Abstract
Rice production needs to increase in the future in order to meet increasing demands. The development of new improved and higher yielding varieties more quickly will be needed to meet this demand. However, most rice breeding programmes in the world have not changed in several decades. In this article, we revisit the evidence in favour of using rapid generation advance (RGA) as a routine breeding method. We describe preliminary activities at the International Rice Research Institute (IRRI) to re-establish RGA on a large scale as the main breeding method for irrigated rice breeding. We also describe experiences from the early adoption at the Bangladesh Rice Research Institute. Evaluation of RGA breeding lines at IRRI for yield, flowering time and plant height indicated transgressive segregation for all traits. Some RGA lines were also higher yielding than the check varieties. The cost advantages of using RGA compared to the pedigree method were also empirically determined by performing an economic analysis. This indicated that RGA is several times more cost effective and advantages will be realized after 1 year even if facilities need to be built. Based on our experience, and previous independent research empirically testing the RGA method in rice, we recommend that this method should be implemented for routine rice breeding in order to improve breeding efficiency.
Classification:
Introduction
There are several main breeding methods to develop new varieties for self-pollinated crops: pedigree, bulk, modified bulk, single seed descent (SSD) and doubled haploid (DH) (Mackill et al., Citation1996; Poehlman & Sleper, Citation1995; Sharma, Citation1994; Stoskopf et al., Citation1993). Briefly, the pedigree method involves extensive single plant selection and trait screening during segregating generations until yield testing. The bulk method involves planting entire populations and delaying single plant selection until later generations, followed by yield testing. The pedigree method has by far been the most popular method used in rice breeding (Khush & Virk, Citation2005), followed by the bulk breeding method (Collard et al., Citation2013). The DH method requires a tissue culture-based stage in order to fix lines (i.e. make genetically homozygous) in a single step.
The SSD method is used to fix lines during early generations making them homozygous (i.e. make genetically stable prior to extensive testing in field trials). As the name implies, the method generally refers to the use of a single seed per line (i.e. one seed from a single plant) in a segregating population to advance one generation by self-pollination and produce ‘fixed lines’. This method is applicable to self-pollinated field crops for which growth conditions can be manipulated to enforce earlier flowering and seed set compared to normal field conditions. Typically, plants are grown in a greenhouse or screenhouse facility and several generations or cycles are completed between F2 and F6 generations within a quicker time compared to normal field conditions. This is the reason why this method has also been referred to as rapid generation advance (RGA) because, as the name implies, the method can enable several generations to be completed within a shorter time. In the literature, SSD and RGA are typically used interchangeably. The method is generally well known to breeders and has been described in plant breeding text books for many decades (Fehr, Citation1984; Jensen, Citation1988; Mackill et al., Citation1996; Poehlman & Sleper, Citation1995; Sharma et al. Citation1994; Simmonds, Citation1979; Stoskopf et al., Citation1993).
The advantages of RGA as a breeding method include the technical simplicity, requirement of less field and labour resources leading to savings in money and speed of the method if off-season or quicker generation advancement can be achieved (Poehlman & Sleper, Citation1995; Stoskopf et al., Citation1993). Compared to the pedigree method, record keeping is not required for RGA. Usually, a seed increase step in the field is required to produce sufficient seed for yield testing in plots. Disadvantages reported in textbooks include the retention of poor lines during generation advancement, the identity of F2 plants is not retained and within-family selection cannot be practised. There is also a perception by breeders that the genetic variation captured by this method is not as wide as the pedigree method. Furthermore, RGA lines are considered to be inferior compared to other main breeding methods using early-generation visual selection. The advantages and disadvantages of the RGA method based on findings from multiple crops were reviewed by Jensen (Citation1988).
It may surprise many breeders to learn that the RGA method was first reported in 1939, (Goulden, Citation1939) but it was not popular until the 1960s and 1970s after its use in barley, soybean and oats (Brim, Citation1966; Grafius, Citation1965; Kaufmann, Citation1971), although it was not called SSD or RGA. In recent years, this method has gained more wide-spread use in molecular genetics research to develop mapping populations called recombinant inbred lines (RILs) for quantitative trait loci (QTL) mapping (McCouch & Doerge, Citation1995). These populations are ideal for QTL mapping because they are genetically homozygous and seed can be multiplied in large amounts permitting phenotyping for many traits over many years (Collard et al., Citation2005).
Justification from selection theory and some guidelines from computer simulation
Considering one of the fundamental formulas in selection theory provides the first indication of the advantage of using RGA. The formula for genetic gain (ΔG, or response to selection), which is also known as the ‘breeders’ equation’, is given by:
where i is the selection intensity, h2 is the trait heritability, σP is the square root of the phenotypic variance and L is the length of the breeding cycle or generation interval (Ceccarelli, Citation2015; Moose & Mumm, Citation2008). Simple consideration of this formula indicates that the smaller the denominator (i.e. shorter the length of time of the breeding cycle), the higher the genetic gain, assuming the variables in the numerator remain the same (Fehr, Citation1984; Moose & Mumm, Citation2008). It is generally considered that one of the simplest ways to increase genetic gain is to reduce the time of the breeding cycle (Atlin et al., Citation2017; De La Fuente et al., Citation2013).
Plant breeding theory suggests that delaying selection after several cycles of inbreeding may have advantages. The response to selection using fixed lines will be many times larger compared to a F2 population because genetic variation is completely partitioned between lines and selection accuracy (i.e. the ability to correctly identify superior lines that can be effectively commercialized) is greatly enhanced. Furthermore, selection of families for highly heritable traits is enhanced as well because family means are not confounded by within-line non-additive genetic variation. In fact, Kearsey and Pooni (Citation1995) stated that ‘unless the heritability is high, selecting in the F2 generation gives very little return on effort’ and ‘inbreeding (i.e. SSD) followed by selection on family means can improve selection response by up to a factor of five or more’ (Kearsey & Pooni, Citation1995).
Computer simulations are often useful to gain insights into plant breeding and they have been used to compare breeding methods in self-pollinated crops. Van Oeveren and Stam (Citation1992) compared pedigree and SSD methods by varying trait heritability, number of genes and population size. They concluded that there was little difference between methods when multiple crosses were considered and when more loci were assumed to segregate. SSD was superior at low and moderate trait heritability. Their simulations also showed the advantage of SSD in terms of requiring less land. One interesting result was that the SSD method performed poorly in computer simulations when populations were less than 100 F2 plants (Van Oeveren & Stam, Citation1992).
