ABSTRACT
Partial submergence of Oryza sativa deepwater rice elicits enhancement of internodal elongation, referred to as deepwater response, conferred by three types of genes, SNORKEL1/2 (SK1/2), SEMIDWARF1 (SD1), and ACCELERATOR OF INTERNODE ELONGATION 1 (ACE1). We investigated the presence and expression of these genes in the African cultivated rice Oryza glaberrima and the relationship between these genes and the deepwater response of O. glaberrima. In 49 of the 50 accessions tested, one or two SK genes were identified, which could be divided into three types of SK1 and four types of SK2. The accessions with the SK2 type whose expression was induced by submergence demonstrated rapid internodal elongation under submergence. In most of these accessions, submergence also increased the expression of SD1 and ACE1 genes. However, the accessions did not possess the haplotype of SD1 that is associated with high deepwater response in O. sativa. In contrast, they possessed the type of ACE1 gene similar to that in O. sativa deepwater rice. These results indicate that the molecular mechanisms underlying induction of deepwater response in O. glaberrima are similar to that found in deepwater rice of O. sativa and suggest that most O. glaberrima cultivars, including upland cultivars, can exhibit rapid internodal elongation under submergence.
GRAPHICAL ABSTRACT
Introduction
Two cultigens, Oryza sativa L., Asian rice, and Oryza glaberrima Steud., African rice, exist in the genus Oryza. O. sativa is cultivated worldwide, including in Africa, whereas O. glaberrima is cultivated mainly in West Africa. Some O. glaberrima strains are adaptable to various abiotic and biotic stresses, such as drought, high soil iron, nematodes, and African rice gall midge (Diop et al., Citation2020; Jones, Dingkuhn, et al., Citation1997; Ndjiondjop et al., Citation2018). Therefore, O. glaberrima is a vital genetic resource for conferring stress tolerance to O. sativa grown in Africa (Jones, Mande, et al., Citation1997).
Flooding is an environmental stress that constrains the growth of most crops, thereby severely reducing crop yield. However, in the basins and deltas of large rivers in South and Southeast Asia and the inland delta of the Niger River in West Africa, deepwater rice is cultivated by taking advantage of seasonal flooding (Catling, Citation1992).
The adaptation of plants to flooding by promoting shoot elongation and thereby maintaining part of their foliage above the rising water surface is called deepwater response. This deepwater response, referred to as the ‘escape strategy’ for deepwater flooding, enables plants to continue aerobic respiration and photosynthesis even in flood environments, allowing them to survive. In the genus Oryza, cultivars and strains with deepwater response reportedly exist in the cultivated and wild species (Okishio et al., Citation2014; Sasayama et al., Citation2018, Citation2022), and the mechanism of the reaction has been investigated using the deepwater rice cultivars of O. sativa (Kende et al., Citation1998; Kuroha & Ashikari, Citation2020). In deepwater rice, shoot elongation is largely stimulated in submerged internodes, whose enhanced growth results from increased cell division in the intercalary meristem and increased elongation of the newly formed cells (Kende et al., Citation1998). This submergence-induced internodal elongation is triggered by ethylene accumulation in submerged tissues, which promotes gibberellin (GA) biosynthesis and/or signaling.
Three major quantitative trait loci (QTL) for submergence-induced internodal growth were detected on chromosomes 1, 3, and 12 using the progeny of the non-deepwater rice Taichung 65 (T65) and the deepwater rice C9285 (Hattori et al., Citation2007, Citation2008). The QTL pyramiding line possessing the three QTLs in the T65 genetic background exhibited almost the same internodal growth as C9285, and the QTL on chromosome 12 was the most effective (Hattori et al., Citation2009). Subsequently, the genes responsible for each QTL were identified. The first identified genes were SNORKEL1 (SK1) and SNORKEL2 (SK2), which are located on chromosome 12 in deepwater cultivars of O. sativa and not in non-deepwater cultivars (Hattori et al., Citation2009). SK1/2 genes, which encode ethylene response factor (ERF)-type transcription factors, are upregulated by ethylene and thereby may act directly or indirectly to promote GA biosynthesis and/or signaling, leading to the induction of internodal elongation through GA.
The second identified gene, located on chromosome 1, was SEMIDWARF1 (SD1)/GA20ox2 (Kuroha et al., Citation2018). SD1 is a gibberellin biosynthetic gene encoding GA20-oxidase, and its null mutants, selected for the rice Green Revolution, display a semi-dwarf phenotype (Sasaki et al., Citation2002; Spielmeyer et al., Citation2002). The nucleotide sequences of SD1 in O. sativa cultivars can be classified into six haplogroups (Hap-1 to −6) according to single nucleotide polymorphisms (SNPs) present in the promoter, exon, and intron regions (Kuroha et al., Citation2018). Rice cultivars without SK genes demonstrated no significant internodal elongation under submergence, regardless of the type of haplogroup of SD1, whereas cultivars harboring Hap-2 or Hap-6 with SK genes displayed significant promotion of elongation under submergence. In particular, the deepwater rice cultivars harboring Hap-6 exhibited higher SD1 transcript accumulation under submergence, thereby exhibiting a high deepwater response.
The last identified gene, located on chromosome 3, is ACCELERATOR OF INTERNODE ELONGATION 1 (ACE1), which encodes a protein of unknown function (Nagai et al., Citation2020). There are two genotypes of ACE1, deepwater and non-deepwater rice types. The genomic sequence of the deepwater rice ACE1 has a 1-bp-deletion at position 73 compared to that in the non-deepwater rice, causing a frameshift that disrupts the nuclear localization signal in the predicted amino acid sequence. The expression of deepwater rice ACE1 confers cells of the intercalary meristem with competence for cell division, leading to internodal growth in the presence of GA.
