ABSTRACT
We examined the effect of panicle position relative to canopy and of panicle angle on pollination and floret sterility in rice at anthesis in a paddy field. We set panicles of pot-grown rice at the flowering stage either upright (0°) or inclined (30°), either above or at canopy height (Experiment 1) and either beneath or at canopy height (Experiment 2), in three replications each. In Experiment 1 and Experiment 2, panicle inclination at 30° increased the percentage of florets with <20 total pollen grains on the stigma (TP20) by 21% and 27%, increased the percentage of florets with <10 germinated pollen grains on the stigma (GP10) by 21% and 30%, and increased floret sterility by 10% and 26%, respectively. Floret sterility was correlated with GP10 in all trials. GP10 was correlated with TP20. The effect of position on floret sterility was not significant. We conclude that panicle inclination at 30° significantly increased floret sterility even under the canopy condition. Uprightness of panicles should be an important objective in rice breeding and production.
Abbreviations
TP20, percentage of florets with <20 total pollen grains on the stigma; GP10, percentage of florets with <10 germinated pollen grains on the stigma.
Graphical Abstract
Introduction
Rice is the primary staple food for more than half of the world’s population (FAO, Citation2014). Its pollination is indispensable for seed set. Anthesis of rice occurs in late morning in many cultivars under normal condition. Each flower opens once for around 1 h. At the start of the floret opening, anther dehisces right above the stigma and pollen grains drop from the anther dehiscence to the stigma (Hoshikawa, Citation1993). There is seemingly little risk of disruption of rice pollination by direct external factors.
However, we found that small panicle inclination reduced pollination stability in isolated pot-grown rice probably by changing the relative position between anther and stigma, and delaying pollen release (Win et al., Citation2020). Panicle inclination of 30° increased percentage of florets having less than 10 germinated pollen grains on the stigma after flowering by 24%. Since rice fertilization requires 10 or more germinated pollen grains (Satake & Yoshida, Citation1978), increase in such insufficiently pollinated flowers may induce floret sterility. Panicle inclination can be observed in agricultural scene. Inclined tillers, long weak culms and huge panicles can cause the inclination of panicle at flowering. However, the effect of inclination of panicle on the seed set has not yet been examined.
In rice production, the flowering occurs with canopy. The meteorological conditions such as wind speed and solar radiation change dramatically with height, particularly around the canopy surface (Monteith & Unsworth, Citation2008; Yoshimoto et al., Citation2005). Matsui et al. (Citationin press) showed that pollination stability and floret sterility of rice depend on the height of florets at flowering. Poorly pollinated florets and floret sterility was minimum around the surface of canopy and increased as increase in the distance from the surface to the floret height (Matsui et al., Citationin press). The canopy may affect the relationship between the inclination of panicle and pollination stability in practical rice production.
The purpose of this study is to clarify if the small inclination of panicle really induces the floret sterility in rice and if the canopy affects the relationship between inclination of panicle at flowering and pollination stability. We conducted model experiments using pot-grown rice plants set in the rice canopy at different heights and inclinations and examined pollination stability, pollen germination and seed set.
Materials and methods
General concept
We conducted two experiments with pot grown-rice plants at the heading stage set in canopy with four treatments in each experiments: two steps of panicle height × two inclinations (upright and 30°). In Experiment 1, we set the panicle at 25 cm above the canopy surface and at the height of the canopy surface to detect the effect of canopy on the relationship between panicle angle, pollination and floret sterility. In Experiment 2, we set the panicle around 40 cm below the canopy surface and at the height of the canopy to know the effect of depth of the panicle in the canopy on that relationship.