In an earlier simulation-based study by Snape and Riggs (Citation1975), the authors reported that transgressive segregation can be fixed whether it is observed in the F2 or at the F6 stage, regardless of the genetic architecture of the traits. Snape and Riggs (Citation1975) stated that ‘clearly the savings in time and effort which the method offers should make it (SSD) attractive to many plant breeders’ (Snape & Riggs, Citation1975).
A review of the use of RGA in rice breeding
Overall, the use of RGA in rice breeding has been very limited. Some of the earliest reports of using RGA in rice were from Japan and Korea in the 1960s and 1970s (Heu et al., Citation1982; Ikehashi, Citation1977; Maruyama, Citation1989). According to Ikehashi and Fujimaki (Citation1980), 24 leading Japanese varieties which covered over 40% of the total rice area were bred using RGA by 1977. In such temperate countries, rice is only grown during one season each year due to the cold weather, and therefore, this method would have had dramatic time savings by off-season generation advancement. The authors specifically advocated that this method could be used to shorten the breeding cycle and applied this method for cold tolerance breeding (Heu et al., Citation1982). The use of RGA in Japan led to the development of the extremely popular rice variety called Nipponbare, which was used to generate the first rice genome sequence.
RGA was popularized at IRRI during the 1980s, especially for rain-fed rice and even deep-water rice. One advantage of using SSD was to manage photoperiod sensitivity present in many parents used for breeding for rain-fed ecosystems (Ikehashi, Citation1977; ), because some parents would not flower in field conditions. Vergara et al. (Citation1982) published a detailed report which described the use of strict day length control using dark chambers and moving rice seedling trays using trolleys. The system established at IRRI could advance 200,000 plants per batch or planting and advance three cycles per year (i.e. 600,000 lines per year) (Vergara et al., Citation1982). The authors even proposed (based on several RGA experiments) that screening for highly heritable agronomic traits (e.g. earliness and plant height; Patena et al., Citation1980; Vergara et al., Citation1980; Roy et al., Citation1982), diseases (e.g. bacterial leaf blight; Eunus et al., Citation1980a) and submergence tolerance could also be performed during RGA (Bardhan Roy et al., Citation1980; Vergara et al., Citation1982).
To the best of our knowledge, only three published research studies have specifically compared pedigree, RGA and other breeding methods in rice (Fahim et al., Citation1998; Kanbar et al., Citation2011; Mishra et al., Citation1994). In the first study comparing breeding selection methods in rice, Mishra et al. (Citation1994) reported that bulk and SSD were superior in terms of generating higher yielding lines compared to the pedigree method. This conclusion was based on evaluating selections using a randomized complete block design (RCBD) trial with three replications in 1990. It is noteworthy that this conclusion was based on comparing selections at the F3 or F4 stage and only one population (derived from the cross ARC10372/IR36) and only a small number of lines were included in the study (25 pedigree lines, 10 SSD-derived and 10 bulk lines) in different environments (irrigated and upland).
In the study by Kanbar et al. (Citation2011), pedigree, modified bulk and SSD methods were compared using F3, F4 and F5 and F6 populations (20 families produced from each method) derived from a single cross (Moroberekan/IR20). Interestingly, the pedigree-based selections were performed by breeders and farmers. For the pedigree method, the initial number of F2 plants selected was 92, followed by 90 F3:4 and 126 F4:5 families. For SSD, 1240 F2 plants were initially grown in the field. A total of 480 F5 plants were obtained by the F5 stage as many plants died due to high temperature during the summer. From this, 112 families were randomly selected. Twenty families produced by each breeding method was tested in a randomized complete block design trial with four replications and observed for eight traits in 2005. The results indicated that the pedigree method was superior to SSD for grain yield (Kanbar et al., Citation2011). However, it should be noted that the basis of the conclusion was comparison of population means of the selected lines and multiple comparison tests were not performed, which was also the case for Mishra et al. (Citation1994). We believe that it is more appropriate to compare the best lines produced from each of the different methods together, rather than specifically compare population means, because one of the ultimate goals of breeding is to identify transgressive segregants.
The landmark study by Fahim et al. (Citation1998) empirically compared different breeding methods in great detail. The authors compared the performance of F6 breeding lines developed using pedigree, bulk, modified pedigree and SSD using two breeding populations (derived from Bg850/IR50 and 88-5328/Ob2552 crosses). F6 selected lines were directly compared from each of the four breeding methods in a field trial. The initial population sizes used varied depending on the breeding method (Fahim et al., Citation1998). For the pedigree method, 6000 F2 plants were grown from which 73 lines were selected by the F5 stage. For SSD, 1000 F2 plants were grown and 100 randomly selected F5 lines were selected. A total of 12 quantitative traits were scored including grain yield. The most critical finding was that none of the best lines produced by methods involving selection (i.e. pedigree, bulk or modified pedigree) were significantly better for any trait from either cross than the lines produced using SSD. In fact, some of the best lines for grain yield were derived from the SSD method (Fahim et al., Citation1998). Obviously this result depends heavily on the genetic variance for each of the 12 traits in the two crosses in question, but if we consider the SSD lines as a random sample of the potential recombinants from a cross, the fact that the lines that underwent selection through pedigree were not significantly better implies that the selection accuracy during the segregating generations was extremely low. Selection was effective for pedigree, modified pedigree, bulk to eliminate or cull poor performing lines (as evidenced when population means of families were compared). This indicates that ‘poor’ lines are carried forward during the line fixation process in RGA, which is well known from theory and practice. However, because the cost of line fixation associated with RGA is so much cheaper, the budgetary burden of bringing poor performing lines forward is offset by the increased selection accuracy of evaluating fixed lines in a properly replicated yield trial. Overall, the authors concluded that SSD was as effective as the other methods, less costly and more rapid (Fahim et al., Citation1998).
In a more recent study, Janwan et al. (Citation2013) used SSD to develop 271 RILs and investigated 11 quantitative traits. In a field trial with five checks and both parents, transgressive segregation was observed for all 11 quantitative traits. Three lines were significantly higher yielding than the best check variety (Janwan et al., Citation2013). We believe that these results provide further independent verification that RGA is an effective breeding method that should be more widely used.
Details regarding the use of rice breeding methods have rarely been reported. This may be attributable to the fact that breeders do not usually publish results arising from their breeding programs. A literature search indicated that RGA is currently (or has recently been) used in the Philippines, Thailand, India, Bangladesh and Japan (Das, Citation2013; Eunus et al., Citation1980b; Janwan et al., Citation2013; Manigbas & Lambio, Citation2015; Maruyama, Citation1989). Some released varieties developed using RGA are shown in Table , which implies that RGA has had extremely limited use mostly during the last 10–20 years.
