ABSTRACT
Soybean is a short-day plant and is highly sensitive to photoperiod. How photoperiod regulates soybean flowering is well known, whereas how it regulates pod setting is poorly known. In this study, short-day treatment decreased the number of days from flowering to pod setting. The duration of short-day treatment and the number of days from flowering to pod setting were negatively correlated. Additionally, short-day treatment of flowers after flowering did not promote pod setting, whereas that of leaves significantly shortened the period from flowering to pod setting. Vascular tissue of the two stems of Y-shaped plants was not connected at the stem junction, and short-day treatment of leaves on one of the two stems did not promote pod setting on the other stem. It is likely that a signal produced in leaves under short-day condition moves to the nodes and promotes pod setting after flowering.
ABSTRACT
KEYWORDS:
Introduction
Soybean (Glycine max (L.) Merr.) is one of the most economically important plant oils and protein crops (Graham & Vance, Citation2003). Since the 1970s, the area of soybean production has had the highest percentage increase among those of major crops; production increased from 17 million tonnes in 1960 to 230 million tonnes in 2008 (Hartman, West & Herman, Citation2011). Soybean production is expected to increase more than that of other crops, owing to the expanded production area and higher yields.
Soybean yield is determined by the pod number, seed number, and individual seed weight; it correlates more strongly with the number of pods than with the other two components (Schou, Jeffers & Streeter, Citation1978). Therefore, analysis of the pod setting mechanism would contribute to increasing pod number and possibly yield.
Recently, we reported that pod setting rate in soybean is regulated by vegetative growth after flowering through gibberellic acid biosynthesis (Taniguchi et al., Citation2018). Soybean is a short-day plant and is highly sensitive to photoperiod; its flowering is promoted by short-day conditions, and the period from flowering to pod setting is also shorter under short-day conditions than under natural conditions (Zheng, Maeda & Fukuyama, Citation2003). In other plants, pod setting of bambara groundnut (Vigna subterranea (L.) Verdec.) was promoted by short-day conditions (Nishitani, Muraki & Inoue, Citation1988), onions (Allium cepa L.) develop bulbs in response to long-day photoperiods (Garner & Allard, Citation1920), and potato (Solanum tuberosum ssp. andigena) requires a short day for tuber formation, and grafting experiments have confirmed that the photoperiod for tuberization is perceived in the leaves (Martínez-García, García-Martínez, Bou & Prat, Citation2001). Therefore, understanding of the relationship between photoperiods and fructification is important in several crop yields. In soybean, however, little is known about the relationship between photoperiod and pod setting. In this study, we investigated the effect of short-day treatment duration on pod setting time, the organs that sense photoperiod associated with pod setting, and the mobility of the signal related to pod setting in soybean.
Materials and methods
Plant material and growth conditions
Soybean (Glycine max (L.) Merr.) cv. Fukuyutaka was grown at Kyushu University (33°67′N, 130°42′E). Paddy soil mixed with 5 g compound fertilizer (N:P:K = 3:10:10) and 5 g magnesium lime was packed in a 1/5000-a Wagner pot or 4-L plastic pot before sowing.
Flowering and pod setting dates were recorded in all experiments. Flowering day and pod setting day were defined as the day when the first flower opened and the first pod reached 1 cm regardless of the place in the stem, respectively. The Tukey–Kramer method was used to compare the number of days from flowering to pod setting among treatments in each experiment.
Experiment 1: Seeds were sown on 26 May 2017 and the plants started flowering on 17 July in this experiment. When the first flower opened, plants were moved into an apparatus with a roof that automatically opened at 08:00 and closed at 18:00 every day for short-day treatment, exposing plants to natural light and temperature (10-h light/14-h dark), and were treated for 2, 4, 6, 8, and 11 days, respectively. Data presented are the averages of at least 12 replicates.
Experiment 2: Seeds were sown on 26 May 2015 and the plants started flowering on 20 July in this experiment. For short-day treatment (10-h light/14-h dark) of leaves or the floral organ from flowering to pod setting, all branches were removed to make it easy to elucidate the effect of short-day treatment on pod setting. Leaves and the main stem were covered with aluminum foil and black cloth, respectively, for short-day treatment. Data presented are the averages of at least 10 replicates.
Experiment 3: Seeds were sown on 1 July 2016 and the plants started flowering on 9 August in this experiment. Y-shaped plants were produced by epicotyl cutting when the primary leaves opened; such plants grew two main stems. After flowering, leaves of one main stem were covered with aluminum foil for short-day treatment (10-h light/14-h dark) from flowering to pod setting. Data presented are the averages of at least four replicates.
Morphological features of y-shaped plant
The stem junction in Y-shaped soybean sampled at the seed filling stage was fixed in 80% ethanol: 100% acetic acid: formalin = 90:5:5 (v/v/v). Tissue was cut into 20-µm-thick sections on a cryostat (Leica CM1950, Leica, Wetzlar, Germany) and stained with toluidine blue. The morphological features were observed under a microscope (BZ-X710, Keyence, Osaka, Japan).
