ABSTRACT
Approximately 30% of harvested yams are used in subsequent plantings as seed tubers, which reduces the harvest size; however, planting tuber pieces (setts) potentially represents a viable alternative. To determine how sett size affects yam production, different sett sizes were compared for sprouting, shoot growth, and tuber yield. Larger setts exhibited faster sprouting with greater shoot biomass due to larger shoot growth rates during the early growth period. Tuber yield from 200 g setts was higher than that from 50 g setts; however, this yield advantage was not enough to compensate for the cost of larger sett size. Thus, planting 50 g setts might be the most cost-effective. Even the 50 g setts yielded higher than 1 kg tuber per plant which were available for selling in markets. Thus, planting with smaller setts can be promising method for efficiently improving yam production.
Graphical Abstract

1. Introduction
White guinea yam (Dioscorea rotundata) is a widely cultivated tuber crop in the gulf region of West Africa, in a region from west Cameroon to central Côte d’Ivoire that is called the yam belt. This region produces over 90% of the global yam production. Harvested fresh tubers are used for local consumption and can be boiled, fried, roasted, or pounded. Because yam plants reproduce via vegetative propagation using tubers, the harvest also supplies planting materials for subsequent cultivation. In a traditional farming system, approximately 10,000 seed yams, each weighing between 200 and 1000 g, are needed to plant one hectare (Orkwor & Asadu, Citation1998). Seed yams represent up to 30% of the harvest, generating competition over the use of tubers as either food or planting material (Agbarevo, Citation2014).
As an alternative, 200–500 g pieces from a large tuber (sett) can be used as planting material. A minisett method has also been developed that uses tuber pieces under 50 g for seed yam production in a process that is separate from food yam production (Nwekw, Citation1997). Another advantage of using the sett method is that it can reduce the number of tubers needed for planting. This method also facilitates breeding with large cross population or genetic resources, in which propagation cost is one of the key obstacles. Igwilo and Okoli (Citation1988) reported that 25 g setts yielded larger than 1 kg tuber per plant in D. alata; thus, the minisett method could be used to produce not only seed yam but also food yam.
Using smaller setts would be more favorable for reducing production costs; however, smaller setts or minisetts are infrequently adopted by farmers owing to low sprouting rates and unstable tuber yields (Emokaro & Law-Ogbomo, Citation2008; Onemolease & Adisa, Citation2005). Ayankanmi, Shiwachi and Asiedu (Citation2005) reported that sprouting rates were higher in larger setts compared to smaller setts, with larger setts also producing higher yields. There is empirical evidence that sett size affects tuber yield due to differences in shoot growth; however, this phenomenon has not been well studied. To determine the possibility of using smaller setts in food yam production, this study compared different sett sizes with respect to sprouting, shoot growth, and tuber yield.
2. Materials and methods
2.1. Plant materials and growth conditions
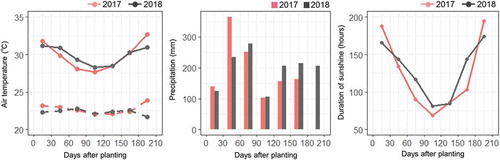
The field study was conducted in 2017 and 2018 in the experimental field of the International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria (7° 29′ N, 3° 54′ E). D. rotundata accession (TDr 95/01932) from IITA was used to create setts of different sizes. A normal sized tuber weighing approximately 1–2 kg was cut horizontally to remove the head and tail parts. Next, the center part was cut into small pieces, each with a skin surface where a shoot bud could emerge. The seed setts were cut into three sizes: 50 g, 100 g, and 200 g. The setts were treated with fungicide before planting for presprouting. The setts were planted on 12 May 2017 and 3 May 2018, in plastic pots (12 cm diameter and 10 cm height) filled with topsoil (sandy loam, pH 7.6). After one month, the plants that had adequate sprouts were selected and transplanted to a field with stakes set at 1 m × 1 m density. For each sett size, a 2 m line plot was established containing three plants in a randomized block design with five replications. Weeding was performed manually when required. Fertilizers were not applied. Meteorological conditions during the growth period (from planting to seven months after planting) are presented in . Total precipitation, average minimum/maximum temperatures, and average duration of sunshine for this period was 1217 mm, 22.7/30.5°C, and 5.0 h day−1 in 2017 and 1384 mm, 22.2/30.7°C, and 5.5 h day−1 in 2018.