Table 1. Rice varieties developed by RGA.
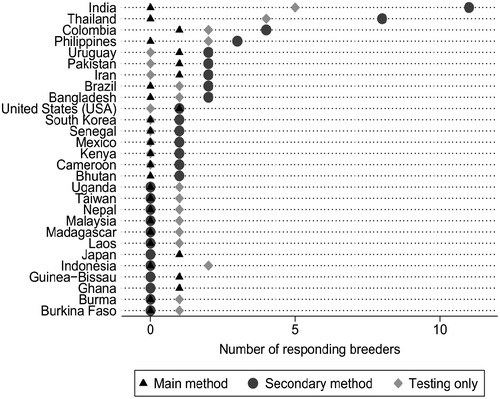
In order to obtain current information on rice breeding methods, an online survey was conducted by IRRI in 2015 (Lenaerts, Citation2016). The results indicated that the vast majority of rice breeders still use the pedigree method (79%), however RGA is used in some Asian, South American and African countries (Figure ). The full details and interpretation of the survey, which also obtained characteristics and attributes of the world’s rice breeders, can be found in Lenaerts (Citation2016), and will be published elsewhere.
Economic benefits of quicker variety release
An essential feature of breeding is the fact that costs of breeding occur early, while benefits from adoption of improved varieties occur later in the breeding programme. Due to forgone opportunities, economic theory dictates that late monetary streams are always of less value than those accruing earlier, whether they are public (i.e. social and environmental) or private profits. This topic has rarely been investigated in the plant breeding literature.
Only one study has tried to quantify the public benefits of reducing the breeding cycle for rice in North-east Thailand and discusses some strategies to reduce the adoption lag (Pandey & Rajatasereekul, Citation1999). The key finding in this paper was that forgone benefits are substantial, even for small savings in time. Reducing the breeding cycle by only one year resulted in $19 million and $39 million of additional benefits over two years, for a standard discount rate of 5%. This points to a crucial responsibility among breeders in developing a breeding programme. It is important that breeders weigh up the economic losses of taking one year longer when considering the potential genetic gain. Pandey and Rajatasereekul (Citation1999) also suggested potential strategies to reduce the adoption lag besides reducing the breeding cycle, such as addressing the length of the release process, the time to reach the ceiling adoption rate and the initial adoption level. Lenaerts (Citation2016) extended this method to a meta-analysis of recent rice breeding impact assessments and found that for varieties with a large geographical spread and for large breeding programmes, incremental benefits of shorter breeding cycles in rice breeding can add up to several billion US dollars over a period of 20–25 years. These incremental benefits accrue due to a higher valuation of earlier benefits compared to later ones through the process of discounting.
Comparisons with the doubled haploid (DH) method
DH is another widely-used method in plant breeding for species with well-developed methods in tissue culture (Poehlman & Sleper, Citation1995). The advantages are that fixed lines can be produced in the shortest currently possible time. Both SSD and DH methods were compared for three populations (Courtois, Citation1993). The author concluded that both methods were equally effective. This result was also confirmed by an independent study using a japonica population (Moon et al., Citation2003). It is noteworthy that the germplasm used in the study by Courtois (Citation1993) involved mostly japonica germplasm which is more amenable to tissue culture. In practice, tissue culture method using indica germplasm is more problematic although there have been successful examples. Based on technical simplicity and cost, we believe that RGA has advantages and is preferable compared with doubled haploids especially for NARS in South and SE Asia to adopt.
Brief description of RGA method used in IRRI’s irrigated breeding program
Screenhouse system
The method described below was developed after months of trial and error, followed by specific optimization of various parameters. An overview of the method is shown in Figure and full details are included in the Electronic Supplemenary Material (ESM) section 1. In brief, our method used seedling trays to grow rice plants. Seedling trays had 8 rows x 13 columns, 104 cells per tray; 36 cm × 56 cm) and each cell contained <40 cm3 of soil for a single breeding line (i.e. originally derived from a single F2 plant). Direct-dry seeding was performed using our system. Initially, about four–five seeds were seeded per cell to ensure 95–100% germination rate and plants are then thinned to one plant per cell around 10–14 days after seeding. Minimal fertilizer was applied. In our conditions, harvesting usually began around –90 DAS depending on the population, and was completely harvested by 95–105 DAS. Harvesting was done within a ~2 week window, which was beneficial to distribute work over time with fixed staff. Once the target generation had been achieved (e.g. F5 or F6), the panicles were planted in the field (i.e. ‘panicle rows’) as a seed increase step (i.e. usually F5:6 seed). However, this stage was also used to check for uniformity of each line (i.e. check for segregation due to outcrossing or mixtures) and apply selection. Repeated check varieties were used for observation and disease spreader rows were also used to eliminate highly susceptible lines to insect pests. We called this stage the ‘line stage testing (LST)’ because each line was evaluated using at least 12 plants per line planted in short dual rows.
Figure 2. RGA system used in irrigated breeding programme at IRRI. (a) seeding (b) seedling stage (c) vegetative stage (d) flowering stage (e) glasshouse set-up (f) showing tillering (g) showing panicles (h) harvesting stage with inset showing harvested panicles (i) inside view of screenhouse (CS-08B).

Field RGA (FRGA)
Ideally RGA is best performed in screenhouse or greenhouse facilities where the environment can be easily manipulated. However when such facilities are not available, or if screenhouse/greenhouse facilities are insufficient, RGA can be performed in the field, and plants can be seeded or transplanted directly into the soil of the designated field area. This is possible when the daily temperatures in a location do not fluctuate much during the year. A variant of this was method previously described by Fahim et al. (Citation1998) who used seedling trays and sowed seeds at high density, but did not directly sow seeds into the soil of the field area. In both cases, these breeding populations need to be carefully protected from animals such as rats and birds in a designated field area, and the selected areas must have access to irrigation.
There are several advantages of FRGA. Firstly, this method can obviously be used when screenhouse/greenhouse facilities are unavailable. Secondly, plants grown in the field may avoid artificial micro-climates often encountered in greenhouse or screenhouse facilities which can reduce risks of pest outbreaks. Thirdly, temperature may be more suitable compared to RGA in greenhouses without adequate temperature control or shading. The disadvantages of FRGA include genetic drift and possible extensive loss of material due to field pests or natural disasters such as typhoons, although this is also possible for material grown in screenhouses/greenhouses.
