Abstract
Oligochitosan (OC) is effective biostimulant on growth promotion and elicitation against disease infection for plants. However, the range of OC molecular weight that exhibits the most effective activity is not fully understood and requires further investigation. In this study, OCs with different weight average molecular weight (Mw) were prepared by gamma Co-60 irradiation degradation of chitosan in solution and the effect on growth promotion and enhancement of fruit yield of chili plant (Capsicum frutescens L.) by foliar application of OCs particularly with Mw of 7.8, 5.0, and 2.5 kDa was investigated. Chili plants, cultivated in a greenhouse were sprayed with OC concentration of 50 mg/L for three times. Results indicated that among treatments, OC with 2.5 kDa proved to be the best, which increased the shoot fresh weight by 71.5%, shoot dry weight by 184%, total chlorophyll content by 12%, and fruit fresh weight by 49.8% for the control. Thus, OC with low Mw (2.5 kDa) that can be suitably produced on large scale by gamma Co-60 ray irradiation degradation of chitosan solution is potentially promising to apply as a biostimulant to enhance chili fruit yield significantly.
Classification:
Introduction
Oligochitosan (OC) is effective not only on promotion of plant growth such as on rice (Chamnanmanoontham et al., Citation2015), wheat (Ma et al., Citation2014; Zhang et al., Citation2016; Zou et al., Citation2015), soybean (Costales et al., Citation2016; Dzung & Thang, Citation2002; Luan et al., Citation2006; Phu et al., Citation2017), coffee (Dzung et al., Citation2011), faba bean (El-Sawy et al., Citation2010), orchid tissue (Nge et al., Citation2006), chrysanthemum (Hien, Citation2004), etc. but also on elicitation against disease infection for plants such as for Pisum sativum (Kendra & Hadwiger, Citation1984), tomato (Walker-Simmons et al., Citation1983), wheat (Vander et al., Citation1998), potato (Vasyukova et al., Citation2001), soybean (Khan et al., Citation2003), grapevine (Aziz et al., Citation2006), rice (Agrawal et al., Citation2002; Rodríguez et al., Citation2007), tobacco (Falcón et al., Citation2008; Zhao et al., Citation2007), etc. More information of the elicitation effect of OC on plants can be referred to the papers reviewed by Yin et al. (Citation2010) and Das et al. (Citation2015). According to Yin et al. (Citation2010), the OC acts as a plant vaccine that is similar with general vaccine for animal. It is worth to note that in certain conditions, the impacts of elicitors on plants physiology and defense response against pathogenic diseases may translate into yield increase (Al-Tawaha et al., Citation2005). Chitosan and OC are classified as plant biostimulants (du Jardin, Citation2015; Pichyangkura & Chadchawan, Citation2015). A biostimulant is defined as an organic material applied in small quantities that can enhance the plant growth and development (Sharma et al., Citation2014). According to Calvo et al. (Citation2014), the global market for biostimulant is increasing to exceed $2200 million by 2018. Therefore, chitosan and OC have attracted increasing interest in agricultural applications, especially in development of organic agriculture that do not harm to environment (Das et al., Citation2015; Garg & Balodi, Citation2014; Katiyar et al., Citation2014). The chitosans with molecular weight less than 10 kDa are called OC (Xia et al., Citation2011). The biological activity of OC is known to depend mainly on size (molecular weight), degree of deacetylation (DDA), and dosage (Cabrera et al., Citation2006; El-Sawy et al., Citation2010; Hien, Citation2004; Kendra & Hadwiger, Citation1984; Khan et al., Citation2003; Luan et al., Citation2006; Nge et al., Citation2006; No et al., Citation2002; Vasyukova et al., Citation2001; Zhang et al., Citation2016; Zou et al., Citation2015). For instance, Luan et al. (Citation2006) studied the effect of radiation-degraded chitosan, which was fractionated to prepare different molecular weight fractions on growth promotion of barley and soybean plants. The results revealed that the OC fraction with molecular weight of 1–3 kDa showed the highest effect compared to either smaller or higher OC molecular weight fractions. Results of El-Sawy et al. (Citation2010) also indicated that OC of 5–10 kDa exhibited better effect on growth promotion and increase in seed yield of faba bean plant compared to that of chitosan with higher molecular weight. Zou et al. (Citation2015) studied the effect of chitooligosaccharides (COS) with degree of acetylation (DA) from 2% to 68% on the wheat seedlings’ defense response under salt stress. The results showed that COS with DA 50% was the most effective in alleviating salt stress to wheat seedlings. Hien (Citation2004) found that the optimal concentration of OC for growth promotion of chrysanthemum plant