Figure 1. Air temperature, precipitation, and duration of sunshine during the growth periods. For air temperature (left box), solid lines and dashed lines represent maximum and minimum air temperatures averaged for every 30 days after planting. For precipitation (central box) and duration of sunshine (right box), data are the cumulative values for every 30 days after planting.
2.2. Measurements
The sprouting date was recorded for all plants before transplanting. In the field, time-course changes in shoot dry weight were evaluated using the non-destructive method described in Iseki and Matsumoto (Citation2019). The normalized difference vegetation index (NDVI) was measured using a handheld sensor (GreenSeeker, Nikon Trimble, Tokyo, Japan) with simultaneous measurements of plant height also being recorded. Shoot dry weight was estimated using an equation including NDVI and plant height as explanatory variables. Shoot dry weight was evaluated for the three plants in each plot. The shoot growth rate was calculated by dividing differences in shoot dry weight between the two different sampling time-points by the duration of the sampling interval and was expressed as g m−2 day−1. In late December, at full senescence of the above ground parts, the tubers of each plant were harvested and the fresh weight measured. A subset of tubers from each plot was used to measure moisture content. Fresh tuber yield was calculated as 65% of the moisture content. The tuber multiplication rate was calculated as the ratio of tuber yield to the sett size.
2.3. Statistical analysis
One-way analysis of variance and multiple comparison analysis using a Tukey’s HSD test were performed to detect any statistically significant differences in days to sprouting, tuber yield, tuber multiplication rate, and shoot growth among the sett sizes. These analyses were conducted using statistical software R version 3.4.1 (R Core Team, Citation2018).
Results and discussion
The effect of sett size on sprouting differed between the two years (). In 2017, the days to sprouting were similar for the three sett sizes. Faster sprouting was observed in the larger sett sizes in 2018; however, these differences were not statistically significant. The sprouting resulted from the activation of meristematic cells near the tuber surface (Onwueme, Citation1973). Fast sprouting suggests that cell activation is faster in larger setts, indicating the possible existence of signaling mechanisms that perceive available resources in meristematic cells. In comparison, the 2017 results show that factors other than sett size influence sprouting, such as dormancy or disease status (Craufurd, Summerfield, Asiedu & Vara Prasad, Citation2001).
Figure 2. Days to sprouting (a), tuber yield (b), and tuber multiplication rate (c) of the different sett sizes. Data are average ± standard errors for 15 plants. Bars with different letters represent significant differences at the p < 0.05 level.
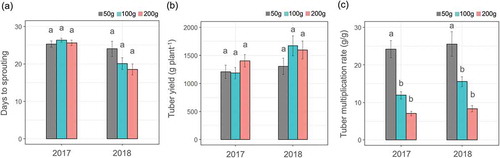
Tuber yield from the 200 g sett was, on average, 16% and 30% higher than the that by using 50 g sett in 2017 and 2018, respectively (). The yield response of the 100 g sett differed across years, being lower than the 50 g sett in 2017 and higher than the 200 g sett in 2018. However, differences in yield among the sett sizes were not statistically significant in either year, and the yields of the 200 g or 100 g setts were not enough to compensate for the larger sizes. This was clearly shown in the tuber multiplication rates, where values for the 50 g sett were more than three times larger than the 200 g sett (). Although a previous study showed that the sprouting rate among sett sizes differed by a maximum factor of two (Ayankanmi et al., Citation2005), it was still smaller than the difference in the multiplication rate found in the present study. Therefore, when considering the negative effects of smaller sett sizes on sprouting rates, it might be more cost effective to use a 50 g sett for planting.