At IRRI, we modified the FRGA method by inserting the seedling trays directly into the soil. The reason for this was because the plastic trays were used to constrain plant growth by restricting root development and keep the plants smaller and with fewer tillers than they would have if planted directly into the soil using 20 cm x 20 cm spacing. This also minimized edge effects. Seeds were pre-germinated in seedling trays before placing on top of the soil, which permitted roots to grow into the ground from the hole in the cells of the seedling tray. From our experience, a rat fence was essential. When harvesting, only a single panicle was taken with sufficiently filled spikelets and processed in the same way as for the screenhouse method (as described above). The main steps are shown in Figure .
Early adoption at the Bangladesh Rice Research Institute (BRRI)
BRRI has used the pedigree method for breeding for many decades. The field-based rapid generation advancing system (FRGA) system was modified and adopted by BRRI. Segregating populations were seeded directly in the soil in the field in raised beds with very close spacing and low fertilizer (Figure ). Full details are provided in ESM section 2. The length of time from F2 to F5 generations which generally takes about 4 years was shortened to 2 years. Efforts to optimize this system in terms of minimizing labour and costs, and shorter generation times are ongoing. Further empirical evaluation of RGA breeding lines is ongoing at BRRI and these results will be reported elsewhere.
Preliminary evaluation of RGA lines at IRRI -field trials and data analysis
RGA-derived breeding lines (from seven different elite x elite crosses) were then tested in an observational yield trial (OYT) in the dry season of 2015 which used an augmented design (i.e. unreplicated entries with repeated checks). A total of 185 RGA lines, representing the first batch of newly developed RGA lines within the irrigated breeding programme, were included in the OYT in addition to parents. The top performing breeding lines were promoted into the preliminary yield trial (PYT). Best trial management practices at IRRI were followed. Yield and agronomic trait data (flowering time and plant height) were collected and analysed. Full details regarding breeding populations, trials and data analysis are reported in Electronic Supplementary Material section 3.
General experiences at IRRI
The main screenhouse facility used for RGA at IRRI had a capacity over 100,000 lines could be handled at one time. This was managed by an Assistant Scientist who led the overall operations including data management, and three Research Technicians who were responsible for seeding, maintenance and harvesting. Casual labour was used if large batches of populations were seeded or harvested at the same time. In order to optimize use of labour, seeding and harvesting of populations were staggered over time to avoid spikes in labour demand.
On average, populations flowered around 75–85 DAS and were completely harvested by 105 DAS. Earlier flowering and quicker harvesting were observed by Manigbas and Lambio (Citation2015) in their RGA system. Therefore on average, 3.5 generations were comfortably completed in 12 months. Some populations, especially with early or medium duration parents, and early lines from almost all populations were advanced four generations within 12 months. In our experience, staggered planting and harvesting were effective to efficiently use fixed labour resources (i.e. staff) and minimize costs. In order to accelerate line fixation for specific populations, we established an ‘express lane’ for the highest priority crosses specifically targeting four generations/year.
The LST stage was an important step for seed increase and permits selection of highly heritable traits using short rows, because there is no previous selection for plant type, disease resistance or other traits. Large phenotypic variation was observed within RGA populations observed at this stage, which has been a concern of some sceptical rice breeders. There were advantages to select lines based on a panicle-row compared to selecting single plants. We quickly eliminated at least 10–20% of the poor lines with obvious defects by eye and by comparison with check varieties. Lines were also scored for flowering time and also selected for grain yield to eliminate a large number of the poorest performing lines.
The population size used typically consisted of several 100 lines per population. Although a relatively small number of RGA lines was developed and tested in the first batch of RGA-derived material, we believe a minimum of several hundred lines is required to increase the likelihood of identifying transgressive segregants. Subsequently in 2016, a target of a minimum of 400 lines was defined for each breeding population. This target (i.e. ≥400 lines) permitted many lines from many different crosses to be evaluated for yield in the OYT. In other words, the optimal total population size was determined by practical reasons regarding how many lines can be advanced within the RGA system, and how many entries that could be included in the OYT. In our experience, it was desirable to test at least 50 lines/cross in the OYT, which based on Gaussian theory should permit the identification that at least one line that will be two standard deviations (SD) better than the mean for the cross (~2.3% of a normal distribution is more than two SD from the mean in a single ‘tail’ of the population). This also required optimization of the overall dimensions of the breeding programme, especially the number of crosses performed.
Performance of RGA breeding lines
Our main objective was to implement RGA on a large scale within the irrigated breeding program after decades of use of the pedigree method. Pilot RGA experiments to optimize the basic method were initiated in 2013, and RGA was implemented on a large scale as the sole breeding method in 2014. The first RGA-derived populations developed using the system described above were fixed in late 2014, and tested in the OYT during the 2015 dry season (DS) at Los Baños. We used the actual irrigated breeding programme to evaluate breeding lines, because this was the most efficient use of resources and because we wanted to fast-track the incorporation of RGA lines within the irrigated breeding programme. Specifically, we were trying to obtain transgressive segregants for yield or other agronomic traits. For yield, we were also trying to obtain new RGA breeding lines that were superior yielding compared to the high-yielding check variety (e.g. IRRI154 or NSICRc222), which is the ultimate objective of most breeding programmes.
Although only a relatively small number of RGA lines was tested in only a single location (i.e. only 185 lines derived from a total of 553 lines tested in the LST) in the 2015 DS OYT, transgressive segregation was observed for all traits and some RGA lines outperformed check varieties for yield (Figure ). Details of the trials are reported in ESM Table . Parents were deliberately included in the initial OYT trial to permit comparisons with progeny lines. The top performing RGA lines were subsequently promoted to the irrigated PYT trial (results reported for Los Baños trial only) for evaluation during the wet season, or retested within the 2015 WS OYT. Parents were not included in the PYT due to limited space for test entries (total maximum entries = 479 for this trial). However, RGA-lines that were higher yielding compared to check varieties were clearly identified. Results showing RGA-derived lines that outperformed check varieties in both seasons are presented in Table . However, it should be emphasized that these results are preliminary since they are based on only two seasons and with unreplicated trials designs. Ideally, evaluation for yield should be conducted across several locations to properly estimate heritability for yield (Yan, Citation2014).
Figure 5. Histograms of yield and flowering time for 3 different RGA populations evaluated in the 2015 dry season OYT. IR98492: yield (a), flowering time (b). IR98590: yield (c), flowering time (d). IR96915: yield (e), flowering time (f). Means of parents and IRRI154 check variety are indicated by arrows.