was of 100 ppm. Generally, OC has stronger impact on growth promotion and elicitation for plants than chitosan (Chamnanmanoontham et al., Citation2015; Dzung & Thang, Citation2002; Luan et al., Citation2006; Rodríguez et al., Citation2007; Vasyukova et al., Citation2001). For instance, Vasyukova et al. (Citation2001) reported that OC of 5 kDa which was the most potent in protection potato tubers from Phytophthora infestans induced the formation of the greatest amount of the phytoalexin rishitin, while chitosan of 200 kDa was ineffective and low molecular weight chitosan (24 kDa) exhibited intermediate effect. Dzung and Thang (Citation2002) studied on enhancing seed yield of soybean from soybean seeds coated with OC and chitosan. The results showed that coating with OC produced higher yield compared to that of chitosan. In addition, by foliar application of OC and chitosan with the same concentration (60 ppm) for coffee plant, OC had a greater effect on the growth and development of coffee plant than chitosan (Dzung et al., Citation2011). Chitosan and OC are increasingly applied to crops with the aims of reducing or replacing environmentally damaging agrochemicals and farmers could gain substantial benefits from these applications of chitosan and OC to crops due to reduced input cost and the potential for increased yields (Katiyar et al., Citation2014). Furthermore, it is worth to note that beside increased yields, treatment of chitosan and OC also promotes the content of bioactive substances in several agro-products such as isoflavone in soybean seeds (Al-Tawaha et al., Citation2005), polyphenols in Greek oregano (Yin et al., Citation2012), artemisinin in Arteminisia annua L. (Lei et al., Citation2011), trigonelline, an antidiabetic compound obtained from the seeds of Fenugreek (Dar et al., Citation2015), and curcumin in turmeric (Anusuya & Sathiyabama, Citation2016). Therefore, study on finding of the suitable molecular weight of OC to achieve the optimal effect on plant growth promotion and/or elicitation is an essential work particularly for field application. Chili fruit is widely consumed as fresh and dried products mainly as spice for food ingredients. World production of chilies in 2009 was estimated to be about 3 million tons (Tiwari, Citation2009). The top-10 chili producing countries were India, China, Ethiopia, Myanmar, Mexico, Vietnam, Peru, Pakistan, Ghana, and Bangladesh, and accounted for more than 85% of the world chili production in 2007 (Huq & Arshad, Citation2010). Production of chili in Vietnam is currently estimated to be more than 30,000 tons with average productivity of about 15–20 tons/ha. Due to the demand of domestic and foreign market, there is a need to increase the productivity of these chili products appropriately. Up to now, study on foliar application of OC on chili plant has been scarcely published. The objective of this study is to investigate the effect of foliar spaying of OC with different molecular weights on the growth and fruit yield of chili plant.
Materials and methods
Preparation of oligochitosan
Chitosan from shrimp shell with DDA of ~91.4% and weight average molecular weight (Mw) of 44.5 kDa was supplied by a factory in Vung Tau province, Vietnam. OC with Mw of 7.8 and 5.0 kDa was prepared by gamma Co-60 ray irradiation degradation of 4% chitosan solution and 4% chitosan/.5% H2O2 solution at dose of 21 kGy, respectively, as reported in our previous paper (Phu et al., Citation2017). OC with Mw of 2.5 kDa was prepared by gamma Co-60 ray irradiation degradation of 2% chitosan/.5% H2O2 solution at dose of 21 kGy.
Experimental design and OC foliar application
The 30-day-old chili plants (Capsicum frutescens L.) were designed as randomized complete block design including four treatments with three replications of foliar application of OC namely: control (treated with water without OC), OC with Mw of 7.8 kDa (OC-7.8), 5.0 kDa (OC-5.0), and 2.5 kDa (OC-2.5). The OC concentration used for foliar sprays was of 50 mg/L. The experiments were conducted in the research green house of the Hi-Tech Agriculture Center, Cu Chi, Ho Chi Minh City at temperature of 30 ± 2 °C, RH of 60 ± 2%, and applied drip irrigation. The experiment period of four months was carried out from August to December 2016. The number of chili plants used in 4 treatments mentioned above was of 120 plants (30 plants/treatment), which were cultivated in plastic pots contained a mixture of 70% coconut fiber, 20% microbial fertilizer (Perionyx excavatus), and 10% rice husk. The total amount of applied nitrogen fertilizer (N2O) of 120–140 ppm was supplemented to appreciate physiological stages of chili plants. Foliar spraying by a hand sprayer on both surfaces of leaves was carried out three times at 30, 40, and 50 days after sowing with a volume of 50 ml of sample solutions per a chili plant.