In this study, average yields were higher than 1 kg per plant, even in plants from the 50 g sett. This value is higher than reported by a previous study using same sett size for D. rotundata (Ayankanmi et al., Citation2005). In addition, the yield was higher than from 200 g seed tubers reported by Okoli, Opara and Anyoha (Citation1999). Thus, planting 50 g setts could be used in the food yam production of D. rotundata. The higher yield might be attributed to the use of stakes for shoot growth, as previous studies did not use stakes. Because yams are a vine-type of plant, staking is widely used, even in famer’s fields where bamboo sticks and residual stems of harvested maize are used as stakes. Staking improves leaf arrangement and possibly reducing the occurrence of mutual leaf shedding, which might depress leaf photosynthetic capacity (Onwueme & Johnston, Citation2000), and enhances shoot biomass and tuber yield. Therefore, planting smaller setts with stakes is better than planting larger setts or seed tubers without stakes, and results in higher yields from smaller planting materials.
In 2017 and 2018, shoot dry weight was higher in the 200 g sett than in the 50 g sett. This difference was noticeable during the early growth period, from 70 to 85 days after planting (DAP), and was maintained until plant senescence (,). The differences in shoot biomass were accounted by shoot growth rates being the highest during the early growth period (from 40 to 85 DAP) (,)). The higher shoot growth rates in the larger setts were due to initial resource availability. Shoot growth depends on the nutrition of seed tubers until six weeks after sprouting, corresponding to approximately 90–100 DAP in this study (Orkwor & Ekanayake, Citation1998). The faster sprouting in larger setts might be another reason for the higher growth rates; however, this was not found in 2017 when the days to sprouting did not differ among the sett sizes. Thus, this effect may be limited. Shoot growth rate during the early growth period was well correlated with tuber yield in both years (). This correlation was also observed during latter growth periods (around 150 DAP) in 2017. However, the growth rate was much smaller than that found in the other periods (), indicating that this effect was not large.
Figure 3. Time-course changes in shoot dry weight (a, b), shoot growth rate (c, d), and correlation coefficient between shoot growth rate and tuber yield (e). Shoot dry weight was estimated by a non-destructive method (Iseki & Matsumoto, Citation2019). Data are average ± standard errors for 15 plants. *, **, and ns represent significant differences at p < 0.05, p < 0.01 level, and not significant, respectively. Bars with different letters represent significant differences at p < 0.05.
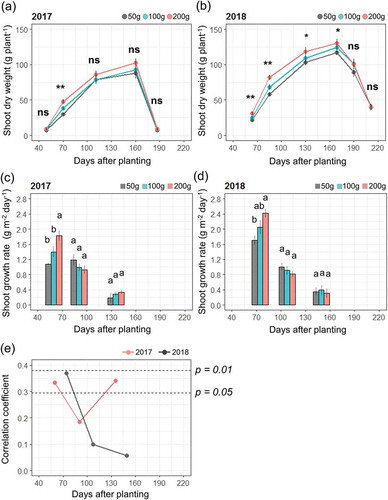
The tuber yield might have been higher in 2018 compared to 2017 due to the higher shoot dry weight in 2018 (,)), which is potentially related to the vigorous shoot growth during the early growth periods (,)) and/or the faster sprouting (). In addition, higher precipitation and hours of sunshine during the late growth period in 2018 () may have also contributed to the higher yields. At 100–130 DAP, increases in shoot dry weight slowed and tubers started to enlarge (Hgaza, Diby, Assa & Ake, Citation2010). The phase change from shoot growth to tuber growth is influenced by day length, with tuber enlargement being stimulated by daylight periods under 12 hours (Sobulo, Citation1972). Also, leaf photosynthetic activity peaks during this period, with assimilated carbohydrates being transferred to tubers (Aighewi & Ekanayake, Citation2004). Thus, higher precipitation and solar radiation values during the maximum growth stage from 130 to 160 DAP are important for increasing yields.
In this study, 50 g setts had slower sprouting and shoot growth rates during early growth periods, resulting in lower maximum shoot biomass. However, these had minor effects on tuber yield. The tuber multiplication rate was much higher in the 50 g sett compared to the 100 g and 200 g setts. Fertilization can be used to increase the shoot growth and tuber yield of the 50 g sett (Law-Ogbomo & Remison, Citation2008). In addition, the smaller shoot biomass in the 50 g sett can result in a higher planting density to increase the base yield of a field. To validate the potential use of smaller setts for food yam production, further studies are needed to elucidate how sett size affects shoot growth in relation to agronomical practices.
