ABSTRACT
Abscisic acid (ABA) is a key factor regulating starch biosynthesis genes and is involved in assimilate partitioning to individual spikelets. The objective of this study was to clarify the effects of high temperature and shading during grain filling on grain ABA content and the grain filling pattern of spikelets located at different positions in a panicle. We grew the rice cultivar ‘Koshihikari’ in pots in 2009 under two temperature regimes and two light conditions during grain filling. We periodically measured grain dry weight and grain ABA content (pmol per grain) and concentration (pmol per grain dry weight). Shading increased a grain weight difference between superior and inferior spikelets while high temperature decreased the difference regardless of light condition. High temperature decreased ABA content and concentration in grains. There was a close correlation between mean grain ABA content and mean grain-filling rate averaged over the first half of grain filling.
Introduction
High temperature during grain filling reduces the grain quality of rice (Oryza sativa L.); in particular, it results in chalky grains, whose direct cause is proposed to be the imbalance of starch biosynthesis and degradation (Mitsui, Yamakawa, & Kobata, Citation2016). High temperature downregulates the expression of starch biosynthesis genes (Yamakawa, Hirose, Kuroda, & Yamaguchi, Citation2007). It has been proposed that some of key genes for starch biosynthesis are regulated by abscisic acid (ABA) (Halford & Hey, Citation2009; Jossier et al., Citation2009; Radchuk et al., Citation2010; Sreenivasulu et al., Citation2006). These observations suggest that grain ABA content is a key factor in grain filling under high temperature. Yang et al. (Citation2004) reported the relationship between grain ABA content and grain-filling rate under water stress condition. However, to the best of our knowledge, no evaluation of the effects of high temperature on grain ABA level has been reported.
In general, grain growth and filling are not synchronous among spikelets located at different positions in a panicle. Early flowering spikelets (superior spikelets) fill fast and produce large and heavy grains, whereas later flowering spikelets (inferior spikelets) fill slowly and poorly. Many studies have suggested that various phytohormones, including ABA, contribute to this dominance of superior spikelets, although its mechanism is not fully understood (Mohapatra, Panigrahi, & Turner, Citation2011). Yang, Zhang, Wang, Liu, and Wang (Citation2006) showed a highly positive correlation between grain ABA content and grain filling rate of individual spikelets in a panicle. If grain ABA content is affected by high temperature, assimilate partitioning to individual spikelets would also be affected. Assimilate partitioning to spikelets depends on the plant assimilate level; superior spikelets are less affected than inferior spikelets (Tsukaguchi, Murakami, & Michimoto, Citation2016).
The objective of this study was to clarify the effect of high temperature on grain ABA content and concentration and on the grain-filling pattern of individual spikelets located at different positions in a panicle, under two light conditions, and also to clarify the relationship between grain ABA content and grain-filling rate of these spikelets under high temperature and shaded conditions in rice. Shading would change a pattern of assimilates partitioning to spikelets by increasing a difference in grain weight between superior and inferior spikelets.
Materials and methods
Plant materials
Experiments were conducted with the cultivar ‘Koshihikari’ at Ishikawa Prefectural University in Nonoichi, Japan, in 2009. Seeds were sown in paper pots (R-5, Nippon Beet Sugar Manufacturing, Tokyo, Japan). On 3 June, seedlings (20 per pot; 23-day-old) were transplanted in a ring into Wagner pots (16.0 cm in diameter, 19.0 cm in depth) filled with sandy soil that received a basal dressing of 0.6 g of N as ammonium sulfate, 1.0 g (P2O5 equivalent) of P as superphosphate, and 1.0 g (K2O equivalent) of K as potassium chloride. An additional 0.2 g of N per pot was topdressed as ammonium sulfate on 28 July. Tillers were pruned to leave only the main stem in each plant. Plants were grown outdoors until the experimental temperature treatment. At heading, we selected pots with at least 10 plants whose first flowering date was 13 August and tagged those plants. The flowering date of each spikelet of the tagged plants was recorded.