Determination of growth and yield attributes
After the third spraying time, the chili plants were continued to grow till to blossom (Figure ). Then the fresh weight, the dried weight of shoot, and the chlorophyll content in fresh leaf were evaluated. Chlorophyll (a, b) content was spectrophotometrically determined using methanol extraction according to the method described by Dere et al. (Citation1998) with slight modifications. Briefly, 1 g of fresh leave samples was homogenized with 50 ml of methanol 96% in a ceramic mortar and pestle. The homogenate was centrifuged at 1150 ×g for 15 min on an EBA-12 centrifuge machine, Hettich, Germany. Then the supernatant was separated and recorded absorbances at 653 and 666 nm on an UV-2401PC spectrophotometer, Schimadzu. Fruit numbers per plant, fresh fruit weight per plant, and 100 fresh fruit weight were determined at the harvesting time (three times harvesting).
Statistical data analysis
All treatments were conducted in triplicate and the means were calculated. All the results were statistically analyzed by analysis of variance (ANOVA, MSTATC, Ver. 1.2, Michigan, USA, 1989). The means were compared using the least significant difference (LSD) at .05 probability level (p < .05).
Results
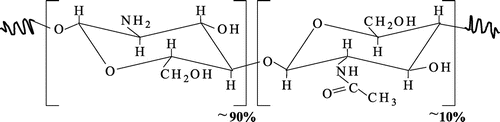
The molecular weight structure of OC with deacetylation degree of about 90% was presented in Figure . The prepared OCs of 7.8, 5.0; and 2.5 kDa had corresponding monomer units of 43, 27, and 14, and the main chemical structures analyzed by FTIR spectra did almost not change compared with initial chitosan (Duy et al., Citation2011; Phu et al., Citation2017).
Effect of OC on growth promotion for chili plant
Foliar application of OC stimulated shoot development and chlorophyll content for chili plant (Table ). The extent of increase in shoot dry weight followed the order: OC-2.5 (184%) > OC-5.0 (123.4%) > OC-7.8 (82.2%) for the control. The results in Table also indicated that treatment of OC-2.5 achieved the highest increase in shoot fresh weight (71.5%), shoot dry weight (184%), and chlorophyll content (12%) compared with that of the control.
Table 1. The growth parameters of chili plant treated by OC with different Mw.
Effect of OC on enhancement of chili fruit yield
The result in Table showed that the chili fruit yield enhanced to 49.8, 13.2, and 8.4% for OC-2.5, OC-5.0, and OC-7.8, respectively, compared to the control. The increase in fruit numbers per plant and fruit fresh weight of chili per plant was in the order of OC-2.5 > OC-5.0 > OC-7.8 > control. While the weight of 100 fresh fruits was not significant difference among four treatments. It was also observed that the OC-2.5 treatment attained the highest increase in fruit numbers per plant (83.4%), fruit fresh weight per plant (49.8%), or the yield increase of 49.8% for the control.
Table 2. The fruit yield attributes of chili plant treated by OC with different Mw.
Discussion
As reported in our previous papers, OC can be potentially produced on large scale by gamma Co-60 ray irradiation degradation of chitosan in solution (Duy et al., Citation2011; Phu et al., Citation2017). Thus, the greatest challenge in the development of efficient methods of OC for application in agriculture mentioned by Das et al. (Citation2015) could have been accordingly satisfied. According to Mourya et al. (Citation2011), the production of OC is of interest not only to agriculture but also to the food and medicine-related biotechnology industries.
