ABSTRACT
Rice bran application is practically used by Japanese organic rice farmers as a common measure in controlling Monochoria vaginalis, a known weed in organic rice production. To elucidate the inhibitory mechanisms of rice bran application on M. vaginalis germination, we focused on volatile fatty acids (VFAs) and aromatic carboxylic acids (ACAs) as organic acids produced through rice bran decomposition in the soil, which can possibly suppress M. vaginalis germination. Both solo solutions of 1 mM VFAs (propionic or isobutyric acid) and 0.2 mM ACAs (3-phenylpropionic or 4-phenylbutyric acid) exhibited M. vaginalis germination suppression, especially at pH 4.6, the lowest pH tested. In the 0.5–3 mM VFA solutions at pH 4.6, germination was strongly suppressed in the order of isobutyric > propionic > butyric > acetic acid. With the 0.05–0.2 mM ACA solutions at pH 4.6, the germination was strongly suppressed in the order of 3-phenylpropionic > 4-phenylbutyric > 2-phenylpropionic acid. Benzoic acid did not exhibit significant germination suppression. The solutions of 0.5 mM VFAs and 0.05 mM ACAs exhibited no suppression when applied separately; however, the certain mixture of a VFA and an ACA at the aforementioned concentration, for example, 0.5 mM isobutyric acid and 0.05 mM 3-phenylpropionic acid, exhibited significant germination suppression. These results showed the several combinations of VFAs and ACAs at even non-effective concentrations synergistically suppressed M. vaginalis germination.
Abbreviations:2PPA: 2-phenylpropionic acid; 3PPA: 3-phenylpropionic acid; 4PBA: 4-phenylbutyric acid; ACA: aromatic carboxylic acid; EC: electrical conductivity; M. vaginalis: Monochoria vaginalis; VFA: volatile fatty acid
Graphical abstract
Introduction
Weed control is one of the most serious problems in organic rice (Oryza sativa L.) production in Japan. Monochoria vaginalis (Burm. F.) Kunth is one of the most problematic lowland weeds to control for organic farmers, because it easily escapes mechanical weeding and adapts to deep-flooded conditions, which is effective to control other noxious weeds, such as Barnyardgrass (Echinochloa crus-galli). For M. vaginalis control, rice bran is sometimes applied on paddy fields after or during rice transplanting, as it can suppress M. vaginalis germination to a certain level (Miura et al., Citation2015; Nakai & Toritsuka, Citation2009). However, the effectiveness often fluctuated when farmers practically use this technique in organic paddy fields. Additionally, it is difficult to effectively improve this technique because of the lack of knowledge regarding the mechanisms that suppress M. vaginalis germination.
The suppression of M. vaginalis by rice bran application is associated with chemical factors indicated by electrical conductivity (EC) increase (Nozoe et al., Citation2021, Citation2012), as well as physical ones, namely, the settled soil volume in water (Nozoe et al., Citation2016). We have shown that rice bran application increased the EC of soil solution, possibly because of the increased ionic compounds from rice bran decomposition, and M. vaginalis germination suppression is increased along with the EC value (Nozoe et al., Citation2021, Citation2012). Organic acids are generally released during rice bran or organic matter decomposition in the paddy soil. These organic acids are composed of several types and varying amounts of organic chemicals, and the accumulation of those substances serves as a factor that suppresses M. vaginalis germination. In the soil solution taken from the paddy field with rice bran, volatile fatty acids (VFAs), such as acetic and isobutyric acids, were detected, and the latter especially remained for approximately 2 weeks after rice bran application (Ueno & Suzuki, Citation2005). Moreover, several aromatic carboxylic acids (ACAs), such as phenylacetic acid, 2-phenylpropionic acid (2PPA), 3-phenylpropionic acid (3PPA), and 4-phenylbutyric acid (4PBA), were detected in the paddy soil solution after wheat straw application (Tanaka, Citation2002; Tanaka et al., Citation1990). ACAs exhibited strong inhibitory effects on rice root elongation. These VFAs and ACAs can be candidates possessing physiologic properties that suppress M. vaginalis germination. The integrated power of these organic acids is presumably responsible for germination suppression. Therefore, it is necessary to investigate whether there is interaction among these substances.