Temperature and shading treatments
Treatments were applied 6 days after first flower (DAFF) in a partitioned temperature-gradient chamber (Nakagawa, Tsukaguchi, Yamada, & Yamamura, Citation2009). The chamber was partitioned into three rooms with different temperatures (T1, T2, T3). The temperature in T1 was maintained at ambient. That in T3, which was used for high-temperature treatment, was approximately 4°C higher. Mean air temperature in T1 and T3 between 6 and 30 DAFF was 25.3°C and 29.7°C. Each room housed a waterbed (3.7 m in length and 1.7 m in width), in which we placed the pots. The water level was 2–5 cm above the top of the pots throughout the treatment. We used four conditions: high temperature with shading (HS), high temperature without shading (HC), ambient temperature with shading (AS), and ambient temperature without shading (AC). For shading, we placed a tunnel (1.8 m in height, 1.2 m in length, 0.9 m in width) covered with two layers of polyethylene shading mesh (DIO Chemicals Ltd., Tokyo, Japan). The bottom of the shading mesh was approximately 30 cm above the water level. The photosynthetically active radiation level at panicle height was 22% of that without the tunnel. The air temperature at panicle height was measured and recorded with a thermo recorder (Ondotori Jr. RTR53. TANDD, Tokyo, Japan) at logging intervals of 10 min. Five pots as replication were housed in each treatment.
Grain dry weight and grain-filling rate
One panicle was sampled from one plant per pot at 6, 9, 12, 14, 16, 19, 22, and 26 DAFF and at maturity. Panicles sampled between 6 and 22 DAFF were frozen in liquid nitrogen and freeze dried to a constant weight. The other panicle samples were oven dried at 80°C for 3 days. Dried spikelets were divided into six groups: U1, U2, U3, L1, L2, and L3. We grouped primary (1), secondary (2), and tertiary spikelets (3) (Matsuba, Citation1991) of upper (or apical, U) and lower (or proximal, L) rachis branches of a panicle (namely U1, U2, U3, L1, L2, and L3) and determined the grain-filling pattern and ABA content in grains of U1, L2, and L3 spikelets. Final grain weight was measured in all groups, along with grain filling rate in U1, L2, and L3 spikelets. The mean flowering date (DAFF) and the number of grains in each position of a panicle sampled at maturity in 2009 are listed in . Grains of each group were hulled, counted, and weighed.
Grain dry weight increase was fitted by the equation of Richards (Citation1959) as described by Yang et al. (Citation2006):
Grain-filling rate was calculated as the derivative of Equation (1):
where W is grain dry weight; Wmax is maximum grain dry weight; t is cumulative temperature; and A, B, and k are coefficients determined by regression.
4. ABA assay
ABA assay was performed with grains of U1, L2, and L3 spikelets sampled between 6 and 22 DAFF in 2009 after the measurement of dry weight. Samples were homogenized in 5 ml of 80% methanol containing 1 mM dibutylhydroxytoluene on ice, and the homogenate was centrifuged at 10,000×g for 5 min. The supernatant was dried in a vacuum centrifuge and the residue was dissolved in 1 ml of phosphate buffer (pH 8.3). The solution was washed by partitioning with 1 ml hexane and then with 1 ml of ethyl acetate, each three times. The buffer phase was adjusted to pH 2.5–3.0 with 1 N HCl, and ABA was extracted with ethyl acetate. The ethyl acetate phase was dried in a vacuum centrifuge; the residue was dissolved in 5 ml of Tris buffer saline and used for ABA analysis by quantitative immunoassay with the Abscisic Acid Immunoassay Detection Kit (SIGMA-ALDRICH, St. Louis, USA). Grain ABA content was calculated as pmol ABA per grain and grain ABA concentration was calculated as pmol ABA per grain dry weight. The mean grain ABA content of each spikelet group was determined by dividing the cumulative ABA content between 6 and the day at which cumulative temperature reached 500°C days. Cumulative temperature reached 500°C days approximately at 16.2 DAFF in HS and HC and at 18.8 DAFF in AS and AC, respectively. ABA contents of these DAFF were determined by linear interpolation. The variation among spikelets in grain-filling rate was maximum when it was calculated between 6 DAFF and the day at which the cumulative temperature reached approximately 500°C in all the treatments.
5. Statistical analysis
Significance of the differences between mean values of spikelets located at different positions was determined by Tukey’s test in SPSS v. 21 software (SPSS Inc., Chicago, IL, USA). Significance was defined as P < 0.05. R2 for Equation (1) in each spikelet was calculated as follows:
where yi is measured grain dry weight, Wi is estimated grain dry weight by Equation (1), and is the mean of yi.
Results
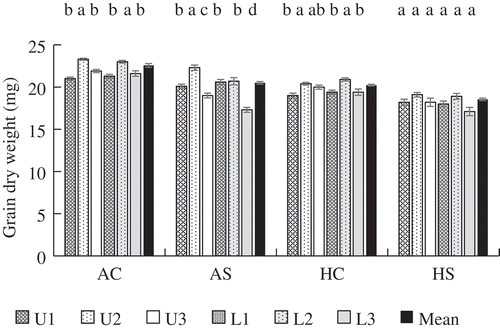
Differences in final grain weight among spikelet positions varied widely among treatments (). In HS, grain weight of all the spikelets was <20 mg and there was no significant difference. In AS, grain weight of tertiary spikelets was significantly lower than that of primary and secondary spikelets and the difference between U2, the highest, and L3, the lowest, was approximately 5 mg. In HC, differences in grain weight tended to be smaller than those in AC.