Most deepwater rice cultivars of O. glaberrima reportedly have strong elongation capacity, with some cultivars reaching a height of up to 6 m during gradual submergence (Catling, Citation1992). Although few reports exist on the elongation response of O. glaberrima deepwater rice compared to that of O. sativa deepwater rice (Inouye et al., Citation1989; Mochizuki et al., Citation1998; Watarai & Inouye, Citation1997), the mechanism of submergence-induced elongation in O. glaberrima deepwater rice has not been elucidated. Thus, we aimed to investigate the involvement of SKs, SD1, and ACE1 in the growth response of African rice O. glaberrima to submergence. To clarify the involvement of these genes in multiple O. glaberrima cultivars, we determined the DNA sequence of each of these genes and examined the relationship between the sequences and expression of the genes, as well as the submergence-induced elongation response of the internodes.
Materials and methods
Plant materials
The accessions of O. glaberrima and O. barthii, O. glaberrima’s wild ancestral species, used in this study are listed in Supplementary Table S1. Two O. sativa indica cultivars, Habiganj Aman II (HA II, a deepwater rice cultivar with strong internode elongation capacity under submergence) and T442–57 (a deepwater rice cultivar with moderate internode elongation capacity under submergence) from Bangladesh and Thailand, respectively, were used as controls.
For O. glaberrima and O. barthii accessions, seeds were pretreated at 42°C in the dark to break dormancy, after which the outer and inner glumes of the seeds were removed. Seeds were surface-sterilized in 1% sodium hypochlorite solution for 30 min and then rinsed several times with tap water, after which they were germinated by soaking in water at 30°C in the dark for 2–3 days. The germinated seeds were then sown in pairs in 1 L plastic pots filled with paddy soil containing 0.2 g N, 0.2 g P2O5, and 0.2 g K2O per liter of soil. At 40 days after germination, additional fertilizer was applied at 0.07 g N, 0.07 g P2O5, and 0.07 g K2O per pot. The plants were grown outdoors under natural conditions in an experimental field at Kobe University, Hyogo, Japan. The ambient temperature during the experiment was within a range of 27 to 33°C. The plants remained in the vegetative growth stage throughout the experimental period.
DNA analysis
Genomic DNA was extracted from the leaves of three-month-old plants using 200 mM Tris-HCl (pH 7.5) containing 250 mM NaCl, 25 mM EDTA, and 0.5% SDS. PCR analyses for SK, SD1, and ACE1 were performed using TaKaRa Ex Taq (Takara Bio, Shiga, Japan). Primer sets and reaction conditions are listed in Supplementary Table S2. PCR amplification was performed for sequence analysis using Tks Gflex DNA Polymerase (Takara Bio). To obtain the full-length sequences of some O. glaberrima SK2 homologs, gene-specific primers were designed based on the sequences obtained by 5′ and 3′ rapid amplification of cDNA ends (RACE) PCR. The homology of SK1 and SK2 genomic DNAs of O. sativa, O. glaberrima, and O. barthii was determined using the GENETYX software (GENETYX, Tokyo, Japan). A phylogenetic tree representing the relationship between the predicted SK1 and SK2 amino acids in O. sativa, O. rufipogon, O. nivara, O. glumaepatula, O. glaberrima, and O. barthii was constructed using UPGMA. Bootstrap analysis with 1,000 replicates was performed to estimate the statistical support for the nodes.
Gradual submergence treatment of plants
Fifty days after germination, two pots with two plants were submerged at a depth of 10 cm in 200 L plastic tanks. After two days, the water level was raised at a rate of 5 cm per day for 15 days to a final depth of 85 cm. The control plants were grown under non-submerged conditions during the same period. Plant length and total internode length on the main stem were measured before and after submergence. The position of the nodes before submergence was determined by the difference in texture of the leaf sheath with fingers. After submergence, the plants were dissected to measure total internode length.
RNA isolation and expression analysis
For the expression analysis, 50-day-old plants were partially submerged to a level where 70% of the height of each plant was below water. Total RNA was extracted from the basal 1 cm portion of the uppermost internodes of the plants grown in the air or submerged for 12 h using ISOSPIN Plant RNA (NIPPON GENE, Tokyo, Japan) according to the manufacturer’s protocol. Total RNA was extracted from the basal 1 cm portion of the main culms for plants that did not form internodes. First-strand cDNA was synthesized from 1 μg total RNA using a PrimeScript II 1st Strand cDNA Synthesis Kit (Takara Bio). Expression analysis was performed as follows with three biological replicates.
Expression analysis using quantitative RT-PCR for SK1, SK2, and SD1 was performed using TB Green Premix Ex Taq GC (Takara Bio) with MyGo Pro (Funakoshi, Tokyo, Japan) according to the manufacturer’s instructions. The relative expression levels of the target genes were calculated based on their initial expression levels. Expression analysis using semi-quantitative RT-PCR for the ACE1 gene was performed using Tks Gflex DNA Polymerase (Takara Bio). PCR products were separated on 1.8% agarose gels and visualized under ultraviolet light with ethidium bromide. Gene-specific primers and PCR conditions are listed in Supplementary Table S2. The 17s rRNA gene was used as an internal control.