Results of the present study demonstrated the potential of foliar application of OC for growth promotion as well as for enhancement of fruit yield of chili plant (Tables and ). It is important to note that the increase in chili fruit yield was not due to the increase in individual fruit weight but due to the increase in fruit numbers per plant. The same results were also obtained by Phu et al. (Citation2017) that the effect of OC on the increase in soybean seed yield was also due to the increase in the number of seeds per plant. Based on the results obtained in this study and the results of Phu et al. (Citation2017), it can be deduced that foliar application of OC for chili as well as for soybean plants made them to bloom better. That means there were more flowers to bear fruits per plant compared to that of the control. The highest increase of chili fruit yield was found for the treatment of OC-2.5, the smallest Mw among three studied OCs. Luan et al. (Citation2006) studied the effect of irradiated chitosan by molecular weight fractionation on the growth of barley and soybean. They also concluded that the fraction of OC with Mw in the range of 1–3 kDa exhibited the highest growth promotion effect. Thus, our results for chili plant in this study were in good agreement with the results reported by Luan et al. (Citation2006). However, the optimal concentration of OC-2.5 applied for chili plant should be further specified in a next study. Falcón-Rodríguez et al. (Citation2011) studied on plant elicitation effect of OC, and the obtained results indicated that OC caused a 10-fold small difference in concentration needed to induce the highest enzymatic activity compared to that of chitosan. The reason for the difference was that OC with small size (~.8–1.5 kDa) can be more easier than chitosan in passing plant physical barriers and accessing receptors at the plasma membranes in epidermal cells. Results of this study was also showed that OC with small Mw (2.5 kDa) was the most effective biostimulant for enhancement of crop yields particularly chili plant. The increase in chlorophyll content of OC-treated chili plants generates the higher photosynthetic rate. Photosynthesis converts light energy to chemical energy for plant growth (Chamnanmanoontham et al., Citation2015). Similar results were also obtained by Zhang et al. (Citation2016) for wheat seedlings, Chamnanmanoontham et al. (Citation2015) for rice seedlings, and Dzung et al. (Citation2011) for coffee plant. According to Zhang et al. (Citation2016), photosynthesis was the primary process for crops to form their grain yields and the reason for the increase in chlorophyll content may be that OC reduced the gene expression and enlarged the main photosynthesis apparatus chloroplast. In addition, Dzung et al. (Citation2011) reported that the OC treated coffee plant increased accumulation of mineral nutrients (N, P, K, Ca, and Mg). Mondal et al. (Citation2013) reported that the foliar application of 100–120 ppm chitosan at early stages improved the morphological, physiological parameters, key enzyme activities of nitrogen metabolism, yield components, and enhanced plant growth and development thereby increased seed yield of maize. However, the exact growth promotion mechanism was not clearly understood and needed to be further studied fundamentally.
According to Darvill et al. (Citation1992), the plants have the ability to recognize oligosaccharides which in turn regulates growth, development, and defense response in plants. Thus, besides OC, oligoalginate, oligo-β-glucan, and oligocarrageenan were also reported to possess growth promotion effect for plants (Abad et al., Citation2016; Hien et al., Citation2000; Luan & Uyen, Citation2014).
In conclusion, the foliar spray of OC for chili plant promoted the growth and enhanced the fruit yield. It was found that among three OCs studied, the OC with the lowest Mw (2.5 kDa) exhibited the most effective on growth promotion and enhancement of fruit yield for chili plant. Further study on finding optimal concentration of OC (2.5 kDa) to achieve the highest biological activities for chili plant should be carried out. Moreover, these prepared OCs can be also applied for other plants to promote the growth and enhance the crop yield, owing to the beneficially biological activities of OC for plants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Vietnam National Foundation for Science and Technology Development (NAFOSTED) [grant number 106-NN.03-2015.84].
Acknowlegements
The authors also would like to thank the Research and Development Center for Radiation Technology, VINATOM and the Research and Development Center for Hi-Tech Agriculture for favorable conditions for carrying out this work.
