T., Inoue, H., Nakagawa, H., Murakami, K., & Kita, E. (2012). The response of the occurrence of milky white kernels with different cross-sectional patterns of chalkiness in the endosperm to grain-filling temperature and to assimilate supply in Koshihikari. Japanese Journal of Crop Science, 81, 267–274**.
- Wada, T., Miyahara, K., Sonoda, J., Sonoda, J., Tsukaguchi, T., Miyazaki, M., Tsubone, M., Ando, T., et al. (2015). Detection of QTLs for white-back and basal-white grains caused by high temperature during ripening period in japonica rice. Breeding Science, 65, 216–225.
- Wakamatsu, K., Sasaki, O., Uezono, I., & Tanaka, A. (2007). Effects of high air temperature during the ripening period on the grain quality of rice in warm regions in Japan. Japanese Journal of Crop Science, 76, 71–78**.
- Wakamatsu, K., Sasaki, O., Uezono, I., & Tanaka, A. (2008). Effect of the amount of nitrogen application on occurrence of white-back kernels during ripening of rice under high-temperature conditions. Japanese Journal of Crop Science, 77, 424–433**.
- Wakamatsu, K., Sasaki, O., & Tanaka, A. (2009). Effects of the amount of insolation and humidity during the ripening period on the grain quality of brown rice in warm regions of Japan. Japanese Journal of Crop Science, 78, 476–482**.
- Wassmann, R., Jagadish, S. V. K., Heuer, S., Ismail, A., Redoña, E., Serraj, R., Singh, R. K., Howell, G., Pathak, H., & Sumfleth, K. (2009). Climate change affecting rice production: the physiological and agronomic basis for possible adaptation strategies. Advances in Agronomy, 101, 59–122.
- Weerakoon, W. M. W., Maruyama, A., & Ohba, K. (2008). Impact of humidity on temperature-induced grain sterility in rice (Oryza sativa L.). Journal of Agronomy and Crop Science, 194, 135–140.
- Xiao, Y., Pan, Y., Luo, L., Zhang, G., Deng, H., Dai, L., Liu, X., Tang, W., Chen, L., & Wang, G. (2011). Quantitative trait loci associated with seed set under high temperature stress at the flowering stage in rice. Euphytica, 178, 331–338.
- Yamakawa, H., Hirose, T., Kuroda, M., & Yamaguchi, T. (2007). Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiology, 144, 258–277.
- Yamakawa, H., Ebitani, T., & Terao, T. (2008). Comparison between locations of QTLs for grain chalkiness and genes responsive to high temperature during grain filling on the rice chromosome map. Breeding Science, 58, 337–343.
- Ye, C., Argayoso, M. A., Redoña, E. D., Sierra, S. N., Laza, M. A., Dilla, C. J., Mo, Y., et al. (2012). Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breeding, 131, 33–41.
- Ye, C., Tenorio, F. A., Argayoso, M. A., Laza, M. A., Koh, H., Redoña, E. D., Jagadish, K. S. V., & Gregorio, G. (2015a). Identifying and confirming quantitative trait loci associated with heat tolerance at flowering stage in different rice populations. BMC Genetics, 16, 41.
- Ye, C., Tenorio, F. A., Redoña, E. D., Morales-Cortezano, P. S., Cabrega, G. A., Jagadish, K. S. V., & Gregorio, G. B. (2015b). Validating and characterizing qHTSF4.1 to increase spikelet fertility under heat stress at flowering in rice. Theoretical and Applied Genetics, 128, 1507–1517.
- Zakaria, S., Matsuda, T., Tajima, S., & Nitta, Y. (2002). Effect of high temperature at ripening stage on the reserve accumulation in seed in some rice cultivars. Plant Production Science, 5, 160–168.
- Zhang, T., Yang, L., Jiang, K., Huang, M., Sun, Q., Chen, W., & Zheng, J. (2008). QTL mapping for heat tolerance of the tassel period of rice. Molecular Plant Breeding, 6, 867–873.
- Zhao, X. & Fitzgerald, M. (2013). Climate change: Implications for the yield of edible rice. PLoS ONE, 8, e66218.
- Zhao, Z., Zhang, L., Xiao, Y., Zhang, W., Zhai, H., & Wan, J. (2006). Identification of QTLs for heat tolerance at the booting stage in rice. Acta Agronomica Sinica, 32, 640–644*.
- Zhong, L., Cheng, F., Wen, X., Sun, X., & Zhang, G. (2005). The deterioration of eating and cooking quality caused by high temperature during grain filling in early-season indica rice cultivars. Journal of Agronomy and Crop Science, 191, 218–225.