Results
Genomic DNA analysis of SK genes
We screened 50 O. glaberrima accessions (Supplementary Table S1) for SK1 and SK2 by amplifying genomic DNA using gene-specific primers (Supplementary Table S2). PCR products amplified with SK1- and SK2-specific primers were obtained from 34 and 49 accessions, respectively. Sequence analysis of these amplified products established the presence of three types of sequences in the SK1 products, which share 82.5–95.5% identity with OsSK1, and the presence of four types in the SK2 products, which share 66.6–98.0% identity with OsSK2 (Supplementary Table S3). Therefore, the three amplified products for SK1 were named OglaSK1-A, OglaSK1-B, and OglaSK1-C in descending order of their identity to OsSK1 (Supplementary Tables S1, S3). Similarly, the four amplified products for SK2 were named OglaSK2-A, OglaSK2-B, OglaSK2-C, and OglaSK2-D (Supplementary Tables S1, S3). The OsSK2 gene, which contains an intron, possesses a single ERF domain in the 5′ half of the second exon. The OglaSK2-A, OglaSK2-B, and OglaSK2-D genes possess a similar gene structure to OsSK2, but the OglaSK2-C gene lacks the sequence of the entire first exon and the 5′ half of the second exon, thereby not possessing the ERF domain (Supplementary Figures S2, S3). Although the coding region length of OglaSK2-C was approximately 40% of that of OsSK2, the sequence of OglaSK2-C was 79.5% identical to that of OsSK2. (Supplementary Figure S2).
OglaSK1-A was identified in 17 O. glaberrima accessions, 16 of which contained OglaSK2-A (Supplementary Table S1). There were 16 O. glaberrima accessions with OglaSK1-B, all of which contained OglaSK2-B. OglaSK1-C was only observed in C8569, which possessed OglaSK2-A. Conversely, OglaSK2-C or OglaSK2-D was identified in 15 accessions, 14 of which could not confirm the presence of OglaSK1. Notably, C7290, harboring OglaSK2-C, possessed OglaSK1-A. Among the 50 accessions tested, C0025 was the only accession with neither OglaSK1 nor OglaSK2.
In addition to O. glaberrima, we investigated whether the two accessions of O. barthii (W0833 and W0844) possessed SK genes. In both accessions, two PCR products were obtained with the SK1-specific primers and SK2-specific primers. The amino acid sequences encoded by SK1 and SK2 amplification products of W0833 were identical to those encoded by OglaSK1-A and OglaSK2-A, respectively (; Supplementary Table S1). Furthermore, the amino acid sequences encoded by the SK1 and SK2 amplification products of W0844 were the same as those of OglaSK1-B and OglaSK2-B, respectively (; Supplementary Table S1).
Table 1. Percentage amino acid identity of SK2 in Oryza sativa (Os), Oryza glaberrima (Ogla), Oryza barthii (Obar), and Oryza glumaepatula (Oglu).
Growth response to submergence
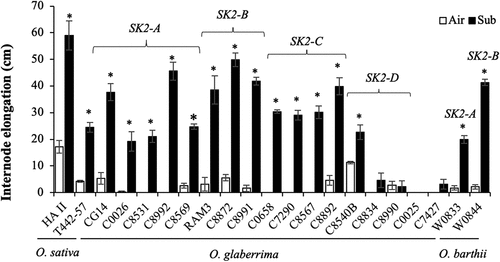
We investigated the growth response to gradual submergence in 17 accessions of O. glaberrima and two accessions of O. barthii with various alleles of SK2 and compared their responses to those of the high-ability deepwater cultivar HA II and the moderate-ability deepwater cultivar T442–57 of O. sativa. When 50-day-old plants were submerged in water at a rate of 5 cm daily to a final depth of 85 cm, significant shoot elongation was observed in 13 O. glaberrima accessions and the O. barthii accessions, as well as in the O. sativa cultivars (). At the end of the experiment, all the submerged plants maintained a portion of their foliage above the water surface.
Figure 1. Effect of gradual submergence on elongation responses of O. glaberrima and O. barthii accessions and O. sativa deepwater cultivars. Fifty-day-old plants were submerged to a depth of 10 cm for two days, and the water level was then increased at the rate of 5 cm day −1 for 15 days. Air-grown control plants were grown in non-submerged conditions during the same period. (a) Shoot length. The horizontal grey line denotes the final water level (85 cm). (b) Total internode elongation. The SK2 allelic form of each accession of O. glaberrima and O. barthii is shown in the figure. Data represent the mean ± SE of four plants. Asterisks indicate a significant difference (P <0.05, Student’s t-test) between the submerged and control plants.
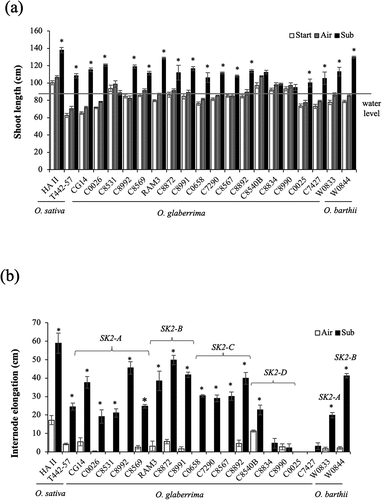
Submergence significantly promoted internodal elongation in 13 O. glaberrima accessions and the O. barthii accessions, as well as in the O. sativa cultivars (). In the accessions of O. glaberrima and O. barthii that demonstrated significant elongation of internodes during submergence, the increase in internodal elongation was approximately equal to or greater than that of T442–57 but less than that of HA II. All O. glaberrima accessions with OglaSK2-A, OglaSK2-B, or OglaSK2-C displayed significant internodal elongation under submergence, whereas the accessions with OglaSK2-D, except for C8540B, did not. Furthermore, accession C0025 without any allele of OglaSK2 genes and accession C7427 with a loss-of-function allele of SK2 failed to demonstrate submergence-induced internodal elongation: the SK2 sequence of C7427 had a 13-bp deletion in the predicted coding region (; Supplementary Figure S3).