References
- Abad, L. V., Aurigue, F. B., Relleve, L. S., Montefalcon, D. R. V., & Lopez, G. E. P. (2016). Characterization of low molecular weight fragments from gamma irradiated κ-carrageenan used as plant growth promoter. Radiation Physics and Chemistry, 118, 75–80. doi:10.1016/j.radphyschem.2015.03.001
- Agrawal, G. K., Rakwal, R., Tamogami, S., Yonekura, M., Kubo, A., & Saji, H. (2002). Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiology and Biochemistry, 40, 1061–1069. doi:10.1016/S0981-9428(02)01471-7
- Al-Tawaha, A. M., Seguin, P., Smith, D. L., & Beaulieu, C. (2005). Biotic elicitors as a means of increasing isoflavone concentration of soybean seeds. Annals of Applied Biology, 146, 303–310. doi:10.1111/j.1744-7348.2005.040106.x
- Anusuya, S., & Sathiyabama, M. (2016). Effect of chitosan on growth, yield and curcumin content in turmeric under field condition. Biocatalysis and Agricultural Biotechnology, 6, 102–106. doi:10.1016/j.bcab.2016.03.002
- Aziz, A., Trotel-Aziz, P., Dhuicq, L., Jeandet, P., Couderchet, M., & Vernet, G. (2006). Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology, 96, 1188–1194. doi:10.1094/PHYTO-96-1188
- Cabrera, J. C., Messiaen, J., Cambier, P., & Van Cutsem, P. (2006). Size, acetylation and concentration of chitooligosaccharide elicitor determine the switch from defense involving PAL activation to cell death and water peroxide production in Arabidopsis cell suspensions. Physiologia Plantarum, 127, 44–56. doi:10.1111/j.1399-3054.2006.00677.x
- Calvo, P., Nelson, L., & Kloepper, J. W. (2014). Agriculture uses of plant biostimulants. Plant and Soil, 383, 3–41. doi:10.1007/s11104-014-2131-8
- Chamnanmanoontham, N., Pongprayoon, W., Pichayangkura, R., Roytrakul, S., & Chadchawan, S. (2015). Chitosan enhances rice seedling growth via gene expression network between nucleus and chloroplast. Plant Growth Regulation, 75, 101–114. doi:10.1007/s10725-014-9935-7
- Costales, D., Falcón, A. B., Nápoles, M. C., de Winter, J., Gerbaux, P., Onderwater, R. C. A., … Cabrare, J. C. (2016). Effect of chitosaccharides in nodulation and growth in vitro of inoculated soybean. American Journal of Plant Sciences, 7, 1380–1391. doi:10.4236/ajps.2016.79131
- Dar, T. A., Uddin, M., Khan, M. M. A., Ali, A., Mir, S. R., & Varshney, L. (2015). Effect of Co-60 irradiated chitosan and phosphorus fertilizer on growth, yield and trigonelline content of Trigonella foenum-graecum L. Journal of Radiation Research and Applied Sciences, 8, 446–458. doi:10.1016/j.jrras.2015.03.008
- Darvill, A., Augur, C., Bergmann, C., Carlson, R. W., Cheong, J. J., Eberhard, S., … Albersheim, P. (1992). Oligosaccharins-Oligosaccharides that regulate growth, development and defense responses in plants. Glycobiology, 2, 181–198. doi:10.1093/glycob/2.3.181
- Das, S. N., Madhuprakash, J., Sarma, P. V., Purushotham, P., Suma, K., Manjeet, K., … Podile, A. P. (2015). Biotechnological approaches for field applications of chitooligosaccharides (COS) to induce innate immunity in plants. Critical Reviews in Biotechnology, 35, 29–43. doi:10.3109/07388551.2013.798255
- Dere, S., Güneş, T., & Sivaci, R. (1998). Spectrophotometric determination of chlorophyll-a, b and total carotenoid contents of some algae species using different solvents. Turkish Journal of Botany, 22, 13–17.
- du Jardin, P. (2015). Plant biostimulants: Definition, concept, main categories and regulation. Scientia Horticulturae, 196, 3–14. doi:10.1016/j.scienta.2015.09.021
- Duy, N. N., Phu, D. V., Anh, N. T., & Hien, N. Q. (2011). Synergistic degradation to prepare oligochitosan by & γ-irradiation of chitosan solution in the presence of hydroperoxide. Radiation Physics and Chemistry, 80, 848–853. doi:10.1016/j.radphyschem.2011.03.012
- Dzung, N. A., Khanh, V. T. P., & Dzung, T. T. (2011). Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydrate Polymers, 84, 751–755. doi:10.1016/j.carbpol.2010.07.066
- Dzung, N. A., & Thang, N. T. (2002). Effects of oligoglucosamine prepared by enzyme degradation on the growth of soybean. Advances in Chitin Science, 5, 463–467.