Figure 1. Final grain dry weight of primary (1), secondary (2), and tertiary (3) spikelets in the upper (U) and lower (L) parts of a panicle and mean grain weight of a panicle. Error bars represent standard error (n = 5). The same letters indicate no significant difference among spikelet positions in a treatment (Tukey’s test). AC: Ambient temperature without shading; AS: ambient temperature with shading; HC: high temperature without shading; HS: high temperature with shading.
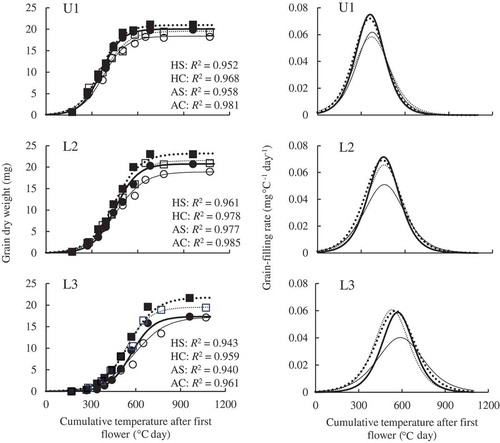
The grain-filling rate peaked and then decreased (). The peaks of U1 spikelets in HS and HC were lower than in AS and AC. The peak of L2 spikelets in HS was lower than in the other treatments. The peak of L3 spikelets in HS was lower than in the other treatments whereas the rapid increase of grain-filling rate in AS was clearly later than in HC and AC.
Figure 2. Changes in grain dry weight and grain-filling rate of primary spikelets in the upper part of a panicle (U1) and in secondary (L2) and tertiary spikelets (L3) in the lower part. ··■·· AC (ambient temperature without shading); —●— AS (ambient temperature with shading); ··□·· HC (high temperature without shading); —○— HS (high temperature with shading).
The grain ABA content of U1 spikelets increased rapidly after 6 DAFF, peaked, and then decreased (). In comparison with AC, HS and HC decreased grain ABA content, but AS had no clear effect. In L2 spikelets, grain ABA content increased similarly in all treatments but started to decrease much earlier in HS and HC. AS delayed the peak. In L3 spikelets, grain ABA content showed trends similar to those of L2 spikelets, but HS peaked at nearly the same time as in AC.
Figure 3. Changes in grain ABA content and concentration in primary spikelets in the upper part of a panicle (U1) and in secondary (L2) and tertiary spikelets (L3) in the lower part. Error bars represent standard error (n = 5). ··■·· AC (ambient temperature without shading); —●— AS (ambient temperature with shading); ··□·· HC (high temperature without shading); —○— HS (high temperature with shading).
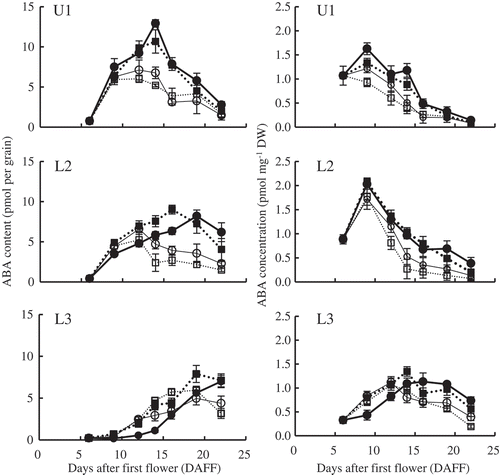
The grain ABA concentration increased, peaked, and then decreased, except in U1 spikelets in HC, in which no peak was observed between 6 and 22 DAFF (). In U1 and L2 spikelets, it tended to be lower in HS and HC than in AC. In L3 spikelets, it increased similarly in AC, HC, and HS but started decreasing earlier in HC and HS. AS delayed the increase.