Table 2. Allelic forms of SK,SD1 and ACE1 genes of O. glaberrima and O. barthii accessions and O. sativa deepwater cultivars used in submergence treatment.
DNA analysis of SD1 and ACE1 genes
In the 17 O. glaberrima and two O. barthii accessions whose growth response to gradual submergence was examined as described above, we determined the genotypes of SD1 and ACE1 by PCR with type-specific primers. Analysis of SD1 indicated that three O. glaberrima accessions possessed SD1 Hap-4, and all other accessions possessed SD1 Hap-2 (; Supplementary Figure S4(a)), but none of the accessions possessed SD1 Hap-6, which exists in O. sativa cultivars exhibiting high deepwater response. Conversely, analysis of ACE1 revealed that the ACE1 gene sequence of all O. glaberrima and O. barthii accessions had a 1-bp-deletion at the same position as the ACE1 gene sequence of O. sativa deepwater rice cultivars, indicating that these O. glaberrima and O. barthii accessions possess the deepwater rice type of ACE1 (; Supplementary Figure S4(b)).
Additionally, the genomic sequences of ACE1 of several O. glaberrima accessions possessing different SK2 genes were determined. The sequences of the ACE1 genes in the accessions examined shared more than 99.9% identity with the sequences of deepwater rice type or non-deepwater rice type of the OsACE1 gene (Supplementary Figure S5). In O. glaberrima accessions possessing SK2-A, SK2-B, or SK2-C, the sequences of ACE1 genes were identical (Supplementary Figure S5).
Expression analysis of SK1/2, SD1, and ACE1 genes
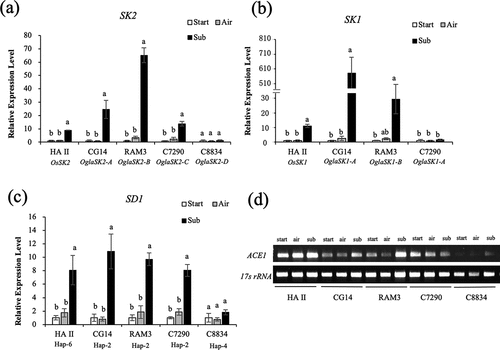
The expression of SK1/2, SD1, and ACE1 was examined in the internodes of air-grown and submerged plants of four O. glaberrima accessions with different alleles of SK2: accessions CG14, RAM3, C7290, and C8834 possessed SK2-A, SK2-B, SK2-C, and SK2-D, respectively.
In the accessions with SK2-A, SK2-B, and SK2-C, SK2 expression was induced by submergence for 12 h, but not in the accession with SK2-D (). SK1 expression in CG14 and RAM3, which possess SK1-A/SK2-A and SK1-B/SK2-B, respectively, was induced by submergence. However, submergence-induced expression of SK1 was not observed in accession C7290, possessing SK1-A/SK2-C (). The expression levels of SK1 in CG14 and RAM3 were much higher than those of OsSK1 in HA II. The expression level of SD1 was significantly increased by submergence in CG14, RAM3, and C7290, all of which possess SD1 Hap-2 (). Their expression levels were comparable to those of SD1 Hap-6 in HA II. In contrast, submergence did not promote SD1 expression in C8834, which possesses SD1 Hap-4. Submergence-induced expression of ACE1 was observed in CG14, RAM3, C8834, and HA II but not in C7290 (); however, these O. glaberrima accessions all possessed the deepwater rice type ACE1 (Supplementary Figure S5).
Figure 2. Expression analyses of the genes involved in the deepwater response in O. glaberrima. Expressions analyses of SK2 (a), SK1 (b), and SD1 (c) were performed by real-time RT-PCR using gene-specific primers on the internodes of the plants submerged or grown in the air for 12 h. 17s rRNA was used as an internal control. Data represent the means ± SE of three measurements. Different letters above bars indicate significant differences (P <0.05) based on the Bonferroni test. (d) ACE1 expression analysis was performed by RT-PCR using gene-specific primers on the internodes of the plants submerged or grown in the air for 12 h.
Discussion
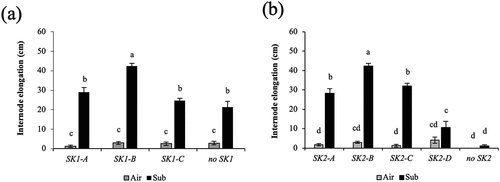
In O. glaberrima, the SK1 and SK2 genes were observed in three and four forms, respectively, whereas in O. barthii, both SK1 and SK2 were present in two allelic forms (Supplementary Figures S1, S2); the amino acid sequence encoded by ObarSK1-A, ObarSK1-B, ObarSK2-A, and ObarSK2-B was identical to that encoded by OglaSK1-A, OglaSK1-B, OglaSK2-A, and OglaSK2-B, respectively. shows the relationship between the presence of the respective alleles of SK1 or SK2 and the submergence-induced internode elongation. When comparing the different types of SK1 alleles, the accessions that have the SK1 genes, regardless of the allele type, displayed submergence-induced internodal elongation. The elongation was greatest in the accessions with SK1-B (). However, accessions without SK1 also exhibited submergence-induced internode elongation, comparable to that observed in accessions with SK1-A or SK1-C. When comparing the different types of SK2 alleles, the accessions with the SK2-A, SK2-B, or SK2-C alleles displayed submergence-induced internodal elongation, and the elongation was greatest in the accessions with SK2-B. In contrast, the accessions with SK2-D or without a functional SK2 gene did not exhibit elongation (). The accessions harboring no SK1 that demonstrated deepwater response possessed SK2, whereas accessions harboring no SK2 did not show deepwater response (; ). These results indicate that SK2 is critical for the deepwater response of O. glaberrima and O. barthii, as in the case of O. sativa and other wild species (Hattori et al., Citation2009). Therefore, although the accessions with SK1-B demonstrated the highest elongation ability compared to the different types of SK1 alleles (), the response is probably attributed to the presence of SK2-B because all of the accessions harboring SK1-B possessed SK2-B, conferring the highest ability (; Supplementary Table S1; . The high elongation ability of accessions with SK2-B may be explained by the fact that SK2-B was more highly expressed by submergence than other types of SK2 alleles ().