- El-Sawy, N. M., El-Rehim, H. A. A., Elbarbary, A. M., & Hegazy, E. A. (2010). Radiation-induced degradation of chitosan for possible use as growth promoter in agricultural purposes. Carbohydrate Polymers, 79, 555–562. doi:10.1016/j.carbpol.2009.09.002
- Falcón, A. B., Cabrera, J. C., Costales, D., Ramírez, M. A., Cabrera, G., Toledo, V., & Martínez-Téllez, M. A. (2008). The effect of size and acetylation degree of chitosan derivatives on tobacco plant protection against Phytophthora parasitica nicotianae. World Journal of Microbiology and Biotechnology, 24, 103–112. doi:10.1007/s11274-007-9445-0
- Falcón-Rodríguez, A. B., Costales, D., Cabrera, J. C., & Martínez-Téllez, M. A. (2011). Chitosan physico-chemical properties modulate defense responses and resistance in tobacco plants against the oomycete Phytophthora nicotianae. Pesticide Biochemistry and Physiology, 100, 221–228. doi:10.1016/j.pestbp.2011.04.005
- Garg, A., & Balodi, R. (2014). Recent trends in agriculture: Vertical farming and organic farming. Advances in Plants and Agriculture Research, 1, 00023. doi:10.15406/apar.2014.01.00023
- Hien, Q. N. (2004). Radiation degradation of chitosan and some biological effect, radiation processing of polysaccharides (IAEA-TECHDOC-1422). (pp. 67–73). Vienna: IAEA.
- Hien, N. Q., Nagasawa, N., Tham, L. X., Yoshii, F., Dang, V. H., Mitomo, H., … Kume, T. (2000). Growth-promotion of plants with depolymerized alginates by irradiation. Radiation Physics and Chemistry, 59, 97–101. doi:10.1016/S0969-806X(99)00522-8
- Huq, A. S. M. A., & Arshad, F. M. (2010). Technical efficiency of chili production. American Journal of Applied Sciences, 7, 185–190. doi:10.3844/ajassp.2010.185.190
- Katiyar, D., Hemantaranjan, A., Singh, B., & Bhanu, A. N. (2014). A future perspective in crop protection: Chitosan and its oligosaccharides. Advances in Plants and Agriculture Research, 1, 00006. doi:10.15406/apar.2014.01.00006
- Kendra, D. F., & Hadwiger, L. A. (1984). Characterization of the smallest chitosan oligomer that is maximally antifungal to Fursarium solani and elicits pisatin formation in Pisum sativum. Experimental Mycology, 8, 276–281. doi:10.1016/0147-5975(84)90013-6
- Khan, W., Prithiviraj, B., & Smith, L. D. (2003). Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. Journal of Plant Physiology, 160, 859–863. doi:10.1078/0176-1617-00905
- Lei, C., Ma, D., Pu, G., Qiu, X., Du, H., Wang, H., … Liu, B. (2011). Foliar application of chitosan activates artemisinin biosynthesis in Artemisia annua L. Industrial Crops and Products, 33, 176–182. doi:10.1016/j.indcrop.2010.10.001
- Luan, L. Q., Nagasawa, N., Tamada, M., & Nakanishi, T. M. (2006). Enhancement of plant growth activity of irradiated chitosan by molecular weight fractionation. Radioisotopes, 55, 21–27. doi:10.3769/radioisotopes.55.21
- Luan, L. Q., & Uyen, N. H. P. (2014). Radiation degradation of (1 → 3)-β-D-glucan from yeast with a potential application as a plant growth promoter. International Journal of Biological Macromolecules, 69, 165–170. doi:10.1016/j.ijbiomac.2014.05.041
- Ma, L. J., Li, Y. Y., Wang, L. L., Li, X. M., Lui, T., & Bu, N. (2014). Germination and physiological response of wheat (Triticum aestivum) to pre-soaking with oligochitosan. International Journal of Agriculture and Biology, 16, 766–770.
- Mondal, M. M. A., Puteh, A. B., Dafader, N. C., Rafii, M. Y., & Malek, M. A. (2013). Foliar application of chitosan improves growth and yield in maize. Journal of Food, Agriculture and Environment, 11, 520–523.