The condition of pH is an important component that affects organic acid toxicity. Dissociation of these organic acids is strongly affected by the solution’s pH, because these substances are generally weak acids (Yoshida, Citation1981). Several weak acids, such as acetic acid, are present in dissociated forms, such as CH3COO−, at high pH conditions, whereas the undissociated form of acids, such as CH3COOH, prevails at low-pH conditions. The toxicity increases with the decrease in pH, because the toxic effect of undissociated forms of acids on rice root elongation is greater than that of their dissociated forms (Tanaka & Navasero, Citation1967). Therefore, it is necessary to determine the effect of pH on the evaluation of organic acids toxicities.
In this study, laboratory experiments were conducted to evaluate three factors. First, the relationship between pH and M. vaginalis germination was investigated with or without organic acids. Second, the suppressive effects of several VFAs and ACAs on germination were assayed. Finally, synergistic activities between VFAs and ACAs in terms of germination suppression were assessed.
Materials and Methods
Seeds and chemicals
M. vaginalis seeds were sampled from the plants grown at the experimental field of the National Agricultural and Food Research Organization (NARO), Central Region Agricultural Research Center (Tsukubamirai-city, Ibaraki, Japan; 36°00′27″N, 140°01′19″E) on October 2016. The seeds were air-dried and kept in a refrigerator at 4°C for more than 18 months.
Four VFAs (acetic, propionic, butyric, and isobutyric acids) and four ACAs (benzoic acid, 2PPA, 3PPA, and 4PBA) were evaluated for their suppressive activity on M. vaginalis germination. These chemicals were obtained from Fujifilm and Wako Chemicals (Tokyo, Japan). Stock solutions were prepared at concentrations of 1 M and 10 mM for VFAs and ACAs, respectively, with sterilized distilled water. We used a citrate–phosphate (McIlvaine’s) pH buffer prepared on the basis of Stoll and Blanchard (Citation1990), with slight modifications by Nozoe et al. (Citation2018) for assay solution to perform the assay under constant condition, because the pH of a solution varies depending on solute composition. Buffers of pH 4.6, 5.2, 5.8, 6.4, and 7.0 were prepared in combination with 20 mM of disodium hydrogen phosphate and 10 mM of citric acid. Each acid solution at the given concentrations was prepared using the stock solution diluted with these buffers.
Assay of suppressive activity on M. vaginalis germination
Ten seeds of M. vaginalis were put into a 1.5-mL microtube, sterilized with 70% ethanol and 0.5% sodium hypochlorite, and then rinsed thrice with sterilized distilled water. Then 1 mL of the test solution containing the organic acid was added into the microtube, followed by incubation at 30°C under a 12-h light/dark cycle in the incubator (LPH-350S, Nippon Medical & Chemical Instruments Co., LTD., Osaka, Japan). After incubation for 3 days, germination rate was determined by counting the number of seeds that cotyledon emerged outside seeds. Buffers without organic acids were used as a control.
Experiment 1: Effects of pH in suppression assay
We used two VFAs (propionic and isobutyric acids) and two ACAs (3PPA and 4PBA) for evaluating the effect of pH on the assay. The concentration of VFAs and ACAs were 1.0 and 0.2 mM, respectively. Each buffer without acid was used as control. Each treatment was done in triplicate. The suppressive activity of each solution was assayed according to the aforementioned method.
Experiment 2: Suppression assay in single-component solutions of organic acids
Four VFAs (acetic, propionic, butyric, and isobutyric acids) and four ACAs (benzoic acid, 2PPA, 3PPA, and 4PBA) were diluted with pH 4.6 buffer solution at the given concentration. VFA concentrations were 0.5, 0.75, 1.0, 2.0, and 3.0 mM, whereas ACA concentrations were 0.05, 0.1, 0.15, and 0.2 mM. Each treatment was done in triplicate. The suppressive activity of each solution was assayed according to the aforementioned method.
Experiment 3: Suppression assay in mixed solutions of VFAs and ACAs
Four VFAs (acetic, propionic, butyric, and isobutyric acids) were prepared in combination with four ACAs (benzoic acid, 2PPA, 3PPA, and 4PBA) at final concentrations of 0.5 and 0.05 mM, respectively; that is, 2 mL of solo solution of VFAs (1.0 mM) and 0.2 mL of solo solution of ACAs (1.0 mM) were mixed with 1.8 mL of buffer (pH 4.6). Additional to the control solutions (buffer without organic acids), 0.55 mM VFA and 0.05 mM ACA solutions were also assayed. Each treatment was done in triplicate. The suppressive activity of each solution was assayed according to the aforementioned method.