Figure 3. Comparison of internode elongation under submergence in O. glaberrima and O. barthii accessions with different SK1 (a) and SK2 (b) allelic forms. Fifty-day-old plants were submerged to a depth of 10 cm for two days, and then the water level was increased at the rate of 5 cm day −1 for 15 days. Air-grown control plants were grown in non-submerged conditions during the same period. Data represent the means ± SE of 10–23 plants. Different letters above bars indicate significant differences (P < 0.05) based on the Bonferroni test.
On the other hand, Kawano et al. (Citation2008) reported that the seedings of O. glaberrima cultivar Saligbeli exhibited enhanced leaf elongation with increased dry matter accumulation during submergence. In the present study, two O. glaberrima accessions without SK1/SK2 genes, C0025 and C7427, showed enhanced shoot elongation () without internode elongation () under deepwater condition. Therefore, the shoot elongation exhibited by these two accessions is thought to be due to the promotion of leaf elongation. Such a growth response might also function as adaptation to deepwater in O. glaberrima.
In O. glaberrima accessions possessing SK2-C, submergence induced internodal elongation with increased expression of SK2-C ()). This observation suggests that this gene, as well as SK2-A and SK2-B, may function in the induction of internode elongation under submergence; however, unlike SK1 and other SK2 genes, SK2-C lacks the ERF domain (Supplementary Figures S1, S2). Transactivation activity assays of different regions of the OsSK2 protein demonstrated that the C-terminal region, which does not contain the ERF domain, has transactivation activity but not the ERF domain or N-terminal region (Hattori et al., Citation2009). Because OglaSK2-C covers 87% of the C-terminal region of OsSK2 (Supplementary Figure S2), the OglaSK2-C protein was expected to exhibit transactivation activity. In addition, OglaSK2-C shared 94% amino acid identity with the corresponding region of OgluSK2-2, which was identified in accessions with the high deepwater response of the wild rice species O. glumaepatula. However, OglaSK2-C shared 67.7% amino acid identity with that of OsSK2 (). Notably, the transcription of OgluSK2-2 was induced by submergence but not by ethylene; however, the gene has an ERF domain (Sasayama et al., Citation2018).
The genomic sequence of OglaSK2-D was 100% identical to that of SNORKEL-LIKE1(SKL1) on chromosome 12 of O. sativa subsp. japonica cv. Nipponbare () (Nagai et al., Citation2022). Therefore, OglaSK2-D is unlikely to confer deepwater response to O. glaberrima. However, among the three accessions with OglaSK2-D investigated in the present study, one accession (C8540B) demonstrated significant internodal elongation induced by submergence, whereas the other accession did not (). The reason for this finding remains unclear.
Of the 17 O. glaberrima accessions examined, all accessions possessed SD1 Hap-2 or Hap-4 (; Supplementary Figure S4(a)): no accessions possessed SD1 Hap-6, which was observed in O. sativa cultivars with high deepwater response, such as HA II. In O. sativa deepwater rice, the expression of SD1 Hap-6 was much more enhanced by submergence than that of SD1 Hap-2 and Hap-4, which may help explain the high deepwater response of the cultivar harboring SD1 Hap-6 (Kuroha et al., Citation2018). In O. glaberrima, the accessions with SD1 Hap-2 exhibited internodal elongation induced by submergence (; ) with increased expression levels of SD1, which was comparable to that of SD1 Hap-6 in O. sativa HA II (), whereas the accession with SD1 Hap-4 did not show internodal elongation or induction of expression of SD1. Therefore, these results suggest that submergence-enhanced expression of SD1 may be involved in rapid internodal elongation in O. glaberrima, as observed in O. sativa deepwater rice (Kuroha et al., Citation2018). Furthermore, the accession with the Hap-4 did not display deepwater response, likely because OglaSK2-D possessed by the accession is not a functional analog of SK2. In O. sativa accessions with OsSD1 Hap-2 or Hap-4, the presence of SK2 is required for submergence-induced expression of OsSD1, thereby promoting internodal elongation (Kuroha et al., Citation2018). These observations also support the view that OglaSK2-A, OglaSK2-B, and OglaSK2-C function in the induction of internode elongation under submergence because accessions possessing the SK2 genes exhibited submergence-induced expression of OglaSD1 ().