Preparation of background canopy
The experiments were conducted in a research field at Gifu University, Gifu Prefecture, Japan (35°27′ N, 136°44′ E, 13 m a.s.l.). We used ‘Khao Nok’ to create a background canopy. It is a tall and late cultivar, and rather tolerant to lodging. Moreover, its leaves are kept nearly upright at narrow angle until the heading stage. Therefore, we chose ‘Khao Nok’ as a background cultivar. The seeds were sown in nursery beds on 30 May 2017 and on 25 April 2018. Seedlings at around the five-leaf stage were transplanted at a spacing of 30 cm (east–west) × 15 cm (north–south) in the field. The field had already received a basal dressing of compound fertilizer at 80 kg/ha N, P2O5-equivalent and K2O-equivalent. The field soil was kept submerged until the ripening stage. The locations of the plots followed a randomized complete block design. The field had three bays as blocks, each 15 m (north–south) × 2.5 m (east–west). Each block was divided into four plots.
Pot preparation
We used rice variety ‘IR72ʹ as a tested variety. Its plant height is shorter than that of ‘Khao Nok’. With the combination of tall ‘Khao Nok’ and short ‘IR72ʹ, we can easily adjust the different panicle heights from beneath to above the background canopy surface. Seeds were sown in nursery beds on 14, 21 and 28 April 2017, and on 6, 16 and 26 April 2018 for three trials in each experiment. Seedlings at around the five-leaf stage were transplanted into 4-L pots (15 cm diameter, 20 cm height) in a circular pattern at 20 seedlings per pot. The pot contained 2.5 kg equivalent of air-dried sandy loam (pH 7.64). Each pot received a basal dressing of slow-release compound fertilizer at 0.5 g N, P2O5-equivalent and K2O-equivalent and was puddled in water the day before transplanting. The plants were grown outdoors with the soil surface submerged until treatment. Tillers were removed as they appeared during the vegetative stage to achieve uniform (Satake, Citation1972), straight panicles on the main culms.
Canopy treatment in Experiment 1, 2017
Pots were set on wooden stands to position the panicles at or above the canopy surface in one of two inclinations: at the canopy height (Canopy) or at 25 cm above the canopy (Above); either upright (Upright) or inclined 30° from the vertical (Inclined). Panicle heights were set and background canopy heights were measured at the start of each trial. Leaf area index (LAI) was measured with an LAI 2000 plant canopy analyser positioned at the bottom of the canopy on the middle day of each trial. The background canopy height ranged from 0.95 to 1.20 m across the trials. LAI ranged from 3.2 to 4.0 across the trials.
Panicle depth treatment in Experiment 2, 2018
Pots were set to position the panicles at or under the canopy surface in one of the two inclinations as above: at the canopy height or under the canopy (Under); either upright or inclined 30°. The pots were placed either on wooden stands or on the soil surface.
The panicle height of the pot-grown plants and the background canopy height were measured at the start of each trial (). From each pot that was set in the canopy, we randomly selected four panicles at anthesis and measured their heights (from the ground to the tips of the panicles). From the hills surrounding each pot, we selected one hill randomly and measured the height of the highest tips of the leaves. LAI was measured at the bottom of the canopy and at flowering height (). The measurements were conducted on the middle day of each trial.
Table 1. Background canopy height and panicle height of pot-grown rice in Experiment 2.
Table 2. Leaf area index (LAI) and cumulative LAI from the surface of the canopy to the height of the florets at the flowering stage in Experiment 2.
Common procedures and data collection in Experiments 1 and 2
Five pots were used in each plot. In the evening of the day before the start of data collection in each trial, pots with 40–50% of plants at heading were inserted into each plot on a north–south line at the centre of the bay, about 0.6 to 0.7 m apart, on wooden bases set to adjust the inclination and panicle height. Each pot was set in a bucket (32 cm deep, 22 cm wide) to keep the soil submerged in water. Every evening during the treatment period, inclined pots were rotated to avoid gravitropic recovery and to maintain the inclination. Three trials of 5 to 7 days were performed, totalling 16 days in Experiment 1 and 20 days in Experiment 2. After the treatment periods, pots were collected from the field and grown as before.