Statistical analysis
Statistical analysis was conducted with JMP® 12 (SAS Institute Inc., Cary, NC, USA). Germination rate was converted to the arc sine and used for Tukey’s honestly significant difference test and Dunnet’s test.
Results and Discussion
Experiment 1: Effects of pH in suppression assay
In the buffers ranging from pH 4.6 to 6.4 without the addition of any organic acid, the germination rate of M. vaginalis was almost constant between 63% and 72% (). In the buffer with pH 7.0, the germination rate was decreased to 42%. These findings indicated that a weak acid condition was more conducive for M. vaginalis germination than a neutral condition. This supports the finding of Nozoe et al. (Citation2018), who reported that germination increased in low-pH soil solutions, as long as the pH ranged from 4.0 to 7.0.
Figure 1. Monochoria vaginalis germination under several pH conditions with or without volatile fatty acids and aromatic carboxylic acids. Different lower-case letters indicate significant differences at 5% level by Tukey’s honestly significant difference test. Same lower-case letters indicate insignificant differences. Vertical bars indicate standard deviation (n = 3); 3PPA: 3-phenylpropionic acid; 4PBA: 4-phenylbutyric acid
In the presence of 1.0 mM VFAs or 0.2 mM ACAs, germination was strongly inhibited at lower pH values. All the tested acids kept the germination rate under 30% in solutions with pH 4.6 (–e), and about half of the control’s germination, especially with 0.2 mM 3PPA and 4PBA, was suppressed to almost no germination (, e). Up to pH 6.4, germination rate increased to approximately 80% along with increased pH, indicating that the suppressing effect of organic acids nearly disappeared. This may have been due to the low germination potential at pH 7.0, as shown in the buffer without organic acids, although germination rate declined again at pH 7.0.
Lynch (Citation1980) showed that 5.0 mM acetic acid inhibited the root extension of barley seedlings, and inhibition was greater at pH 5.5 than at pH 6.5, because of the increased permeability of plant tissue to the undissociated form of the acid. The findings obtained in this report indicated that low-pH conditions were conducive for germination inhibition by organic acids (–e), even though it enhanced germination without the presence of organic acids (). However, a low pH value is not always beneficial for rice cultivation. First, low pH stimulates M. vaginalis germination based on the findings of this study () and the report of Nozoe et al. (Citation2018). Subsequently, the optimum pH for rice growth is reported to be approximately between 5 and 6 (Alam, Citation1981; Fageria & Baligar, Citation1999), indicating that a pH value that is lower than 5 can retard rice growth. Finally, low soil pH can enhance ferrous (Nozoe et al., Citation2009) and aluminum (Tanaka & Navasero, Citation1966) toxicities to rice. The suitable soil pH in rice field that complements the efficiency of rice bran application should be determined according to these other factors.
Experiment 2: Suppression assay in single-component solutions of organic acids
In Experiment 1, organic acids in lower pH conditions suppressed M. vaginalis germination more extensively. In the current experiment, therefore, the suppressive activity of organic acids was evaluated using a pH 4.6 buffer solution to evaluate each activity clearly. In the 0.5 mM solo solutions of the four VFAs (acetic, propionic, butyric, and isobutyric acids), the germination rate was the same as the control (buffer without organic acids) (). However, propionic and isobutyric acids strongly suppressed the germination rate to lower than 30% and 10%, respectively, in the 1.0 mM solutions (, d), whereas acetic and butyric acids did not exhibit significant suppression (, c). In the 2.0 mM solutions, propionic, butyric, and isobutyric acids almost completely suppressed germination, but acetic acid did not significantly suppress germination. In the 3.0 mM solutions, all of the four VFAs suppressed germination to almost 0%. The suppressive activity of VFAs was observed in the order of isobutyric > propionic > butyric > acetic acid.