All O. glaberrima accessions examined possessed ACE1 harboring a 1-bp-deletion at the same position found in the ACE1 of O. sativa deepwater rice cultivars (; Supplementary Figure S4(b)); however, not all accessions with this deepwater rice type of ACE1 exhibited deepwater response (). This suggests that the presence of deepwater rice type ACE1 alone may not be sufficient for inducing internode elongation during submergence in O. glaberrima. ACE1 expression is induced in response to GA, leading to the formation of elongated internodes (Nagai et al., Citation2020). In three O. glaberrima accessions (CG14, RAM3, and C7290), the expression of ACE1 in internodes was already induced before submergence treatment (), and the elongation of the internodes was promoted by submergence (). Conversely, under accession C8834, expression of ACE1 was not observed in air-grown plants but increased in submerged plants (), which had elongated internodes (). These results suggest that deepwater rice type ACE1 may be related to submergence-induced internode elongation in O. glaberrima as well as O. sativa.
The sequence of OglaSK2-D identified in this study was present in the genomes of many O. sativa cultivars, including indica and japonica (data not shown). In the O. glaberrima accessions examined here, all accessions harboring SK2-D possessed SD1 Hap-4, whereas the other O. glaberrima accessions, and two accessions of the ancestral species O. barthii, possessed SD1 Hap-2 (). Furthermore, the ACE1 genes of three accessions harboring SK2-D contain thymine at position 62, as found in ACE 1 of O. sativa. In contrast, the ACE1 of the other O. glaberrima accessions and O. barthii accessions contained adenine at the corresponding position (Supplementary Figure S5). Therefore, these findings suggest that SK2-D of O. glaberrima was derived from O. sativa. To test this possibility, we compared the accessions with OglaSK2-D and other accessions in terms of the morphologies of glume hair, ligule shape, and secondary rachis-branch structure, which are generally known to be different between O. sativa and O. glaberrima. The results indicated that all the morphologies of the accessions with OglaSK2-D, unlike those of the other accessions, were characteristic of O. sativa rather than O. glaberrima (Supplementary Figure S6). Furthermore, as a result of examining Osprog1, specific to O. sativa, and Oglaprog7, specific to O. glaberrima, the accessions with OglaSK2-D possessed Osprog1 but not Oglaprog7 (Supplementary Figure S7) (Hu et al., Citation2018). These observations support our hypothesis that SK2-D of O. glaberrima was derived from O. sativa. Linares (Citation2002) reported that since the late 20th century, rice cultivation in Africa had been based on a farming system in which several cultivars of O. sativa and O. glaberrima are grown in a mixed manner. In addition, Nuijten et al. (Citation2009) reported that descendants of interspecific hybridization between these species were observed in African farms. These reports would also support the possibility that SK2-D of O. glaberrima was derived from O. sativa.
Catling (Citation1992) reported that the African rice O. glaberrima was cultivated as upland or deepwater rice. Among the 50 O. glaberrima accessions tested in the present study (Supplementary Table S1), six accessions were registered as deepwater rice cultivars, and four accessions were upland rice cultivars; however, whether the remaining 40 accessions were upland or deepwater rice cultivars remains unknown. Three of the four upland accessions (CG14, C7265, and C7493) contained the OglaSK2-A gene (Supplementary Table S1). All accessions with SK2-A, including CG14, displayed enhanced internodal elongation under submergence (). Of the 40 accessions without registration of cultivation type, 22 possessed SK2-A, SK2-B, or SK2-C, which can confer the deepwater response to O. glaberrima plants (Supplementary Table S1; ). Because some accessions had been collected from Ivory Coast and Tanzania (Supplementary Table S1), where deepwater rice cultivation systems have not been practiced (Catling, Citation1992), they must have been cultivated in upland areas. Thus, many O. glaberrima cultivars grown as upland rice could possess functional SK genes, thereby showing rapid internodal elongation under submergence. In contrast, the existence of non-deepwater rice cultivars of O. sativa carrying OsSK1/2 genes remains unknown. The domestication process of O. glaberrima began around 3,000 years ago, whereas that of O. sativa began around 9,000 years ago (Fornasiero et al., Citation2022). The difference in time from domestication between O. sativa and O. glaberrima might explain why O. glaberrima cultivars that have been cultivated in non-deepwater areas still preserve the SK genes.
All the known deepwater rice cultivation areas in Africa are in West Africa, and three-quarters of West Africa’s deepwater rice is grown along the Niger River, mainly in Mali, Guinea, and Nigeria (Catling, Citation1992). Notably, most accessions collected in Mali, which has the most extensive deepwater rice cultivation in Africa (Catling, Citation1992), possess the OglaSK2-B gene (Supplementary Table S1), which can confer the strongest deepwater response to O. glaberrima (). The main deepwater rice-growing area in Mali is the inland delta of the Niger River, where floodwaters may reach a height of 2.5–3 m during the rainy season (Catling, Citation1992). Such a severely flooded environment may have resulted in the selection of the most effective SK2-B allele.
Only the accessions collected in Guinea, Gambia, and Ivory Coast, located on the southwest coast of West Africa, possessed OglaSK2-C (Supplementary Table S1), which exhibited 94.0% amino acid identity to OgluSK2-2 of the wild rice species O. glumaepatula in South America (; ). Although O. glaberrima was introduced to the Americas during the slave trade years and grown by enslaved Africans for decades (Carney, Citation2005), van Andel (Citation2010) recently observed that O. glaberrima is still cultivated in the rice fields of Suriname, South America. Analysis of genetic distance calculated across the whole genome indicates that the O. glaberrima of Suriname is most closely related to the O. glaberrima accessions collected in the coastal Guinean lowlands and central highlands in O. glaberrima accessions collected from countries in West Africa (van Andel et al., Citation2016). Therefore, the presence of OgluSK2-2 in O. glumaepatula, which is highly homologous to OglaSK2-C, might have been due to gene flow from O. glaberrima of Guinea origin. This may also be supported by the fact that O. glumaepatula strains with OgluSK2-2 have another SK2 gene, OgluSK2-1gene, which shows higher sequence similarity to OsSK2 (Sasayama et al., Citation2018) and that, except for certain strains of O. glumaepatula, no Oryza species with two SK2 genes have been identified.