The site’s microclimate was measured at its centre with multiple sensors (WXT520, Vaisala Inc., Helsinki, Finland) set at 230 cm above the soil surface. Air temperature, relative humidity (RH), and wind speed were measured every 10 s, and 1-min averages were recorded on a data logger (CR10X, Campbell Scientific Inc., Logan, UT, USA). Plants flowered earlier in Experiment 2 (09:00 h to 12:00 h) than in Experiment 1 (10:00 h to 12:00 h), probably because of the higher temperature in Experiment 2 ()).
One of the five pots in each plot was used to collect floret samples for observation of anther characters and the other four were used for examination of pollination and fertility. To examine the fertilization of the florets, we randomly selected and tagged three panicles on which florets started anthesis on the day before the start of data collection in each trial, in each of the latter four pots. Their fertilities were examined at maturity by manual inspection of ovarian development.
To observe pollination, we randomly sampled 10 florets from the four pots in each plot every day during the treatment period, except on rainy days. We sampled the florets from the dispersed panicles 1 h after anthesis without regard to the location of the florets on the panicles. We did not get samples from the tagged panicles and off-type plants. Stigmata were detached from the florets and stained with cotton blue solution. The numbers of total and germinated pollen grains on each stigma were counted at 100× magnification under an optical microscope (Model BX51, Olympus Corporation, Tokyo, Japan). Fertilization in rice requires at least 10 germinated pollen grains or at least a total of 20 pollen grains on the stigma after anthesis (Matsui et al., Citation2001; Satake & Yoshida, Citation1978). We calculated ‘TP20’ (percentage of florets having <20 total [germinated and ungerminated] pollen grains on the stigma after anthesis) and ‘GP10’ (percentage of florets having <10 germinated pollen grains on the stigma after anthesis) as indices of pollination stability.
To observe the anther dehiscence characters, we sampled seven florets from the designated pot in each plot at anthesis and used three randomly selected florets. From each floret, we randomly selected four anthers to observe at 80× magnification under a digital microscope (KH-7700, Hirox Co., Ltd., Tokyo, Japan). We measured the lengths of dehiscence formed at basal and apical parts of anther (Win et al., Citation2020).
Statistical analysis
We used a split plot design (combined with the randomized block design) with two sub-factors without replication in data analysis, in which trial and (randomized) block were the main effects, and panicle position and inclination were the sub-plot effects for all parameters. We calculated the mean values of anther characters, numbers of total and germinated pollen grains, GP10, TP20 and percentage of floret sterility. Analysis of variance (ANOVA) was conducted to test the statistical differences among the treatment effects. Treatment means were compared using Tukey’s honestly significant difference (HSD) test at the 5% probability level. Analysis of covariance (ANCOVA) was performed in TIBCO Statistica v. 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA) to verify the effect of GP10 on floret sterility. Floret sterility, TP20 and GP10 were analysed after logit transformation. Since TP20 and GP10 included 0 values, we used empirical logit transformation.
Results
Experiment 1
Microclimate conditions during treatment
In Experiment 1, the daily maximum temperature varied from 24.9°C to 35.5°C during the treatment period ()) and from 27.1°C to 33.5°C on the sampling days. The RH at the time of maximum temperature on the sampling days varied from 45.1% to 76.3% ()). Wind speed around flowering time (10:00–12:00 h) on the sampling days varied from 0.54 to 1.56 m s−1 ()).
Effect of treatment on sterility and pollination
The effects of trial and inclination on floret sterility were significant but that of position was not (). Sterility was significantly higher in the inclined treatment (69.5%) than in the upright treatment (59.8%). It was significantly higher in Trial 1 than in Trial 3. The position × inclination interaction was significant. The difference in increment of sterility by the inclined treatment between panicle positions was small (8.8% above the canopy, 10.5% at the canopy surface). The other interactions were not significant.