Figure 2. Monochoria vaginalis germination with various concentrations of volatile fatty acids at pH 4.6. *, **, and *** indicate significant differences to control (buffer only) at 5%, 1%, and 0.1% levels, respectively, by Dunnet’s test. Vertical bars indicate standard deviation (n = 3)
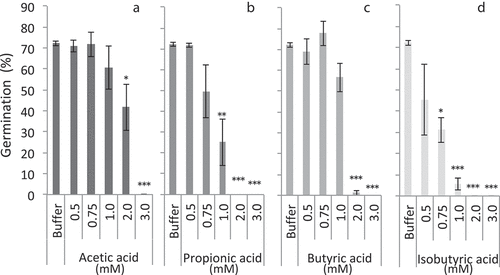
Practically, Ueno and Suzuki (Citation2005) reported that approximately 3 mM of acetic acid was detected in the paddy water on the third day after rice bran application. After 5 days, however, acetic acid was not detected anymore. Moreover, isobutyric acid concentration in the paddy water increased to approximately 4.5 mM at 1 week after rice bran application and kept 3 mM over approximately 2 weeks. That is, acetic acid is produced shortly after rice bran application and readily disappears through volatilization or decomposition and metabolization by microorganisms, whereas isobutyric acid increases gradually and stays for a longer period. M. vaginalis sequentially emerges in paddy fields after transplanting and continues to germinate for more than 1 week in Japanese rice fields (Koarai & Shibayama, Citation2001). Therefore, at least two factors, namely, the shortly existent but high-dose substances, such as acetic acid, and the gradually increasing but highly suppressive substances, such as isobutyric acid, may suppress M. vaginalis germination in combination. Notably, the suppression ability of each VFA was evaluated at pH 4.6, and their abilities reduced in higher pH than in pH 4.6, indicating that the tested concentration (3 mM) of isobutyric acid may not be enough to suppress M. vaginalis germination at the optimum pH level of between 5 and 6 for rice growth in actual paddy fields. Therefore, more unknown factors must be elucidated to explain the suppression effects of rice bran application.
In the case of ACAs, in the 0.05 mM solutions of the four ACAs (benzoic acid, 2PPA, 3PPA, and 4PBA), germination was not significantly suppressed. In the 0.1 mM solutions, 3PPA and 4PBA kept germination rate under 10% and 20%, respectively (, d), whereas benzoic acid and 2PPA did not significantly suppress germination rate, compared with that of the control solution (buffer without organic acids) (, b). In the 0.15 mM 2PPA solution, germination rate was under 30%, and those in 3PPA and 4PBA solutions were almost completely zeroed out, indicating that these ACAs significantly suppressed germination. In the 0.2 mM solutions, 2PPA almost completely suppressed the germination as well as 3PPA and 4PBA. In contrast to these three ACAs, benzoic acid did not suppress germination significantly within the 0.05–0.2 mM solution (). On the basis of these results, three ACAs suppressed the germination in the order of 3PPA > 4PBA > 2PPA, and benzoic acid showed weak suppressive activity against germination.
Figure 3. Monochoria vaginalis germination with various concentrations of aromatic carboxylic acids at pH 4.6. *, **, and *** indicate significant differences to control (buffer only) at 5%, 1%, and 0.1% levels, respectively, by Dunnet’s test. Vertical bars indicate standard deviation (n = 3); 2PPA: 2-phenylpropionic acid; 3PPA: 3-phenylpropionic acid; 4PBA: 4-phenylbutyric acid
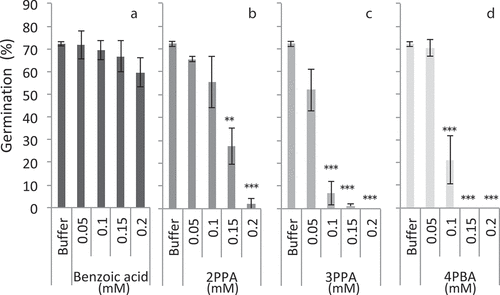
Tanaka et al. (Citation1990) reported that the inhibitory effect of benzoic acid on rice root elongation was weaker than that of 2PPA and 3PPA. The same tendencies were observed in this study (). In relation to the suppression of both M. vaginalis germination and rice root elongation, the spectrum of acid contents overlapped. For instance, more than 0.1 mM of 3PPA retarded both M. vaginalis germination () and rice root elongation (Tanaka et al., Citation1990). Rice bran is applied on the surface of soil, whereas straw is incorporated into the topsoil. It remains to be analyzed whether rice bran application on soil surface increases the organic acid contents in the entire topsoil.