Acknowledgments
We thank Mr. Oyedele Sunday O., Oketokun Abass, and Ms. Obaude Oyebola O. for assisting with field evaluations, and Ms. Olajumoke Olaleye for field management.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agbarevo, M. N. B. (2014). An evaluation of farmers’ adoption of yam mini-sett technique in cross-river state, Nigeria. European Journal of Research in Social Sciences, 2(4), 1–9.
- Aighewi, B. A., & Ekanayake, I. J. (2004). In-situ chlorophyll fluorescence and related growth of white Guinea yam at different ages. Tropical Science, 44, 201–206.
- Ayankanmi, T., Shiwachi, H., & Asiedu, R. (2005). Sprouting and yield of yam (Dioscorea spp.) minisetts in relation to sett size, soil moisture and agro-ecology. Tropical Science, 45, 23–27.
- Craufurd, P. Q., Summerfield, R. J., Asiedu, R., & Vara Prasad, P. V. (2001). Dormancy in yams. Experimental Agriculture, 37, 147–181.
- Emokaro, C. O., & Law-Ogbomo, K. E. (2008). The influence of minisett size on the profitability of yam production in Edo state, Nigeria. Research Journal of Agriculture and Biological Sciences, 4(6), 672–675.
- Hgaza, V. K., Diby, L. N., Assa, A., & Ake, S. (2010). How fertilization affects yam (Dioscorea alata L.) growth and tuber yield across the years. African Journal of Plant Science, 4, 53–60.
- Igwilo, N., & Okoli, O. O. (1988). Evaluation of yam cultivars for seed yam production, using the minisett technique. Field Crops Research, 19, 81–89.
- Iseki, K., & Matsumoto, R. (2019). Non-destructive shoot biomass evaluation using a handheld NDVI sensor for field-grown staking yam (Dioscorea rotundata Poir.). Plant Production Science, 22(2), 301–310.
- Law-Ogbomo, K. E., & Remison, S. U. (2008). Growth and yield of white guinea yam (Dioscorea rotundata Poir.) influenced by NPK fertilization on a forest site in Nigeria. Journal of Tropical Agriculture, 46, 21–24.
- Nwekw, F. I. (Ed.). (1997). Yam in west Africa. Michigan, MI: Michigan State University Press.
- Okoli, O. O., Opara, M. U., & Anyoha, C. O. (1999). Effect of seed weight on yield determinants, yield components and intra-plot variability in yield of yams (Dioscorea spp.). African Journal of Root and Tuber Crops, 3(2), 44–48.
- Onemolease, E. A., & Adisa, T. (2005). Factors associated with use of yam mini sett technology in Egbeda local government area of Oyo state, Nigeria. International Journal of Agriculture and Rural Development, 6, 12–18.
- Onwueme, I. C. (1973). The sprouting process in yam (Dioscorea spp.) tuber pieces. Journal of Agricultural Science, 81, 375–379.
- Onwueme, I. C., & Johnston, M. (2000). Influence of shade on stomatal density, leaf size and other leaf characteristics in the major tropical root crops, tannia, sweet potato, yam, cassava and taro. Experimental Agriculture, 36, 509–516.
- Orkwor, G. C., & Asadu, C. L. A. (1998). Agronomy. In G. C. Orkwor, R. Asiedu, & I. J. Ekanayake (Eds.), Food yams: Advances in research (pp. 105–141). Ibadan: International Institute of Tropical Agriculture.
- Orkwor, G. C., & Ekanayake, I. J. (1998). Growth and development. In G. C. Orkwor, R. Asiedu, & I. J. Ekanayake (Eds.), Food yams: Advances in research (pp. 39–62). Ibadan: International Institute of Tropical Agriculture.
- R Core Team. (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. URL https://www.R-project.org
- Sobulo, R. A. (1972). Studies on white yam (Dioscorea rotundata) I. Growth analysis. Experimental Agriculture, 8, 99–106.