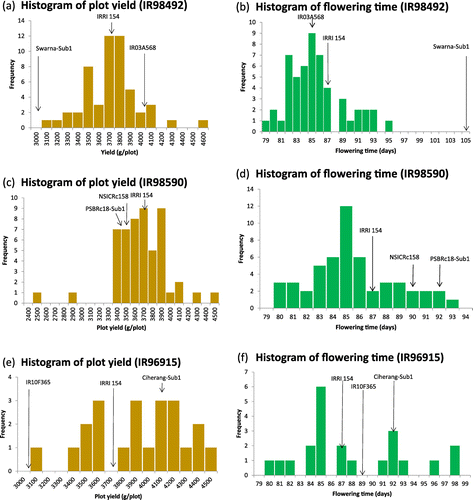
Table 2. Performance of selected RGA lines in 2015 DS OYT and 2015 WS PYT. (a) RGA lines tested in group 1 (early/medium maturity) of the PYT (2015 WS) sorted by yield performance.
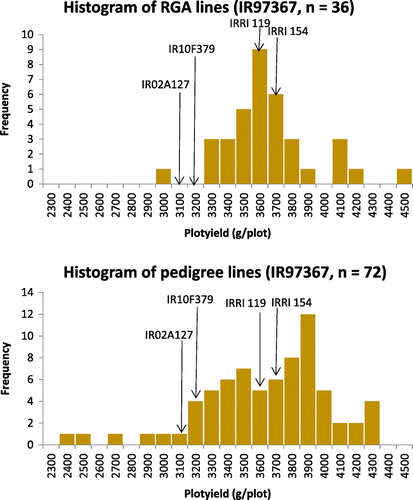
Fortuitously, both RGA and pedigree lines were available from one population (Cross IR97367; parentage: IRRI119/IR02A127//IR10F379) and were included in the 2015 DS OYT. Although unequal numbers lines were available, it permitted a cursory comparison of lines derived from pedigree and RGA methods. The results indicated transgressive segregation was observed from both methods and breeding lines outperformed check varieties (Figure ). Interestingly the top yielding line (IR15A1716) from this population was developed using RGA. These results were consistent with the results of Fahim et al. (Citation1998). Further empirical evaluation of RGA breeding material is ongoing at IRRI and these results will be reported elsewhere.
Cost considerations
Plant breeding textbooks usually highlight the low cost of the RGA breeding method as the main advantage. Indeed, apart from savings in time, RGA also generates more direct benefits to breeders through savings in input resources (which can be considered as institutional costs), as already noted by Ikehashi (Citation1977). Following this, another important objective from the study by Fahim et al. (Citation1998) was to empirically compare costs of each method. Factors considered included land preparation, planting, weeding, chemicals and fertilizers, harvesting and post-harvesting costs. FRGA was five to 10 times cheaper compared to the pedigree method (Fahim et al., Citation1998). More specifically, their cost estimate for the FRGA method ranged between $12 and $24 per line. This is one of the few reports investigating the cost with empirical evidence. Note that Fahim et al. (Citation1998) used seedling trays in boxes that were grown in the field (which differs from the FRGA method reported in our paper, because they were not grown directly in the soil) and consequently their cost estimate differs with the one from the RGA method discussed below.
Facing budget constraints and changing economic conditions, these direct benefits (i.e. cost savings) might be more important to breeders than maximizing the rate of genetic gain in their programme. However, breeders in general do not look at the costs of their breeding operations per variety produced. Breeders manage their operations as a continuous process where the budget has to cover costs on a yearly or seasonal basis. For that reason, the lifetime of a greenhouse or screenhouse might be a more appropriate timeframe than that of a single variety. In this section, we investigated factors that differed significantly between pedigree and RGA. The justification for this lies in conventional investment theory, more specifically in the use of a net present value (NPV) approach (i.e. this discounted cost analysis looks only at the additional cash flows from RGA).
Budget reductions to IRRI’s irrigated breeding programme in 2012 were a major reason as to why RGA was adopted, replacing the pedigree breeding method that had been successfully used for many decades. A cost comparison between RGA and pedigree was performed using a spreadsheet-based partial budgeting approach (Table ) (Lenaerts, Citation2016). This cost audit showed that land use, seasonal labour and the cost of a screen- or greenhouse (i.e. construction and maintenance costs) differed the most, while staff, supplies and equipment were assumed to be more or less the same for both breeding methods. More specifically, this analysis was done for breeding operations in the nursery stage with an output of approximately 1000 breeding lines for inclusion in observational yield trials. Other assumptions and details are indicated in Electronic Supplementary Material section 4.
Table 3. Operational and investment costs for the RGA and pedigree method.
Table shows the NPV for an interest rate of 5% for different scenarios for both a screen- and greenhouse. Given the timeframes proposed by the IRRI data, total discounted benefits from a greenhouse are approximately twice that of a screenhouse. Using a more conventional timeframe, benefits are comparable. Thus, depending on the timeframe, a greenhouse may be the profit-maximizing (or public welfare-maximizing) option.
Table 4. Measures of project value for RGA.
However, the NPV does not take into account the possibility of capital rationing. This can be especially important for small breeding institutes who lack the funding for a greenhouse but can afford to build a screenhouse. Therefore, the (discounted) benefit-cost (B/C) ratio is also calculated, which might be useful in ranking projects under rationing of capital. As Table shows, this ratio gives a different picture both for the 50 year and the 20 year timeframes. This could suggest that for budget-constrained institutes, a screenhouse is the better option to choose. For institutes with limited funds, FRGA might also be a more suitable option due to the low investment costs. However, due to lower benefits (besides lower costs), the NPV and B/C-ratio might be lower than for the greenhouse or screenhouse scenario.
Finally, it is noteworthy that switching to RGA using a screenhouse will repay itself within one year, as the extra costs for construction of the screenhouse ($73,199) are outweighed by the labour and land saved ($101,568). In other words, using RGA does not require an additional budget as the budget for existing resources – labour and land – could be redirected to pay for a screenhouse.
It must be emphasized that despite efforts to be accurate, the numbers in this analysis are only used to give an impression and answer the question whether or not RGA operations are more expensive than pedigree. Price for land use, seasonal labour and costs of a greenhouse and screenhouse are taken from IRRI operations at Los Baños, Philippines and can therefore not be generalized to other breeding institutions. Labour, land and construction costs may also differ significantly between countries. Lastly, the scale of the breeding operations may also influence profitability. Building a smaller greenhouse or screenhouse may be more expensive expressed in cost per area due to economies of scale. Although variation in per unit costs are expected to be large and specific relationships cannot be derived from the example presented here, we believe that this example provides a realistic ballpark estimate about the benefits and profitability of RGA compared to the pedigree method.