Experiment 3: Suppression assay in mixed solutions of VFAs and ACAs
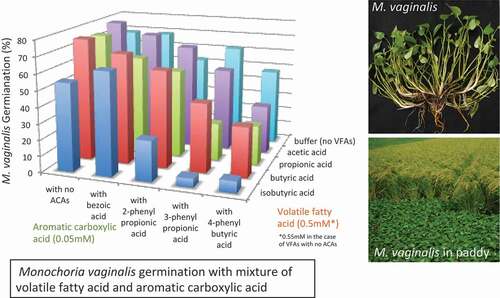
We used combinations of one of each of four VFAs and four ACAs solutions at 0.5 and 0.05 mM concentrations, respectively. These concentrations did not suppress germination in Experiment 2 (). Additionally, none of the solo solutions of VFAs at 0.55 mM indicated significant suppression. In the mixed solutions, however, all four VFAs in combination with 4PBA significantly suppressed germination (). In addition, propionic and isobutyric acids also suppressed germination with 3PPA. Suppressive activity was remarkably observed in the combination of isobutyric acid and 3PPA or 4PBA, showing higher suppressive activity in each solo solution, compared with other VFAs or ACAs (). The combination of isobutyric acid and 2PPA also showed significant germination suppression. Thus, the four VFAs showed synergistic effects against M. vaginalis germination in combination with 3PPA or 4PBA, and isobutyric acid also showed a synergistic suppressive effect with 2PPA. [ near here]
Table 1. Monochoria Vaginalis germination with VFA and ACA mixture solutions at pH4.6
Though 4PBA seems to have a higher potential of enhancing the effectiveness of the four VFAs in this study, 4PBA was detected at a lower concentration than 3PPA and 2PPA in the soil solution of a pot experiment using paddy soil and wheat straw (Tanaka, Citation2002). Moreover, 3PPA was detected at a higher concentration than 2PPA in the soil solution collected from paddy approximately 2 weeks after rice transplanting into soil 3 days following wheat straw application (no detection of 4PBA in the experiment) (Tanaka, Citation2002). 3PPA production was also observed in the laboratory experiment using soil solution applied with rice straw and glucose (Tanaka, Citation2002). Hence, 3PPA possibly has a crucial role to enhance the suppressive ability of isobutyric acid or other VFAs against M. vaginalis germination, although the reported concentration in the soil solution (1–5 µM) is not enough to suppress germination in a solo solution (). In addition, the suppressive effects of 4PBA’s presence in a soil solution of paddy water with rice bran should be investigated in future studies.
When fresh organic materials are applied into paddy soil, the amount of organic acids, as well as ferrous iron (Fe2+), increases in the soil. Fe2+ is one of the factors that suppress paddy weed germination (Nozoe et al., Citation2009). The interaction between Fe2+ and organic acids would be presumably present, and their cooperation could enhance germination suppression. However, this synergism has not been fully elucidated. In addition, several types of organic acids practically exist at various concentrations in the paddy soil solution. Even if one substance alone is not enough to induce toxicity, a synergistic interaction with other substances can effectively suppress weed germination. Further analysis of pH changes, different kinds and concentrations of organic acids, and other factors such as Fe2+ in the soil solution are expected on actual paddy fields to construct stable weed control methods using rice bran application.
In conclusion, we showed the three points as follows: (1) low pH intensified germination suppression by organic acids as long as it ranged between 4.6 and 6.4; (2) the suppressive activity of ACAs was stronger than that of VFAs, and the toxicity of VFAs was shown as isobutyric > propionic > butyric > acetic acid, whereas that of ACAs was 3PPA > 4PBA > 2PPA > benzoic acid (no activity); and (3) several combinations of VFAs and ACAs, for instance, isobutyric acid and 3PPA, which were individually ineffective concentrations, synergistically suppressed M. vaginalis germination. These results imply that the combinations of VFAs and ACAs were the basis of M. vaginalis suppression by rice bran application.