Table 3. Summary of analysis of variance (ANOVA) and Tukey’s HSD test for mean values of floret sterility, numbers of total and germinated pollen grains, TP20 and GP10 as affected by panicle position and inclination in Experiment 1.
The effects of trial and position on pollination parameters (numbers of total and germinated pollen grains, TP20 and GP10) were not significant (). The effect of inclination on all pollination parameters was significant: numbers of total and germinated pollen grains were significantly higher and TP20 and GP10 were significantly lower in the upright treatment than in the inclined treatment. The effect of the position × inclination interaction on all pollination parameters was significant: the differences in TP20 and GP10 between inclined and upright treatments were larger above the canopy than at the canopy surface. The other interactions were not significant.
Effect of treatment on anther dehiscence
The effects of panicle position and inclination treatments on anther dehiscence were not significant (Supplementary Table 1).
Relationship between sterility and pollination
In the ANCOVA of percentage of sterility with GP10 as a covariate (P < 0.001, F (1, 8)) and trial as an independent variable (P < 0.0001, F (2, 8)), sterility increased with GP10 (R2 = 0.940, P < 0.0001; ). GP10 was, in turn, strongly correlated with TP20 (R2 = 0.786, P < 0.001; ).
Experiment 2
Microclimate conditions during treatment
In Experiment 2, the daily maximum temperature varied from 28.5°C to 38.3°C during the treatment period ()) and from 29.5°C to 38.3°C on the sampling days. The RH at the time of maximum temperature on the sampling days varied from 30.8% to 46.4% ()). Wind speed around flowering time (09:00–12:00 h) on the sampling days varied from 0.98 to 1.97 m s−1 ()).
Effect of treatment on sterility and pollination
The effects of trial and inclination on floret sterility were significant but that of position was not (). Sterility was the highest (significantly) in Trial 3. It was significantly higher in the inclined treatment (65.5%) than in the upright treatment (39.5%). The position × inclination and trial × inclination interactions were significant. The difference in sterility between inclined and upright treatments was larger at the canopy surface (29.9%) than under the surface (22.0%). The other two interactions were not significant.
Table 4. Summary of analysis of variance (ANOVA) and Tukey’s HSD test for mean values of floret sterility, numbers of total and germinated pollen grains, TP20 and GP10 as affected by panicle position and inclination in Experiment 2.
The effect of trial on the number of germinated pollen grains was significant (); the number was highest in Trial 1. The effects of position and inclination on all pollination parameters were significant. Numbers of total and germinated pollen grains were significantly higher and TP20 and GP10 were significantly lower in the at-canopy and upright treatments than in the under-canopy and inclined treatments. The effects of position × inclination and trial × inclination interactions on numbers of total and germinated pollen grains, TP20 and GP10 were significant. The differences in TP20 and GP10 between inclined and upright treatments were larger at the canopy surface than under the surface. The other two interactions were not significant.
Effect of treatment on anther dehiscence
The effects of panicle position and inclination treatments on anther dehiscence were not significant (Supplementary Table 2).
Relationship between sterility and pollination
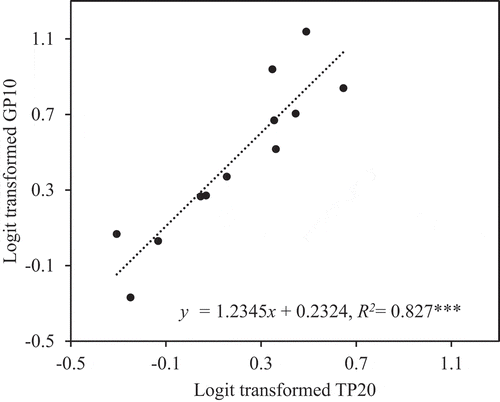
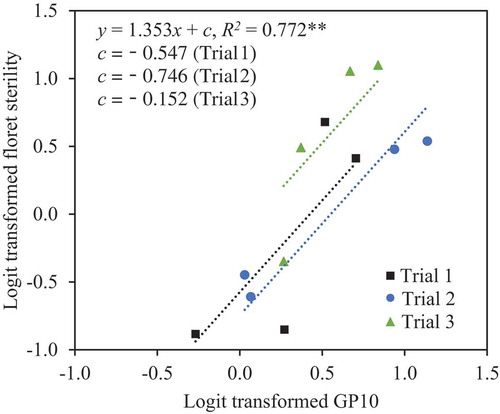
In the ANCOVA of percentage of sterility with GP10 as a covariate (P < 0.01, F (1, 8)) and trial as an independent variable (P = 0.162, F (2, 8)), sterility increased with GP10 (R2 = 0.772, P < 0.01; ). GP10 was, in turn, strongly correlated with TP20 (R2 = 0.827, P < 0.001; ).