Results and discussion
Effect of short-day treatment duration on pod setting in soybean (exp. 1)
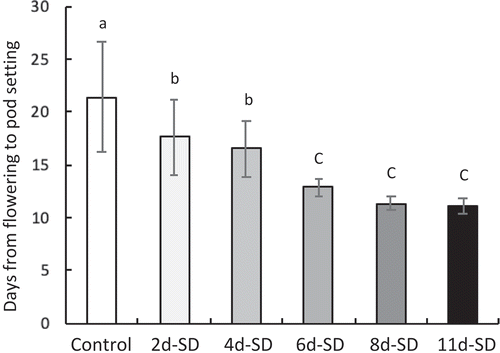
To confirm the relationship between day length and pod setting, we examined the effect of short-day treatment after flowering on the pod setting of ‘Fukuyutaka’. All photoperiod treatments (2–11 short days) significantly promoted pod setting (), in agreement with a previous report (Zheng et al., Citation2003); the effect gradually increased with the duration of short-day treatment. In 2- and 4-day short-day treatments, the period from flowering to pod setting was 4 or 5 days shorter than in control. The effects of short-day treatments that lasted 6 days or more were similar to each other; in these treatments, the period from flowering to pod setting was about 10 days shorter than in control. The negative correlation between duration of short-day treatment and the number of days from flowering to pod setting was observed (r = 0.949) (). These results suggest that the amount of a substance accumulated under short-day conditions may regulate pod setting time in soybean.
Identification of the organ that senses the photoperiod to regulate pod setting (exp. 2)
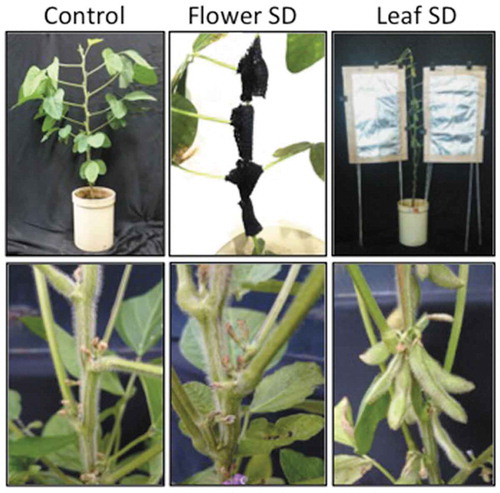
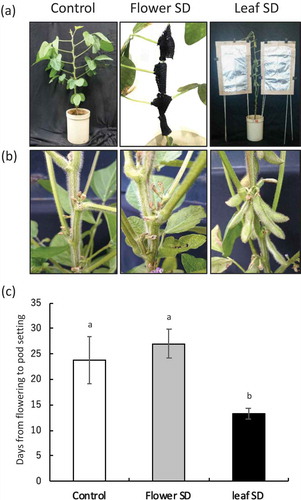
Because the day-length signal related to flowering is perceived in the leaf (Zeevaart, Citation1976), we investigated that whether leaf or floral organ sense the day length associated with pod setting in soybean ()). Short-day treatment of floral organ had no effect on pod setting, whereas treatment of leaves shortened the period from flowering to pod setting in comparison with that in the long-day control (,)). These results suggest that the day-length signal for pod setting in soybean is perceived in the leaves and a substance produced in leaves in response to short-day conditions may move from leaf to floral organ.
Figure 2. Identification of the organ that senses the photoperiod to regulate pod setting in soybean. Appearance of short-day treatments (a) and pod setting after treatments (b). Number of days from flowering to pod setting in each treatment (c). Different lowercase letters indicate significant differences among treatments at P < 0.05 by Tukey–Kramer test.
Mobility of the signal related to pod setting (exp. 3)
Then, we investigated the long-distance transport of signal for pod setting in soybean, because plant adaptive potential is critically dependent on efficient communication and co-ordination of signaling between above- and below-ground parts (Shabala, White, Djordjevic, Ruan & Mathesius, Citation2016). To clarify the long-distance transport of pod setting signal, we created Y-shaped plants and performed short-day treatment of the leaves of one of the two main stems ()). Although the period from flowering to pod setting in the treated stem became shorter than that in control plants, surprisingly the short-day treatment had no effect on the untreated stem ()). Days from flowering to pod setting in Y-shaped plants were a little longer than that in plants treated with short-day in Exp. 1 and 2. Since Exp. 3 started in July, photoperiod conditions became short-day compared with the other two experiments. Therefore, the effect of short-day treatment on pod setting looked like smaller in Exp. 3.
Figure 3. Confirmation of the mobility of the signal related to pod setting in soybean. Appearance of Y-shaped soybean plants (a). The arrow points to the pod. Number of days from flowering to pod setting in each treatment (b). Longitudinal (c) and cross (d) sections of the main stem at the junction in Y-shaped soybean. The black and white bars represent 1.0 mm. The arrows point to the phloem. Different lowercase letters indicate significant differences among treatments at P < 0.05 by Tukey–Kramer test.