Acknowledgments
We thank Dr. Fukuyo Tanaka for her revisions and advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Alam, S. M. (1981). Effects of solution pH on the growth and chemical composition of rice plants. Journal of Plant Nutrition, 4(3), 247–260. https://doi.org/10.1080/01904168109362916
- Fageria, N. K., & Baligar, V. C. (1999). Growth and nutrient concentrations of common bean, lowland rice, corn, soybean, and wheat at different soil pH on an Inceptisol. Journal of Plant Nutrition, 22(9), 1495–1507. https://doi.org/10.1080/01904169909365730
- Koarai, A., & Shibayama, H. (2001). Effects of puddling time on seasonal changes in number and depth of emergence of annual weeds in paddy fields. Journal of Weed Science and Technology, 46(1), 5–12. https://doi.org/10.3719/weed.46.5
- Lynch, J. M. (1980). Effects of organic acids on the germination of seeds and growth of seedlings. Plant, Cell and Environment, 3(4), 255–259. https://doi.org/10.1111/1365-3040.ep11581824
- Miura, S., Uchino, A., Nozoe, T., Tazawa, J., Yoshida, T., Mizukami, T., Jeong, B. H., Wang, X. C., Nakagawa, A., Nakatani, K., Shibuya, T., Shiraishi, A., Imaizumi, T., Aoki, D., & Matsuoka, H. (2015). Weed suppression and rice production by mechanical weeding and rice bran application work in organic rice cultivation system. Bulletin of the NARO Agricultural Research Center, 24, 55–69. https://doi.org/10.24514/00001591
- Nakai, J., & Toritsuka, S. (2009). Inhibitive effect of rice bran treatment of soil surface on paddy-field weeds. Journal of Weed Science and Technology, 54(4), 233–238. https://doi.org/10.3719/weed.54.233
- Nozoe, T., Aoki, D., Matsuoka, H., Matsushima, K., Miura, S., Uchino, A., & Wan, X. C. (2016). Relationship between physical property of soil and growth of Monochoria vaginalis under paddy condition of organic farming – Analysis using settled soil volume in water of superficial layer. Plant Production Science, 19(2), 238–245. https://doi.org/10.1080/1343943X.2015.1128105
- Nozoe, T., Miura, S., Tazawa, J., Uchino, A., & Usui, Y. (2021). Factors destabilizing the control of Monochoria vaginalis by rice bran: Its conflicting powers influence both suppression and promotion of germination in paddy soil. Plant Production Science,24(1), 83–93. https://doi.org/10.1080/1343943X.2020.1794916
- Nozoe, T., Tachibana, M., Uchino, A., & Yokogami, N. (2009). Effects of ferrous iron (Fe) on the germination and root elongation of paddy rice and weeds. Weed Biology and Management, 9(1), 20–26. https://doi.org/10.1111/j.1445-6664.2008.00314.x
- Nozoe, T., Tazawa, J., Uchino, A., & Miura, S. (2018). Promotive effect of soil solution on germination of Monochoria vaginalis under paddy conditions. Soil Science and Plant Nutrition, 64(3), 396–405. https://doi.org/10.1080/00380768.2018.1433957
- Nozoe, T., Uchino, A., Okawa, S., Yoshida, S., Kanda, Y., & Nakayama, Y. (2012). Suppressive effects of rice bran incorporation in paddy soil on germination of Monochoria vaginalis and its relationship with electric conductivity. Soil Science and Plant Nutrition, 58(2), 200–205. https://doi.org/10.1080/00380768.2012.670864
- Stoll, V. S., & Blanchard, J. S. (1990). [4] Buffers: Principles and practice. Methods in Enzymology, Academic Press, 182(C), 24–38. https://doi.org/10.1016/S0076-6879(09)63006-8
- Tanaka, A., & Navasero, S. A. (1966). Aluminium toxicity of the rice plant under water culture conditions. Soil Science and Plant Nutrition, 12(2), 9–14. https://doi.org/10.1080/00380768.1966.10431183
- Tanaka, A., & Navasero, S. A. (1967). Carbon dioxide and organic acids in relation to the growth of rice. Soil Science and Plant Nutrition, 13(1), 25–30. https://doi.org/10.1080/00380768.1967.10431969
- Tanaka, F. (2002). Formation of aromatic acids and growth inhibition of rice (Oryza sativa L.) plants in flooded soil with wheat straw added. Bulletin of the NARO Kyushu Okinawa Agricultural Research Center, 40, 33–78. https://doi.org/10.24514/00001909
- Tanaka, F., Ono, S., & Hayasaka, T. (1990). Identification and evaluation of toxicity of rice root elongation inhibitors in flooded soils with added wheat straw. Soil Science and Plant Nutrition, 36(1), 97–103. https://doi.org/10.1080/00380768.1990.10415714
- Ueno, H., & Suzuki, T. (2005). Herbicidal effects and nutrients supply by applying of distilled spirit waste and rice bran in organic rice cultivation. Japanese Journal of Farming Work Research, 40(4), 191–198. https://doi.org/10.4035/jsfwr.40.191
- Yoshida, S. (1981) Organic Acid. In Fundamentals of rice crop science, International Rice Research Institute, Manila, Philippines, 171–174. https://www.pdfdrive.com/fundamentals-of-rice-crop-science-books-irri-e14909795.html