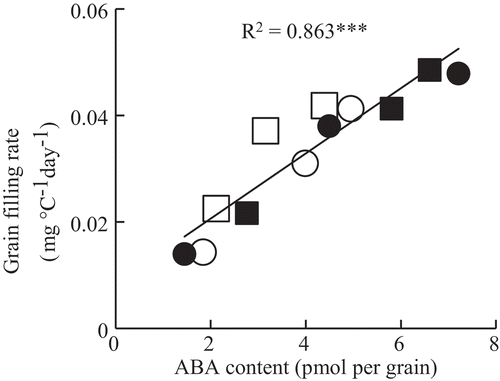
There was a wide variation among spikelets in grain-filling rate between 6 DAFF and the day at which the cumulative temperature reached 500°C days, after that grain weight of U1 spikelets plateaued and the variation became smaller. The mean ABA content during this duration was compared among spikelets located at different positions (). The mean ABA content in U1 spikelets tended to be higher than in L2 and that in L2 tended to be higher than in L3 spikelets. Differences in the mean ABA content among spikelet positions were smaller in HS and HC than in AC. The mean grain-filling rate in this duration was highly and positively correlated with the mean ABA content in the same period ().
Figure 4. Mean grain ABA content of primary spikelets in the upper part of a panicle (U1) and in secondary (L2) and tertiary spikelets (L3) in the lower part of a panicle. The mean grain ABA content was between 6 and the day at which cumulative temperature reached 500°C days. Error bars represent standard error (n = 5). The same letters indicate no significant difference among spikelet positions in a treatment (Tukey’s test). AC: Ambient temperature without shading; AS: ambient temperature with shading; HC: high temperature without shading; HS: high temperature with shading.
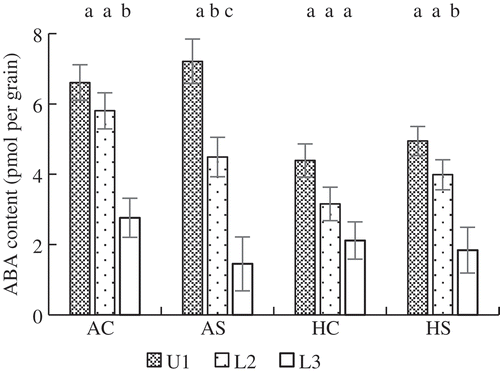
Figure 5. Relationship between the mean ABA content and mean grain filling rate between 6 and the day at which cumulative temperature reached 500°C days. ***Significant at the 0.001 level. ■ AC (ambient temperature without shading); ● AS (ambient temperature with shading); □ HC (high temperature without shading); ○ HS (high temperature with shading).
Discussion
High temperature markedly decreased ABA content and concentration in grains ( and ). ABA produced in the endosperm has been suggested to regulate starch biosynthesis genes. Sreenivasulu et al. (Citation2006) conducted a large-scale transcriptome analysis and revealed that genes for proteins involved in ABA biosynthesis, SNF-related kinase, and starch biosynthesis proteins (such as ADPGP and sucrose synthase) are co-expressed in the mid to late grain-filling period in developing barley seeds. SNF-related kinase plays a key role in sugar and ABA signaling (Jossier et al., Citation2009; Radchuk et al., Citation2010) and is proposed to regulate starch biosynthesis genes (Halford & Hey, Citation2009). In rice grains, the expression of genes related to starch biosynthesis coincides with a rapid increase in grain dry weight both in superior and inferior spikelets (Ishimaru et al., Citation2005; Ishimaru, Matsuda, Ohsugi, & Yamagishi, Citation2003). In our study, the increase in grain ABA content coincided with that in grain-filling rate regardless of spikelet positions and environmental conditions ( and ). These results suggest that ABA accumulation in the endosperm is associated with starch biosynthesis ability. Whether lower ABA content in developing grains is associated with suppressed expression of genes for starch biosynthesis under high temperature stress needs further investigation.
Although mean final grain weight was similar in AS and HC, grain weight of individual spikelets located at different positions was differently affected. The grain weight tended to be more uniform among spikelet positions in HC, whereas grain weight differences were much increased in AS (). Interestingly, high temperature appeared to erase the effect of shading on grain weight difference as there was no significant difference in grain weight among spikelet positions in HS. Similar trends were observed in the mean ABA content in grains. ABA content was more uniform among spikelet positions in HS and HC, whereas differences among spikelet positions seemed to be increased in AS.
There was a close association between mean grain ABA content and mean grain-filling rate during the first half of grain filling, the period when the difference in grain-filling rate among spikelets was maximum, in agreement with a previous study (Yang et al., Citation2006) (). This correlation supports the involvement of ABA in assimilate partitioning to spikelets, possibly through starch biosynthesis ability. A relatively small difference in grain ABA content between superior and inferior spikelets at high temperature may result in the change in assimilate partitioning to individual spikelets located at different positions in a panicle.