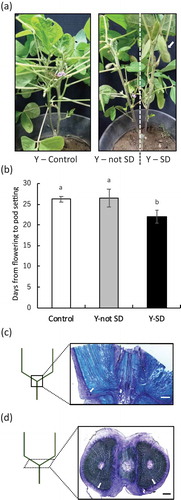
The vascular tissue of the stems in Y-shaped soybean did not cross, that is to say, connect at the junction (,)). These results suggest that the signal related to pod setting does not go through the underground part. It had been reported that lack of assimilate supply after flowering could inhibit pod development (Brun & Betts, Citation1984) and environmental stress from R1 to R3 significantly decreases the pod setting (Liu, Andersen & Jensen, Citation2003). Although short-day treatment on leaves might influence the photosynthesis, pod setting was promoted by short-day conditions. Therefore, photoperiod sensing of leaf regulates pod setting directly in soybean.
Further study is needed to identify this signal and to clarify the molecular mechanism of the regulation of pod setting by photoperiod. Recently, previous reports suggest that GmFT2a and GmFT5a, FLOWERING LOCUS T homologues, which promote flowering in soybean (Nan et al., Citation2014) also regulate pod setting through pollen fertility (Takeshima et al., Citation2017) and E1 gene, a repressor for GmFT2a and GmFT5a inhibit pod setting in long-day conditions (Harigai, Takeshima, Yamada, Kong & Abe, Citation2017). Therefore, in soybean, these genes may be involved with not only flowering but also pod setting.
Conclusion
Short-day treatment of leaves promoted pod setting, and the period from flowering to pod setting became shorter with longer short-day treatment ( and ). In Y-shaped plants, pod setting on one side was not induced by short-day treatment of leaves on the other side, and vascular tissue of the two stems was not connected at the junction (). Our results suggest that the response to short-day condition in leaves after flowering regulate pod setting without moving the signals between nodes passing through the underground part.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Brun, W. A., & Betts, K. J. (1984). Source/sink relations of abscising and nonabscising soybean flowers. Plant Physiology, 75, 187–191.
- Garner, W. W., & Allard, H. A. (1920). Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. Monthly Weather Review, 48, 415.
- Graham, P. H., & Vance, C. P. (2003). Legumes : Importance and constraints to greater use. Plant Physiology, 131, 872–877.
- Harigai, K., Takeshima, R., Yamada, T., Kong, F., & Abe, J. (2017). [Factors inhibiting pod-setting under long day condition in soybean.]. Breeding Research, 19, 48. In Japanese with English title.
- Hartman, G. L., West, E. D., & Herman, T. K. (2011). Crops that feed the World 2. Soybean-worldwide production, use, and constraints caused by pathogens and pests. Food Security, 3, 5–17.
- Liu, F., Andersen, M. N., & Jensen, C. R. (2003). Loss of pod set caused by drought stress is associated with water status and ABA content of reproductive structures in soybean. Functional Plant Biology, 30, 271–280.
- Martínez-García, J. F., García-Martínez, J. L., Bou, J., & Prat, S. (2001). The interaction of gibberellins and photoperiod in the control of potato tuberization. Journal of Plant Growth Regulation, 20, 377–386.
- Nan, H., Cao, D., Zhang, D., Li, Y., Lu, S., Tang, L., … Kong, F. (2014). GmFT2a and GmFT5a redundantly and differentially regulate flowering through interaction with and upregulation of the bZIP transcription factor GmFDL19 in soybean. PLoS One, 9, e97669.
- Nishitani, T., Muraki, K., & Inoue, J. (1988). Effect of daylength on the flowering and fruiting in bambara groundnut (Vigna subterranean (L.) Verdec.). Japanese Journal of Tropical Agriculture, 32, 80–84. In Japanese with English title.
- Schou, J. B., Jeffers, D. L., & Streeter, J. G. (1978). Effects of reflectors, black boards, or shades applied at different stages of plant development on yield of soybeans. Crop Science, 18, 29–34.
- Shabala, S., White, R. G., Djordjevic, M. A., Ruan, Y. L., & Mathesius, U. (2016). Root-to-shoot signalling: Integration of diverse molecules, pathways and functions. Functional Plant Biology, 43, 87–104.
- Takeshima, R., Harigai, K., Zhu, J., Kong, F., Yamada, T., & Abe, J. (2017). Role of soybean florigen genes – FT2a and FT5a – In the control of pod setting. Breeding Research, 19, 100. In Japanese with English title.
- Taniguchi, T., Murayama, N., Hasegawa, M., Nakagawa, A. C. S., Tanaka, S., Zheng, S. H., … Ishibashi, Y. (2018). Vegetative growth after flowering through gibberellin biosynthesis regulates pod setting rate in soybean. Plant Signaling & Behavior, 13, e1473668.
- Zeevaart, J. A. D. (1976). Physiology of flower formation. Annual Review of Plant Physiology, 27, 321–348.
- Zheng, S. H., Maeda, A., & Fukuyama, M. (2003). Genotypic and environmental variation of lag period of pod growth in soybean. Plant Production Science, 6, 243–246.