Figure 4. Phylogenetic tree of SK amino acid sequences in Oryza species. Phylogenetic relationships of SK amino acid sequence of Oryza sativa (Os), Oryza rufipogon (Oru), Oryza nivara (On), Oryza glaberrima (Ogla), Oryza barthii (Obar) and Oryza glumaepatula (Oglu) were constructed using the UPGMA method. The bootstrap support values from 1,000 replicants are shown at the node.
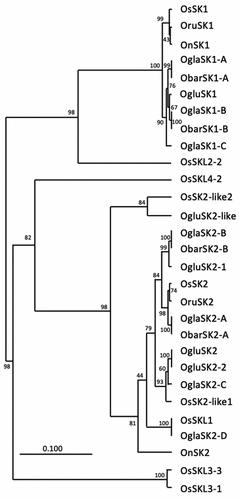
SK genes are reportedly present in the Asian cultivated species O. sativa, its ancestral wild species O. rufipogon, and the South American wild species O. glumaepatula (Hattori et al., Citation2009; Sasayama et al., Citation2018). In the present study, SK genes were also identified in the African cultivated species O. glaberrima and its ancestral wild species O. barthii. Therefore, the common ancestors of these wild rice species distributed on different continents of Africa, Asia, and South America probably already possessed SK genes.
PPS2022_074RP-File006.pdf
Download PDF (7.8 MB)Acknowledgments
We thank Prof. T. Ishii and Dr. R. Ishikawa, Kobe University, for helpful suggestions. The seeds of CG14 and RAM3 used in this study was provided by Dr. Itoh, Kobe University. The accessions of O. glaberrima other than CG14 and RAM3 and O. barthii used in this study were distributed from the National Institute of Genetics supported by the National Bioresource Project, MEXT, Japan. This work was supported by JSPS KAKENHI Grant No.18K05595.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/1343943X.2022.2161401.
References
- Carney, J. (2005). Rice and memory in the age of enslavement: Atlantic passages to Suriname. Slavery & Abolition, 26(3), 325–348. https://doi.org/10.1080/01440390500319562
- Catling, D. (1992). The Niger Basin and West Africa. In Rice in Deep Water (pp. 353–376). Macmillan Press.
- Diop, B., Wang, D. R., Drame, K. N., Gracen, V., Tongoona, P., Dzidzienyo, D., Nartey, E., Greenberg, A. J., Djiba, S., Danquah, E. Y., & McCouch, S. R. (2020). Bridging old and new: Diversity and evaluation of high iron-associated stress response of rice cultivated in West Africa. Journal of Experimental Botany, 71(14), 4188–4200. https://doi.org/10.1093/jxb/eraa182
- Fornasiero, A., Wing, R. A., & Ronald, P. (2022). Rice domestication. Current Biology, 32(1), R20–24. https://doi.org/10.1016/j.cub.2021.11.025
- Hattori, Y., Miura, K., Asano, K., Yamamoto, E., Mori, H., Kitano, H., Matsuoka, M., & Ashikari, M. (2007). A major QTL confers rapid internode elongation in response to water rise in deepwater rice. Breeding Science, 57(4), 305–314. https://doi.org/10.1270/jsbbs.57.305
- Hattori, Y., Nagai, K., Furukawa, S., Song, X. J., Kawano, R., Sakakibara, H., Wu, J., Matsumoto, T., Yoshimura, A., Kitano, H., Matsuoka, M., Mori, H., & Ashikari, M. (2009). The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature, 460(7258), 1026–1030. https://doi.org/10.1038/nature08258
- Hattori, Y., Nagai, K., Mori, H., Kitano, H., Matsuoka, M., & Ashikari, M. (2008). Mapping of three QTLs that regulate internode elongation in deepwater rice. Breeding Science, 58(1), 39–46. https://doi.org/10.1270/jsbbs.58.39
- Hu, M., Lv, S., Wu, W., Fu, Y., Liu, F., Wang, B., Li, W., Gu, P., Cai, H., Sun, C., & Zhu, Z. (2018). The domestication of plant architecture in African rice. The Plant Journal, 94(4), 661–669. https://doi.org/10.1111/tpj.13887
- Inouye, J., Hakoda, H., & NG, N. Q. (1989). Preliminary studies on some ecological characteristics of African deep water rice (Oryza glaberrima Steud.). Japanese Journal of Tropical Agriculture, 33(3), 158–163. https://doi.org/10.11248/jsta1957.33.158
- Jones, M. P., Dingkuhn, M., Aluko, G. K., & Semon, M. (1997). Interspecific Oryza sativa L. × O. glaberrima Steud. progenies in upland rice improvement. Euphytica, 94, 237–246. https://doi.org/10.1023/A:1002969932224
- Jones, M. P., Mande, S., & Aluko, K. (1997). Diversity and potential of Oryza glaberrima Steud in upland rice breeding. Breed Science, 47(4), 395–398. https://doi.org/10.1270/jsbbs1951.47.395
- Kawano, N., Ito, O., & Sakagami, J. -I. (2008). Flash flooding resistance of rice genotypes of Oryza sativa L., O. glaberrima Steud., and interspecific hybridization progeny. Environmental and Experimental Botany, 63(1–3), 9–18. https://doi.org/10.1016/j.envexpbot.2007.12.001
- Kende, H., van der Knaap, E., & Cho, H. -T. (1998). Deepwater rice: A model to study stem elongation. Plant Physiology, 118(4), 1105–1110. https://doi.org/10.1104/pp.118.4.1105