The way forward: new breeding philosophy is needed for rice breeding in the future
Rice breeding programmes across the world are confronted with the fundamentally difficult challenge of integrating technology, theory and logistics into a unified breeding system capable of generating sustainably high rates of genetic gain. Breeding programmes that seek to modernize their approach to genetic improvement have the rather fortunate problem of choosing among a myriad of available tools they can apply. The truly successful programmes will be the ones that apply the right tool for the right job and focus on effective, but necessarily differentiated, strategies for improving quantitative as well as qualitative traits. The preliminary results reported in this study provided empirical evidence that RGA is an effective breeding method, similar to and in many cases superior to pedigree or bulk breeding.
Given the substantial logistical simplification and reduction of costs associated with the deployment of RGA, it is surprising that it has not gained wider adoption, as these benefits have been known for a long time. A comparable innovation in modernized commercial corn breeding programmes would be the advent of DH technology, which was quickly adopted as a way of creating fixed lines in a single step (Prasanna et al., Citation2012). In a naturally self-pollinating crop-like rice, particularly where high-throughput DH technologies are generally not well established, using RGA makes good sense as it achieves many of the same goals of a DH-driven programme, and leverages more meioses during line development.
Further to the logistical and cost related benefits, the capacity to use the RGA system to reduce cycle time comes with substantial advantage. The power of choosing parental material that can be used in the crossing block sooner has unparalleled benefit for increasing genetic gain. For instance, genomic selection as a breeding strategy in plants has been studied carefully for almost a decade (Heffner et al., Citation2009), and the primary advantage it offers is not increased accuracy, but rather reduced cycle time (‘L’ from the breeder’s equation indicated above). RGA carries a much lower cost and shorter learning curve and is capable of making reductions in cycle time, similar to genomic selection, allowing for more quickly realized genetic gains.
Reductions in cycle time due to RGA methods are further exploited due to the enhanced selection accuracies associated with the evaluation of fixed line in a replicated yield trial, a privilege not available in pedigree and bulk methods until after substantial selection for yield in segregating material has taken place. Therefore, we strongly encourage other rice breeders to carefully consider their deployment strategies for quantitative traits (especially yield) and implement this method as a way to evaluate yield and identify superior parents much more quickly. Resources saved using this method could be used for other purposes such as increasing plot size, plot numbers or number of locations for yield trials or by simply testing more recombinants every year, both representing other important variables in the breeder’s equation.
Regarding the RGA systems described in this article, numerous improvements and optimizations are certainly still possible. The method is very flexible due to its technical simplicity and could easily be customized to suit existing resources at practically any breeding station using existing screenhouse/greenhouse facilities or field area. For NARS scientists considering establishing new facilities, screenhouses are much cheaper and enable easier temperature control. Field RGA in particular, adds a measure of flexibility to programmes with uncertain budgets because it allows for the breeding nursery to grow and shrink consistent with the available budget without shackling the breeding programme to the fixed costs of maintaining a greenhouse. Population size is obviously also a key determinant of the genetic variation and the impact of genetic drift on a breeding programme, and needs to be considered carefully and balanced with the number of crosses. Both empirical and simulated data can help determine the ideal values of these parameters on a programme-by-programme basis. RGA methods being cheaper could allow a breeder to maintain the same number of crosses but evaluate more recombinants from a single family. We believe further piloting of earlier harvesting and seeding and further experiments to speed up flowering and seed set would also be worth exploring.
Regarding the critical LST stage, thorough selection for key agronomic traits such as maturity and plant height should be performed, since these traits can be accurately selected for using unreplicated single or dual-row plots. We also propose that the design and layout of LST could also be optimized and empirically tested to enable large-scale selection of RGA lines in the field (i.e. based on the ‘micro-plot concept’), or with complementary trait screening for high-priority traits (e.g. submergence tolerance or BLB resistance).
One feature that we did not test was using photoperiod sensitive material because all (or the vast majority) of elite breeding material used in the irrigated breeding pipeline was photoperiod insensitive. Therefore, in our system we did not control photoperiod at all due to the large scale implemented. It is likely that the use of photo-period sensitive material would require photoperiod control (i.e. dark room treatment or curtain systems) as has been previously reported by Vergara et al. (Citation1982) and Manigbas and Lambio (Citation2015). Dealing with breeding populations derived from highly photoperiod-sensitive parents during RGA was previously managed in field conditions (Roy & Mondal, Citation1985). It should also noted that some varieties with a long basic vegetative phase (BVP) and progeny from crosses may have delayed flowering and may be difficult to advance for three or more generations per year during RGA (Roy et al., Citation1981).
Overlaying selection during RGA
Vergara and co-workers, who pioneered the RGA system at IRRI during the 1980s, reported selection during the RGA process for highly heritable traits. Although we did not specifically try this, we are confident that selection could be overlayed for many target traits that would further improve the efficiency of the RGA system. Given the high correlation observed for maturity and plant height observed in our RGA system and the field, these traits could easily be selected (or at least the extreme phenotypes could be eliminated). One obvious example of that would be the combination of marker-assisted selection (MAS) for major genes/QTLs conducted during generation advancement. This would permit efficient screening of breeding material to eliminate undesirable lines, especially for critical (i.e. ‘make or break’) traits. However depending on the cost of genotyping versus the cost of line fixation in the RGA, the cost/benefit ratio to using MAS in the RGA as opposed to in the LST (immediately following the RGA) could change quite a bit. Higher genotyping costs and lower line fixation costs favour MAS at the end of the RGA workflow as frequencies of favourable alleles in a RIL population approach is 0.5. However cheaper genotyping and more expensive line fixation costs might favour the elimination of genotypes that do not harbour the relevant alleles in the F2, despite the fact that the frequency of favourable alleles is only 0.25 for a single locus. An additional logistical improvement of a combined MAS-RGA system would be to use tubes arrayed in racks to grow single seedlings rather than trays of fixed dimensions. This would allow for the recycling of greenhouse/screenhouse surface area more quickly as a tray of 96 seedlings is, at best, going to contain only 24 seedlings that will be moved forward after MAS. On a related topic, RGA is additionally useful to geneticists and pre-breeders as it is functionally a high-throughput RIL development pipeline. It could easily shorten the development time of bi-parental RIL mapping populations, multi-parent advanced generation inter-cross (MAGIC) populations or nested association-mapping (NAM) populations. With further innovation, it could easily be adapted to create high-throughput backcrossing workflows that empower the rapid development of near-isogenic lines (NILs) and chromosome segment substitution lines (CSSLs).
The power of genomic selection (GS) in a breeding programme is a topic for another discussion, however it’s worth mentioning that the effective implementation of a breeding scheme integrated with a GS approach demands the fingerprinting of fixed lines. Using RGA prior to a GS step in a breeding programme allows for predictions to be made about new recombinants faster than in traditional pedigree schemes. Further if the MAS is done at the terminal step in the RGA (i.e. LST stage), then DNA extraction need only be done once as DNA from lines selected using MAS could easily be used for further high-density genotyping.