Discussion
The temperature was hotter and the wind speed was stronger during Experiment 2 than during Experiment 1 (). The maximum temperature reached 38.3°C in Experiment 2 and 35.5°C in Experiment 1. The average value during the treatment period of average wind speed around flowering time was low, 0.99 and 1.18 m s−1 in Experiment 1 and 2, respectively. A building 20 m high on the northern side of our field might block southerly winds. The low wind speed might be one of the causes of the low stability of pollination and seed set (, ), especially in Experiment 2 under high temperature (Tian et al., Citation2010).
Under these conditions, our results confirmed that panicle inclination significantly increased floret sterility and poor pollination even under the canopy. Inclination increased floret sterility by 10% in Experiment 1 () and by 26% in Experiment 2 (). Panicle inclination, therefore, reduces paddy rice production. GP10 and TP20 were also significantly higher in inclined plants than in upright plants (, ), and floret sterility was well correlated with GP10 ( and ), which was in turn well correlated with TP20 ( and ). These results suggest that the differences in floret sterility between inclination treatments were caused mainly by the differences in pollination stability.
The effect of position on pollination stability parameters (i.e. TP20 and GP10) was significant in Experiment 2 () but not in Experiment 1 (). Matsui et al. (Citationin press) showed that the optimal position for pollination and floret fertility was around the canopy surface, and that pollination stability and seed set became worse as the distance of the floret from the optimal position increased. The distance from the canopy surface was somewhat greater in Experiment 2, so the significance might reflect the distance. In contrast, the effect of position on floret sterility was not significant in Experiment 2 (). Matsui et al. (Citationin press) showed that floret fertility was greater at deeper position within the canopy than shallower position at the same GP10, and suggested that the shallower position reduced floret fertility through processes after pollen germination. Our data agree with their hypothesis.
Anther dehiscence characters are important that determine the stability of rice pollination (Matsui & Kagata, Citation2003). In the present study, we did not detect the effect of treatment on anther dehiscence characters (Supplementary Tables 1 and 2). Therefore, anther dehiscence characters did not affect the stability of rice pollination.
Present study demonstrated that panicle inclination of 30° significantly increased floret sterility under the canopy condition. We sometimes observe inclination of panicle at the flowering stage in agricultural scenes. Huge panicles in high yielding varieties recently developed in China cause panicle inclination at flowering because of long rachides of the panicles and of long flowering period. Excessive application of fertilizer for tall races such as hybrid rice sometimes induces panicle incline. Tall cultivars show inclination of panicle under windy condition. The results show the importance of an erect panicle in rice breeding and growing. Since the primary cause of sterility induced by heat (Kobayashi et al., Citation2011; Matsui et al., Citation1997, Citation2000; Nishiyama & Satake, Citation1981; Weerakoon et al., Citation2008) and drought (Liu et al., Citation2006) at flowering and by cold at booting (Shimazaki et al., Citation1964) and flowering (Zeng et al., Citation2017) is poor pollination, erect panicles may improve the tolerance to floret sterility caused by these stresses through improving stable pollination. We could not collect the data under high wind conditions, which affect the stability of pollination (Matsui et al., Citationin press) and thus might affect the interaction between panicle inclination and panicle height in canopy on the pollination stability and the floret sterility. Further studies are needed for understanding the interactions between panicle inclination and panicle depth under strong wind conditions.
