Abstract
Background: Fluorescence optical imaging with indocyanine-green enhancement (FOI) is a new imaging modality for the assessment of hand arthritis. The objective of this study was to compare performance profiles of clinical examination (CE), US and FOI using MRI as a reference in the same active rheumatoid arthritis (RA) patients.
Methods: CE, US, FOI and MRI were performed on six subjects with active RA. Each sequence of FOI was divided into three phases based on indocyanine-green dynamics and the joints were graded semi-quantitatively. Sensitivities and specificities of CE, US and FOI were calculated using the RAMRIS synovitis score >0 as a reference in a total of 30 joints (the second to fifth metacarpophalangeal (MCP) joints and the wrist of the clinically dominant hand).
Results: FOI showed sensitivities and specificities, respectively, of 85% and of 94% for Phase-1 and 69% and 94% for Phase-2. Sensitivities and specificities were 100% and 35% for CE (tender or swollen), 92% and 41% for gray scale US, and 77% and 100% for color-Doppler US.
Conclusions: The performance characteristics of FOI in detection of synovitis in patients with active RA are comparable to those of US and more specific than CE. FOI has a potential as an assessment modality of RA.
1. Background
Early diagnosis and accurate assessment of disease activity have become more important with recent advances in therapeutic management of rheumatoid arthritis (RA) [Citation1,Citation2]. Magnetic resonance imaging (MRI) and ultrasonography (US) are used in clinical practice to detect early changes and residual inflammation in affected joints [Citation3,Citation4]. In particular, US is being actively investigated as a tool to guide tight disease control and improve relevant clinical outcomes [Citation5,Citation6]. However, operator-dependence and time-consuming procedures are major limitations of US. The routine clinical use of MRI is also limited by availability, cost and the limited area of examination.
Fluorescence optical imaging (FOI) with indocyanine-green (ICG) enhancement is a new imaging modality used to assess hand arthritis. Its technical basis has been described previously; near-infrared fluorescence emitted by intravenously administered ICG are detected from the dorsal side and visualized as signal intensities in a single plane image [Citation7]. ICG acts as a blood flow marker to enhance inflamed joints. Acquiring one image per second, a standard six-minute examination provides a sequence of 360 images. Werner et al. [Citation7] reported that FOI has improved sensitivity for arthritis compared to clinical examination (CE) by identifying three distinctive phases within each sequence. FOI also showed good agreement with US and 1.5-tesla MRI [Citation7]. Krohn et al. [Citation8] performed FOI, US and MRI in the same subjects and reported sensitivity and specificity of three phases of FOI with either MRI or US as a reference. However, sensitivity and specificity of FOI were not compared with those of other modalities with a single reference. As MRI is generally considered as a gold standard for identifying synovitis [Citation9] and US is widely used as an on-site assessment modality of RA, comparison of FOI and US with MRI as a reference is essential for evaluating the performance profile of FOI.
In this study, we performed CE, US, FOI and MRI on the same subjects with active RA and directly compared the sensitivities and specificities of the examinations with 1.5-tesla MRI as a reference standard. FOI was also performed in healthy subjects and false positive findings are reported.
2. Patients and methods
2.1. Patients
Six patients with active RA and three healthy volunteers were recruited for this study. Patients with RA fulfilled the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria [Citation1]. Active RA was defined by presence of at least two swollen joints in the hands at enrollment. To minimize fluctuation in disease activities, changes in treatment (change of disease-modifying antirheumatic drugs (DMARDs) or change in doses of DMARDs or corticosteroid) were not allowed for four weeks prior to and during the two-week study period. All examinations were performed during this two-week study period. Exclusion criteria were contraindications for MRI or gadolinium contrast, hypersensitivity to iodine, pregnancy and breast-feeding. All subjects had to be 20-year-old or older at enrollment. Written informed consent was obtained for each subject. The study was approved by the Institutional Review Board of Tokyo Medical and Dental University Hospital (#25-14) and carried out in compliance with the ethics guidelines for clinical studies in Japan and the Helsinki Declaration (revised in 2008).
2.2. Clinical examination (CE) and disease activity
Joints were examined clinically for swelling and tenderness, and findings were reported as binomials on consensus of two rheumatologists (M. H. and W. Y-K.). Wrists, first to fifth metacarpophalangeal joints (MCP 1-5), first finger interphalangeal joints (IP), second to fifth proximal interphalangeal joints (PIP 2-5), shoulders, elbows and knees of each RA patient were examined. For the healthy volunteers, only the hand joints were examined. Patient global assessment (PGA), physician global assessment (PhGA), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels were also assessed in RA patients to calculate disease activity indices.