- Mourya, V. K., Inamdar, N. N., & Choudhari, Y. M. (2011). Chitooligosaccharides: Synthesis, characterization and applications. Polymer Science Series A, 53, 583–612. doi:10.1134/S0965545X11070066
- Nge, K. L., Nwe, N., Chandrkrachang, S., & Stevens, W. F. (2006). Chitosan as a growth stimulator in orchid tissue culture. Plant Science, 170, 1185–1190. doi:10.1016/j.plantsci.2006.02.006
- No, H. K., Park, N. Y., Lee, S. H., & Meyers, S. P. (2002). Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. International Journal of Food Microbiology, 74, 65–72. doi:10.1016/S0168-1605(01)00717-6
- Phu, D. V., Du, B. D., Tuan, L. N. A., Tam, H. V., & Hien, N. Q. (2017). Preparation and foliar application of oligochitosan and oligochitosan-nanosilica on the enhancement of soybean seed yield. International Journal of Environment, Agriculture and Biotechnology, 2, 421–428. doi:10.22161/ijeab/2.1.53
- Pichyangkura, R., & Chadchawan, S. (2015). Biostimulant activity of chitosan in horticulture. Scientia Horticulturae, 196, 49–65. doi:10.1016/j.scienta.2015.09.031
- Rodríguez, A. T., Ramírez, M. A., Cárdenas, R. M., Hernández, A. N., Velázquez, M. G., & Bautista, S. (2007). Induction of defense response of Oryza sativa L. against Pyricularia grisea (Cooke) Sacc. by treating seeds with chitosan and hydrolyzed chitosan. Pesticide Biochemistry and Physiology, 89, 206–215. doi:10.1016/j.pestbp.2007.06.007
- Sharma, F., Fleming, S. C., Selby, C., Rao, J. R., & Martin, T. (2014). Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. Journal of Applied Phycology, 26, 465–490. doi:10.1007/s10811-013-0101-9
- Tiwari, R. K. (2009). Post harvest profile of chili. Retrieved from http://agmarknet.gov.in/Others/preface-chhilli.pdf
- Vander, P., Vårum, K. M., Domard, A., Gueddari, N. E. E., & Moerschbacher, B. M. (1998). Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves. Plant Physiology, 118, 1353–1359. doi:10.1104/pp.118.4.1353
- Vasyukova, N. I., Zinov’eva, S. V., Il’inskaya, L. I., Perekhod, E. A., Chalenko, G. I., Gerasimova, N. G., … Ozeretskovskaya, O. L. (2001). Modulation of plant resistance to diseases by water- soluble chitosan. Applied Biochemistry and Microbiology, 37, 103–109. doi:10.1023/A:1002865029994
- Walker-Simmons, M., Hadwiger, L., & Ryan, C. A. (1983). Chitosans and pectic polysaccharides both induce the accumulation of the antifungal phytoalexin pisatin in pea pods and antinutrient proteinase inhibitors in tomato leaves. Biochemical and Biophysical and Research Communications, 110, 194–199. doi:10.1016/0006-291X(83)91279-2
- Xia, W., Liu, P., Zhang, J., & Chen, J. (2011). Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids, 25, 170–179. doi:10.1016/j.foodhyd.2010.03.003
- Yin, H., Fretté, X. C., Christensen, L. P., & Grevsen, K. (2012). Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. hirtum). Journal of Agricultural and Food Chemistry, 60, 136–143. doi:10.1021/jf204376j
- Yin, H., Zhao, X., & Du, Y. (2010). Oligochitosan: A plant disease vaccine-A review. Carbohydrate Polymers, 82, 1–8. doi:10.1016/j.carbpol.2010.03.066
- Zhang, X., Li, K., Liu, S., Xing, R., Yu, H., Chen, X., & Li, P. (2016). Size effects of chitooligomers on the growth and photosynthetic characteristics of wheat seedlings. Carbohydrate Polymers, 138, 27–33. doi:10.1016/j.carbpol.2015.11.050
- Zhao, X., She, X., Yu, W., Du, Y., & Liang, X. (2007). Induction of antiviral resistance and stimulary effect by oilgochitosan in tobacco. Pesticide Biochemistry and Physiology, 87, 78–84. doi:10.1016/j.pestbp.2006.06.006
- Zou, P., Li, K., Liu, S., Xing, R., Qin, Y., Yu, H., … Li, P. (2015). Effect of chitooligosaccharides with different degrees of acetylation on wheat seedlings under salt stress. Carbohydrate Polymers, 126, 62–69. doi:10.1016/j.carbpol.2015.03.028