- Kuroha, T., & Ashikari, M. (2020). Molecular mechanisms and future improvement of submergence tolerance in rice. Molecular Breeding, 40, 41. https://doi.org/10.1007/s11032-020-01122-y
- Kuroha, T., Nagai, K., Gamuyao, R., Wang, D. R., Furuta, T., Nakamori, M., Kitaoka, T., Adachi, K., Minami, A., Mori, Y., Mashiguchi, K., Seto, Y., Yamaguchi, S., Kojima, M., Sakakibara, H., Wu, J., Ebana, K., Mitsuda, N., Ohme-Takagi M., and Ashikari, M. (2018). Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science, 361(6398), 181–186. https://doi.org/10.1126/science.aat1577
- Linares, O. F. (2002). African rice (Oryza glaberrima): History and future potential. Proceedings of the National Academy of Sciences of the United States of America, 99(25), 16360–16365. https://doi.org/10.1073/pnas.252604599
- Mochizuki, T., Ryu, K., & Inouye, J. (1998). Elongation ability of African floating rice (Oryza glaberrima Steud.). Plant production science, 1(2), 134–135. https://doi.org/10.1626/pps.1.134
- Nagai, K., Kurokawa, Y., Mori, Y., Minami, A., Reuscher, S., Wu, J., Matsumoto, T., & Ashikari, M. (2022). SNORKEL genes relating to flood tolerance were pseudogenized in normal cultivated rice. Plants, 11(3), 376. https://doi.org/10.3390/plants11030376
- Nagai, K., Mori, Y., Ishikawa, S., Furuta, T., Gamuyao, R., Niimi, Y., Hobo, T., Fukuda, M., Kojima, M., Takebayashi, Y., Fukushima, A., Himuro, Y., Kobayashi, M., Ackley, W., Hisano, H., Sato, K., Yoshida, A., Wu, J., Sakakibara, H., Ashikari, M. … Ashikari, M. (2020). Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature, 584(7819), 109–114. https://doi.org/10.1038/s41586-020-2501-8
- Ndjiondjop, M. N., Semagn, K., Sow, M., Manneh, B., Gouda, A. C., Kpeki, S. B., Pegalepo, E., Wambugu, P., Sié, M., & Warburton, M. L. (2018). Assessment of genetic variation and population structure of diverse rice genotypes adapted to lowland and upland ecologies in Africa using SNPs. Frontiers in plant science, 9, 446. https://doi.org/10.3389/fpls.2018.00446
- Nuijten, E., van Treuren, R., Struik, P. C., Mokuwa, A., Okry, F., Teeken, B., & Richards, P. (2009). Evidence for the emergence of new rice types of interspecific hybrid origin in West African Farmers’ Fields. PLoS One, 4(10), e7335. https://doi.org/10.1371/journal.pone.0007335
- Okishio, T., Sasayama, D., Hirano, T., Akimoto, M., Itoh, K., & Azuma, T. (2014). Growth promotion and inhibition of the Amazonian wild rice species Oryza grandiglumis to survive flooding. Planta, 240(3), 459–469. https://doi.org/10.1007/s00425-014-2100-8
- Sasaki, A., Ashikari, M., Ueguchi-Tanaka, M., Itoh, H., Nishimura, A., Swapan, D., Ishiyama, K., Saito, T., Kobayashi, M., Khush, G. S., Kitano, H., & Matsuoka, M. (2002). Green revolution: A mutant gibberellin-synthesis gene in rice. Nature, 416, 701–702. https://doi.org/10.1038/416701a
- Sasayama, D., Niikawa, M., Hatanaka, T., Fukayama, H., & Azuma, T. (2022). Adaptive responses to flooding in wild rice species with various genomes other than AA. Plant production science, 25(3), 350–358. https://doi.org/10.1080/1343943X.2022.2073896
- Sasayama, D., Okishio, T., Hirano, T., Fukayama, H., Hatanaka, T., Akimoto, M., & Azuma, T. (2018). Internodal elongation under submergence in the Amazonian wild rice species Oryza glumaepatula: The growth response is induced by hypoxia but not by ethylene. Plant Growth Regulation, 85(1), 123–132. https://doi.org/10.1007/s10725-018-0378-4
- Spielmeyer, W., Ellis, M. H., & Chandler, P. M. (2002). Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proceedings of the National Academy of Sciences of the United States of America, 99(13), 9043–9048. https://doi.org/10.1073/pnas.132266399
- van Andel, T. R. (2010). African rice (Oryza glaberrima Steud.): Lost crop of the enslaved Africans discovered in Suriname. Economic Botany, 64(1), 1–10. https://doi.org/10.1007/s12231-010-9111-6
- van Andel, T. R., Meyer, R. S., Aflitos, S. A., Carney, J. A., Veltman, M. A., Copetti, D., Flowers, J. M., Havinga, R. M., Maat, H., Purugganan, M. D., Wing, R. A., & Schranz, M. E. (2016). Tracing ancestor rice of Suriname Maroons back to its African origin. Nature Plants, 2, e16149. https://doi.org/10.1038/nplants.2016.149
- Watarai, M., & Inouye, J. (1997). Effect of water conditions on LEI position in African floating rice (Oryza glaberrima Steud.). Japanese Journal of Crop Science, 66(2), 300–306. https://doi.org/10.1626/jcs.66.300