2.3. Fluorescence optical imaging (FOI)
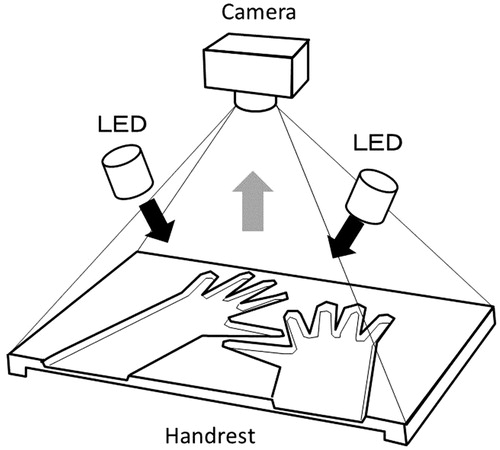
The FOI was performed on RA patients and healthy volunteers using Xiralite®, a commercially available FOI system (Mivenion GmbH, Berlin, Germany). illustrates the device schematically. A sequence total of 360 images was acquired during a six-minute examination following the injection of a bolus of ICG at 0.1 mg/kg. Since severe adverse events reported with ICG were due to anaphylactic reaction (hypotension, bronchospasm, laryngospasm, edema and urticaria) to ICG [Citation10], the patients were carefully observed during the examination. Images were assessed using the method reported by Werner et al. [Citation7]. For each image sequence, a composite image of the first 240 s was generated by the XiraView® software (Mivenion GmbH, Berlin, Germany) and three phases (Phase-1, Phase-2 and Phase-3) were identified based on ICG dynamics in fingertips. Individual joints were graded 0–3 based on the proportional area of signal intensity (0: no signal enhancement; 1: ≦25% of affected joint area; 2: >25% and ≦50% of affected joint area; 3: >50% of the affected joint area) for the composite image and each phase. One reader (F.H.), who learned the technique directly from Dr Werner and her colleagues at RHIO Center Düsseldorf and RHIO Research Institute, Düsseldorf, Germany, graded bilateral wrists, MCP 1-5, IP, and PIP 2-5.
Figure 1. Schematic illustration of the Xiralite® device. A preformed hand rest slides into the device, where light emitting diodes (LED) and a camera (charge-coupled device) are placed overhead. Near-infrared light from LED (black arrows) excites intravenously administered indocyanine-green (ICG). The excited ICG emits fluorescence, near-infrared light of a wavelength slightly different from the excitation light (gray arrow).The fluorescence signal is detected by the camera. The emitted fluorescence signal is distinguished from the excitation light by a long-pass filter (not shown in the figure) placed in front of the camera.
2.4. Ultrasonography (US)
Ultrasonography was performed on the RA patients and healthy volunteers by one of the authors (F.H. or H.Y.). The system used was the EUB-7500 (HITACHI, Tokyo, Japan) with a 6-14 MHz linear probe. Gray scale ultrasonography (GSUS) was assessed with 14 MHz. For color Doppler ultrasonography (CDUS), the pulse repetition frequency was set at 800 Hz. The longitudinal planes of radial sides of the wrists, medial wrists, ulnar sides of the wrists and dorsal sides of the MCP 2-5, IP and PIP 2-5 were assessed. GSUS and CDUS were graded from 0 to 3 in the standard manner [Citation11]. GSUS and CDUS grades in three sides of the wrist were added up to represent the score for the whole wrist.
2.5. Magnetic resonance imaging (MRI)
Fat-saturated coronal proton-density turbo spin-echo (FS-PD-TSE), axial short tau inversion recovery (STIR), non-enhanced and enhanced T1-TSE with subtraction, coronal and axial fat-saturated post-intravenous gadolinium (Magnevist®, 0.2 ml/kg/body weight) (FS-T1-TSE) sequences of the clinically dominant hand were performed on the six RA subjects using a 1.5-tesla MRI unit (Philips Achieva, Best, the Netherlands). MRI findings (MCP 2–5 and the wrist as a whole) were assessed using the RA MRI score (RAMRIS) [Citation12–15] with consensus of two board-certified radiologists (S.S. and T.K.).