Figure 1. Weather conditions at the experimental paddy field during the treatment periods of Experiments 1 (2017) and 2 (2018): (a) daily maximum temperature, (b) relative humidity at the time of maximum temperature, and (c) average wind speed above the canopy (230 cm above the ground) around flowering time (10:00–12:00 h in Experiment 1, 09:00–12:00 h in Experiment 2). Bold, double and triple lines indicate sampling days in first, second, and third trial, respectively.
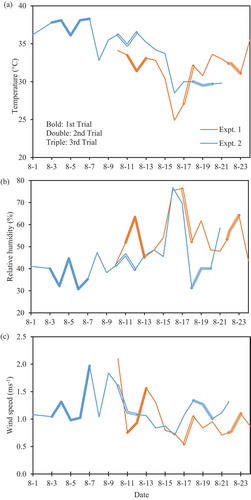
Figure 2. Relationship between logit-transformed percentage of florets with <10 germinated pollen grains (GP10) on the stigma and logit-transformed percentage of floret sterility in Experiment 1. In ANCOVA with GP10 as a covariate and trial as an independent variable, the effects of both GP10 and trial on sterility were significant at the 0.1% level (***).
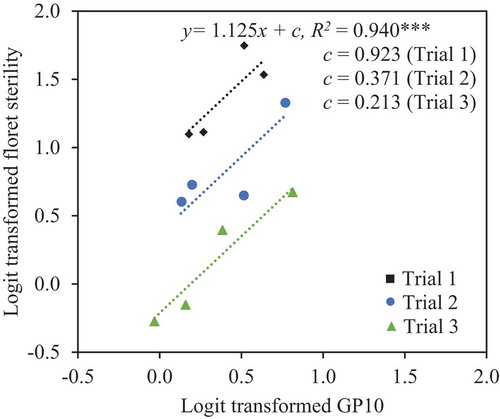
Figure 3. Relationship between logit-transformed percentage of florets with <20 total pollen grains on the stigma (TP20) and logit-transformed percentage of florets with <10 germinated pollen grains on the stigma (GP10) in Experiment 1. ***Significant at the 0.1% level.
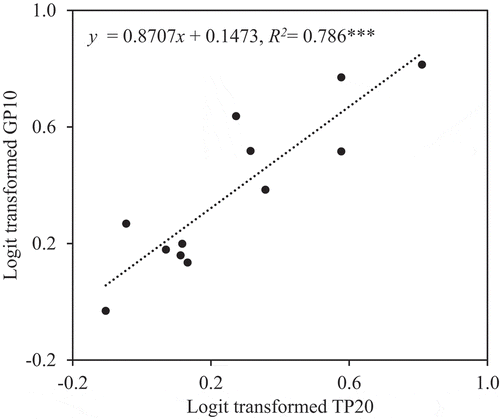
Figure 4. Relationship between logit-transformed percentage of florets with <10 germinated pollen grains on the stigma (GP10) and logit-transformed percentage of floret sterility in Experiment 2. In ANCOVA with GP10 as a covariate and trial as an independent variable, the effect of GP10 on sterility was significant at the 1% level (**), but the effect of trial was not.
PPS2019_168RP-File003.docx
Download MS Word (33.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- FAO. (2014). Statistical yearbook (pp. 634). Rome: Food and Agricultural Organization.
- Hoshikawa, K. (1993). Anthesis, fertilization and development of caryopsis. In T. Matsudo & K. Hoshikawa (Eds.), Science of the rice plant: Vol. 1. morphology (pp. 339–376). Tokyo: Food and Agriculture Policy Research Center.