High temperature during grain filling deteriorates grain quality through increasing chalky grains. Some kind of chalky grains are increased by decreased assimilates supply per spikelet and some are not (Tsukaguchi et al., Citation2012). High temperature decreased grain ABA level and changed assimilates partitioning to spikelets. These results suggest that ABA is a key to understand the mechanism of the occurrence of chalky grains.
In conclusion, high temperature decreased grain ABA content and concentration, suggesting that a lower ABA level at high temperature is related to the decline in starch biosynthesis ability in grains. We also found that high temperature affects assimilate partitioning to individual spikelets possibly through a smaller difference in grain ABA content between spikelets.
Table 1. Mean flowering date (DAFF) and number of grains (±standard deviation of 20 replicates) of primary (1), secondary (2), and tertiary (3) spikelets at upper (U) and lower (L) part of a panicle sampled at maturity.
Acknowledgments
We thank Ayumi Sakuragawa of Ishikawa Prefectural University for her assistance in carrying out the experiments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- *In Japanese with English abstract.
- **In Japanese
- Halford, N., & Hey, S. (2009). Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signaling in plants. Biochemical Journal, 419, 247–259.
- Ishimaru, T., Hirose, T., Matsuda, T., Goto, A., Takahashi, K., Sasaki, H., … Yamagishi, T. (2005). Expression patterns of genes encoding carbohydrate-metabolizing enzymes and their relationship to grain filling in rice (Oryza sativa L.): Comparison of caryopses located at different positions in a panicle. Plant Cell Physiology, 46, 620–628.
- Ishimaru, T., Matsuda, T., Ohsugi, R., & Yamagishi, T. (2003). Morphological development of rice caryopses located at the different positions in a panicle from early to middle stage of grain filling. Functional Plant Biology, 30, 1139–1149.
- Jossier, M., Bouly, J., Meimoun, P., Arjmand, A., Lessard, P., Hawley, S., … Thomas, M. (2009). SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signaling. Arabidopsis Thaliana. The Plant Journal, 59, 316–328.
- Matsuba, K. (1991). The morphogenetic mechanism of formation of the panicle branching system in rice plants (Oryza sativa L.). Bulletin of Chugoku National Agricultural Experimental Station, 9, 11–58.
- Mitsui, T., Yamakawa, H., & Kobata, T. (2016). Molecular physiological aspects of chalking mechanism in rice grains under high-temperature stress. Plant Production Science, 19, 22–29.
- Mohapatra, P., Panigrahi, R., & Turner, N. (2011). Physiology of spikelet development on the rice panicle: Is manipulation of apical dominance crucial for grain yield improvement?. Advances in Agronomy, 110, 333–359.
- Nakagawa, H., Tsukaguchi, T., Yamada, H., & Yamamura, T. (2009). Development of compartmented temperature gradient chamber. Japanese Journal of Crop Science, 78 extra issue 1, 180–181 .
- Radchuk, R., Emery, R., Weiner, D., Vigeolas, H., Geigenberger, P., Lunn, J., … Weber, H. (2010). Sucrose non-fermenting kinase 1 (SnRK1) coordinates metabolic and hormonal signals pea cotyledon growth and differentiation. The Plant Journal, 61, 324–338.
- Richards, F. (1959). A flexible growth function for empirical use. Journal of Experimental Botany, 10, 290–300.
- Sreenivasulu, N., Radchuk, V., Strickert, M., Miersch, O., Weschke, W., & Wobus, U. (2006). Gene expression patterns reveal tissue-specific signaling network controlling programmed cell death and ABA-regulated maturation in developing barley seeds. The Plant Journal, 47, 310–327.
- Tsukaguchi, T., Murakami, K., & Michimoto, T. (2016). A quantitative measure for assimilate partitioning efficiency in rice (Oryza sativa L.). Field Crops Research, 198, 122–130.
- Tsukaguchi, T., Yamamura, T., Inoue, H., Nakagawa, H., Murakami, K., & Kita, E. (2012). The response of the occurrence of milky white kernels with different cross-sectional patterns of chalkiness in the endosperm to grain-filling temperature and to assimilate supply in Koshihikari. Japanese Journal of Crop Science, 81, 267–274.
- Yamakawa, H., Hirose, T., Kuroda, M., & Yamaguchi, T. (2007). Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiology, 144, 258–277.
- Yang, J., Zhang, J., Wang, Z., Liu, K., & Wang, P. (2006). Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. Journal of Experimental Botany, 57, 149–160.
- Yang, J., Zhang, J., Ye, Y., Wang, Z., Zhu, Q., & Liu, L. (2004). Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant, Cell and Environment, 27, 1055–1064.