In the future, greenhouse/screenhouse automation technology may further enhance the throughput of RGA as it would be possible to accelerate generation advance further and increase throughput. There have been remarkable reports of accelerated generation advancement using the ‘biotron system’ which is based on CO2 and day-length control (Ohnishi et al., Citation2011; Tanaka et al., Citation2016). While likely not deployable by individual NARS rice breeding programmes in developing countries, given the proper investment, such systems could become effective business models for genotyping service providers or other entrepreneurial organizations to offer a ‘high-throughput forward breeding service’ where breeders simply submit parental seed and validated trait markers to the service provider, and within a relatively short period of time are returned a set of fully inbred lines, fixed for the alleles of interest, at a pre-determined population size. Although extremely promising, these systems would need to be up-scaled considerably to routinely manage hundreds of thousands of plants per year at a low cost.
In conclusion, we believe that due to the projected increased demand for rice, adverse effects of climate change, and future biotic threats (i.e. new races, pathotypes or biotypes), rice breeders need to consider adopting faster breeding methods to modernize their programmes and specifically to increase the rate of genetic gain, while also minimizing operational costs (Atlin et al., Citation2017). RGA is one of many effective methods that can allow rice breeders and geneticists some ‘quick wins’ to modernize quickly, and with a simple re-allocation of existing resources currently being spent on expensive pedigree selection schemes. Rice breeders – and breeders of other self-pollinated crops – would certainly realize the benefits ‘doing more with less’ by adopting RGA as their main breeding method.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
Funding for this research at IRRI was provided by the Bill and Melinda Gates Foundation for the Transforming Rice Breeding project [grant number OPP1076488], and for a complementary project awarded to BRRI.
Supplemental data
Supplemental data for this article can be accessed https://doi.org/10.1080/1343943X.2017.1391705
TPPS_1391705_Supplementary_material.doc
Download MS Word (451.5 KB)Acknowledgments
Technical assistance within the RGA facility by Edwin Reyes, Mark Javier and Herman Hermanosa is gratefully acknowledged. We would particularly like to thank Dr Gary Atlin from Bill and Melinda Gates Foundation for his encouragement and support of the RGA activities. We also thank two anonymous reviewers for suggestions that were incorporated to greatly improve this paper.
References
- Atlin, G. N., Cairns, J. E., & Das, B. (2017). Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Global Food Security, 12, 31–37. doi:10.1016/j.gfs.2017.01.008
- Bardhan Roy, S. K., Peralta, J., & Vergara, B. S. (1980). Submergence-tolerance screening of rice during rapid generation advance. International Rice Research Newsletter, 5, 3–4.
- Brim, C. A. (1966). A modified pedigree method of selection in soybeans. Crop Science, 6, 220.10.2135/cropsci1966.0011183X000600020041x
- BRRI. (2013). Modern rice cultivation (17th ed.). Gazipur: Bangladesh Rice Research Institute.
- BRRI. (2015). Modern rice cultivation (18th ed.). Gazipur: Bangladesh Rice Research Institute.
- BRRI. (2017). Modern rice cultivation (20th ed.). Gazipur: Bangladesh Rice Research Institute.
- Ceccarelli, S. (2015). Efficiency of plant breeding. Crop Science, 55, 87–97. doi:10.2135/cropsci2014.02.0158
- Collard, B. C. Y., Jahufer, M. Z. Z., Brouwer, J. B., & Pang, E. C. K. (2005). An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica, 142, 169–196. doi:10.1007/s10681-005-1681-5
- Collard, B. C. Y., Ismail, A. M., & Hardy, B. (Eds.). (2013). International Rice Research Institute EIRLSBN : Twenty years of achievements in rice breeding. Los Baños: International Rice Research Institute.
- Courtois, B. (1993). Comparison of single seed descent and anther culture-derived lines of three single crosses of rice. Theoretical and Applied Genetics, 85, 625–631.
- Das, S. R. (2013). Reflections on >40 years of rice breeding for eastern India. SABRAO Journal of Breeding and Genetics, 45, 67–72.
- De La Fuente, G. N., Frei, U. K., & Lübberstedt, T. (2013). Accelerating plant breeding. Trends in Plant Science, 18, 667–672. doi:10.1016/j.tplants.2013.09.001
- Eunus, M., Ikehashi, H., & Vergara, B. S. (1980a). Screening for resistance to bacterial blight of rice under rapid generation advance. International Rice Research Newsletter, 5, 7.
- Eunus, M., Ikehashi, H., Vergara, B. S., & Peralta, J. (1980b). Prevention of lodging in rice plants during rapid generation advance. International Rice Research Newsletter, 5, 3.
- Fahim, M., Dhanapala, M. P., Senadhira, D., & Lawrence, M. J. (1998). Quantitative genetics of rice. II. A comparison of the efficiency of four breeding methods. Field Crops Research, 55, 257–266. doi:10.1016/S0378-4290(97)00090-7
- Fehr, W. R. (1984). Principles of cultivar development. Volume 1. Theory and technique (Vol. 1). New York: Macmillan Publishing Company.
- Goulden, C. H. (1939). Problems in plant selection. In R. C. Burnett (Ed.), Proceeding of the seventh genetics congress (pp. 132–133). Edinburgh: Cambridge University Press.
- Grafius, J. E. (1965). Short cuts in plant breeding. Crop Science, 5, 377.10.2135/cropsci1965.0011183X000500040036x
- Gregorio, G. B., Islam, M. R., Vergara, G. V., & Thirumeni, S. (2013). Recent advances in rice science to design salinity and other abiotic stress tolerant rice varieties. SABRAO Journal of Breeding and Genetics, 45, 31–41.
- Ha, W.-G., Torollo, G. V., Kang, K.-H., Lapiz, M., & Padolina, T. (2011). Breeding temperate japonica rice MS11 for the tropics. Philippine Journal of Crop Science, 36, 111.
- Heffner, E. L., Sorrells, M. E., & Jannink, J.-L. (2009). Genomic selection for crop improvement. Crop Science, 49, 1–12.10.2135/cropsci2008.08.0512
- Heu, M. H., Chung, G. S., Kim, D. K., Sasaki, T., & Vergara, B. S. (1982, April 19–23). Rapid generation advance in breeding rice for low temperature tolerance. International Rice Research Conference, International Rice Research Institute, Los Baños.