- Kobayashi, K., Matsui, T., Murata, Y., & Yamamoto, M. (2011). Percentage of dehisced thecae and length of dehiscence control pollination stability of rice cultivars at high temperatures. Plant Production Science, 14, 89–95.
- Liu, J. X., Liao, D. Q., Oane, R., Estenor, L., Yang, X. E., Li, Z. C., & Bennett, J. (2006). Genetic variation in the sensitivity of anther dehiscence to drought stress in rice. Field Crops Research, 97, 87–100.
- Matsui, T., & Kagata, H. (2003). Characteristics of floral organs related to reliable self‐pollination in rice (Oryza sativa L.). Annals of Botany, 91, 473–477.
- Matsui, T., Kobayasi, K., Yoshimoto, M., Hasegawa, T., & Tian, X. (in press). Dependence of pollination and fertilization in rice (Oryza sativa L.) on floret height within the canopy. Field Crops Research, 249.
- Matsui, T., Omasa, K., & Horie, T. (1997). High temperature induced florets sterility of japonica rice at flowering in relation to air temperature, humidity, and wind velocity. Japanese Journal of Crop Science, 66, 449–455.
- Matsui, T., Omasa, K., & Horie, T. (2000). High temperature at flowering inhibits swelling of pollen grains, a driving force for thecae dehiscence in rice (Oryza sativa L.). Plant Production Science, 3, 430–434.
- Matsui, T., Omasa, K., & Horie, T. (2001). The difference in sterility due to high temperatures during the flowering period among japonica-rice varieties. Plant Production Science, 4, 90–93.
- Monteith, J. L., & Unsworth, M. H. (2008). Micrometeorology (i), turbulent transfer, profiles, and fluxes. In Principles of environmental physics (3rd., pp. 300–334). Burlington, MA: Academic Press.
- Nishiyama, I., & Satake, T. (1981). High temperature damage in the rice plant. Japanese Journal of Tropical Agriculture, 26, 19–25.
- Satake, T. (1972). Circular dense-culture of rice plants in pots: The purpose of obtaining main uniform panicles of main stems. Japanese Journal of Crop Science, 41, 361–362. In Japanese.
- Satake, T., & Yoshida, S. (1978). High temperature-induced sterility in indica rice at flowering. Japanese Journal of Crop Science, 47, 6–10.
- Shimazaki, Y., Satake, T., Ito, N., Doi, Y., & Watanabe, K. (1964). Sterile spikelets in rice plants induced by low temperature during the booting stage. Research Bulletin of the Hokkaido National Agricultural Experiment Station (Japan), 83, 1–9. In Japanese with English summary.
- Tian, X., Matsui, T., Li, S., Yoshimoto, M., Kobayasi, K., & Hasegawa, T. (2010). Heat-induced floret sterility of hybrid rice (Oryza sativa L.) cultivars under humid and low wind conditions in the field of Jianghan Basin, China. Plant Production Science, 13, 243–251.
- Weerakoon, W. M. W., Maruyama, A., & Ohba, K. (2008). Impact of humidity on temperature‐induced grain sterility in rice (Oryza sativa L). Journal of Agronomy and Crop Science, 194, 135–140.
- Win, A., Tanaka, T. S. T., & Matsui, T. (2020). Panicle inclination influences pollination stability of rice (Oryza sativa L.). Plant Production Science, 23, 60–68.
- Yoshimoto, M., Oue, H., & Kobayashi, K. (2005). Energy balance and water use efficiency of rice canopies under free-air CO2 enrichment. Agriculture and Forestry Meteorology, 133, 226–246.
- Zeng, Y., Zhang, Y., Xiang, J., Uphoff, N. T., Pan, X., & Zhu, D. (2017). Effects of low temperature stress on spikelet-related parameters during anthesis in indica–japonica hybrid rice. Frontiers in Plant Science, 8, 1350.