- Ikehashi, H. (1977). New procedure for breeding photoperiod-sensitive deep-water rice with rapid generation advance. In Proceedings of the workshop on deep-water rice. Los Baños: International Rice Research Institute. (pp. 45–54).
- Ikehashi, H., & Fujimaki, H. (1980). Modified bulk population method for rice breeding. In: Innovative approaches to rice breeding. Selected papers from the 1979 International Rice Research Conference (pp. 163–182). Los Baños: International Rice Research Institute.
- Janwan, M., Sreewongchai, T., & Sripichitt, P. (2013). Rice Breeding for high yield by advanced single seed descent method of selection. Journal of Plant Sciences, 8, 24–30.
- Jensen, N. F. (1988). Plant breeding methodology. New York, NY: Wiley.
- Kanbar, A., Kondo, K., & Shashidhar, H. E. (2011). Comparative efficiency of pedigree, modified bulk and single seed descent breeding methods of selection for developing high-yielding lines in rice (Oryza sativa L.) under aerobic condition. Electronic Journal of Plant Breeding, 2, 184–193.
- Kaufmann, M. L. (1971). The random method of oat breeding for productivity. Canadian Journal of Plant Science, 51, 13–16.10.4141/cjps71-002
- Kearsey, M. J., & Pooni, H. S. (1995). The genetical analysis of quantitative traits. Cheltenham: Stanley Thornes (Publishers) Ltd.
- Khush, G. S., & Virk, P. S. (2005). IR varieties and their impacts. Los Baños: International Rice Research Institute.
- Lenaerts, B. (2016). Economics of shorter breeding cycles in rice breeding ( Unpublished master’s thesis). KU Leuven, Leuven.
- Mackill, D. J., Coffman, W. R., & Garrity, D. P. (1996). Rainfed lowland rice improvement. Los Baños: International Rice Research Institute.
- Manigbas, N. L., & Lambio, L. A. F. (2015). Rapid generation advance in developing recombinant and backcrossed inbred lines for high temperature tolerance in rice (Oryza sativa L.). Rice-Based Biosystems Journal, 1, 39–48.
- Maruyama, K. (1989). Using rapid generation advance with single seed descent in rice breeding. Progress in irrigated rice research: Selected papers and abstracts from the international rice research conference, September 21–25, 1987, International Rice Research Institute, Hangzhou.
- McCouch, S. R., & Doerge, R. W. (1995). QTL mapping in rice. Trends in Genetics, 11, 482–487.10.1016/S0168-9525(00)89157-X
- Mishra, D. K., Singh, C. B., & Rao, S. K. (1994). Effectiveness of different selection methods in segregating population of rice (O. sativa L.) in ARC10372 x IR36 in different environments. Indian Journal of Genetics, 54, 402–408.
- Moon, H. P., Kang, K. H., Choi, I. S., Jeong, O. Y., Hong, H. C., Choi, S. H., & Choi, H. C. (2003). Comparing agronomic performance of breeding populations derived from anther culture and single-seed descent in rice. In G. S. Khush, D. S. Brar, and B. Hardy (Eds.), Advances in rice genetics. Supplement to rice genetics IV. Proceedings of the fourth international rice genetics symposium (pp. 3–5), October 22–27, 2000. Los Baños: International Rice Research Institute.
- Moose, S. P., & Mumm, R. H. (2008). Molecular plant breeding as the foundation for 21st century crop improvement. Plant Physiology, 147, 969–977. doi:10.1104/pp.108.118232
- Ohnishi, T., Yoshino, M., Yamakawa, H., & Kinoshita, T. (2011). The biotron breeding system: A rapid and reliable procedure for genetic studies and breeding in rice. Plant and Cell Physiology, 52, 1249–1257. doi:10.1093/pcp/pcr066
- Pandey, S., & Rajatasereekul, S. (1999). Economics of plant breeding: The value of shorter breeding cycles for rice in Northeast Thailand. Field Crops Research, 64, 187–197. doi:10.1016/S0378-4290(99)00059-3
- Patena, G., Bardhan Roy, S. K., & Vergara, B. S. (1980). Selection for earliness or short growth duration during rapid generation advance in rice breeding. International Rice Research Newsletter, 5, 4.
- Poehlman, J. M., & Sleper, D. A. (1995). Breeding field crops (4th ed.). Ames, IA: Iowa State University Press.
- Prasanna, B. M., Chaikam, V., & Mahuku, G. (Eds.). (2012). Doubled Haploid Technology in Maize breeding: Theory and practice. Mexico, DF: CIMMYT.
- Roy, S. K., & Mondal, J. (1985). Rapid generation advance of photoperiod-sensitive rice crosses under field conditions. International Rice Research Newsletter, 10, 3.
- Roy, S. K. B., Vergara, B. S., & Patena, G. (1981). Delay in flowering of some lines during rapid generation advance. International Rice Research Newsletter, 6, 3–4.
- Roy, S. K. B., Patena, G. F., & Vergara, B. S. (1982). Feasibility of selection for traits associated with cold tolerance inrice under rapid generation advance method. International Rice Research Newsletter, 31, 25–31.
- Sharma, J. R. (1994). Principles and practice of plant breeding. New Delhi: Tata McGraw-Hill.
- Simmonds, N. W. (1979). Principles of crop improvement. London: Longman.
- Snape, J. W., & Riggs, T. J. (1975). Genetical consequences of single seed descent in the breeding of self-pollinating crops. Heredity (Edinb), 35, 211–219.10.1038/hdy.1975.85
- Stoskopf, N. C., Tomes, D. T., & Christie, B. R. (1993). Plant breeding: Theory and practice. Boulder: Westview Press.
- Tanaka, J., Hayashi, T., & Iwata, H. (2016). A practical, rapid generation-advancement system for rice breeding using simplified biotron breeding system. Breeding Science, 66, 542–551. doi:10.1270/jsbbs.15038
- Van Oeveren, A. J., & Stam, P. (1992). Comparative simulation studies on the effects of selection for quantitative traits in autogamous crops: Early selection versus single seed descent. Heredity (Edinb), 69, 342–351. doi:10.1038/hdy.1992.134
- Vergara, B. S., Roy, S. K. B., & Patena, G. (1980). Selection for plant height during rapid generation advance in rice breeding. International Rice Research Newsletter, 5, 3.
- Vergara, B. S., Patena, G., Lopez, F. S. S. (1982). Rapid Generation of rice at the International Rice Research Institute. IRRI Research Paper Series No. 84. Los Baños: International Rice Research Institute.
- Yan, W. (2014). Crop variety trials: data management and analysis. Malaysia: Wiley Blackwell.10.1002/9781118688571