2.6. Statistical analysis
Data evaluation and analyses were performed using R version 3.0.3 software (R Development Core Team 2014, Vienna, Austria). Sensitivity and specificity of CE, US and FOI were calculated using the RAMRIS synovitis score with >0 as a reference standard in the aggregate total of 30 joints (MCP 2–5 and the wrist of the clinically dominant hand) of the six patients with RA. Ninety-five percent confidence intervals of sensitivities and specificities were calculated using the score method incorporating continuity correction to avoid inappropriate intervals [Citation16].
For calculation of sensitivity and specificity, grades of FOI and US in each joint were converted to binomial data (presence or absence). Cut-off for presence of synovitis in FOI was set as greater than 0. Two cut-off levels, >0 or >1, were used for both GSUS and CDUS.
False positive rates were calculated as percentage of positive joints in wrists, MCP 1–5, IP and PIP 2–5 of healthy volunteers.
3. Results
3.1. Patient characteristics
Characteristics of the six subjects (five females and one male; median age 59 years; age range 49–65 years) with active RA are shown in . All subjects had disease duration of at least five years. Two subjects had high disease activity and four had moderate disease activity according to the DAS28-ESR. Four subjects were being treated with methotrexate with or without other conventional synthetic DMARDs and two were on tocilizumab monotherapy. No healthy volunteers (three females; median age: 35 years; age range 28–45 years) had tender or swollen joints on CE or positive US finding from GSUS and CDUS.
Table 1. Characteristics of enrolled experimental subjects with active rheumatoid arthritis.
3.2. Sensitivity and specificity
The sensitivities and specificities of CE, US (CDUS and GSUS) and FOI (composite image and 3 phases) using a MRI synovitis score >0 as a reference standard in the 30 total joints of the six RA patients are presented in . Tenderness in CE showed high specificity and low sensitivity while swelling showed high sensitivity with low specificity. When defined by presence of tenderness, swelling or both, CE showed numerically better sensitivity but the specificity remained same as swelling only. GSUS ≥ 0 showed high sensitivity with low specificity. GSUS >1, CDUS >0, CDUS >1 and Phase-1 and-2 of FOI showed numerically higher specificities than those of CE with preserved sensitivity. Notably, Phase-1 showed the highest sensitivity among the four images (the composite image and three phases) of FOI. CI and Phase-3 of FOI were specific while their sensitivities were lowest among all of the modalities. shows an enhanced right second MCP joint in Phase-1 of FOI and corresponding images of MRI and US.
Figure 2. Representative images of FOI and corresponding images of MRI and CDUS. A composite image (A), representative images of Phase-1 (B), Phase-2 (C) and Phase-3 (D). The second MCP joint of the right hand showed grade 3 enhancement in Phase-1. The third and fourth PIP joints of the right hand and the fifth PIP joint of the left hand also showed enhancement in the composite image and all three phases. The second MCP joint of the right hand also showed enhancement in MRI (E) and positive CDUS in the thickened synovium (F, arrows). MC: metacarpal bone, PP: proximal phalanx.
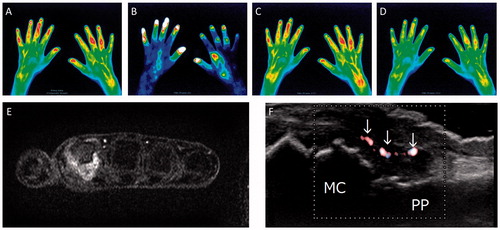
Table 2. Sensitivity and specificity of CE, US and FOI using a MRI synovitis score >0 as a reference standard in 30 joints of the clinically dominant hand of six active RA patients.
3.3. Findings of FOI in the healthy subjects
Two healthy volunteers showed negative findings with FOI (). In remaining one healthy subject, positive findings were found in the wrist and PIP joints (PIP joints were positive as fingers were diffusely enhanced ()). None of the MCP joints in three healthy volunteers showed positive findings ().
Figure 3. Images of FOI in a healthy volunteer. A composite image (A) and representative images of Phase-1 (B), Phase-2 (C) and Phase-3 (D) in a healthy volunteer. No enhancement was seen in the composite image and all three phases.
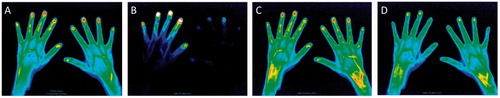
Figure 4. False positive findings in a healthy volunteer. A composite image (A) and representative images of Phase-1 (B), Phase-2 (C) and Phase-3 (D). The right wrist showed enhancement in the composite image and Phase-2. The PIP joints were also enhanced along with diffusely enhanced fingers in the composite image and Phase-2.
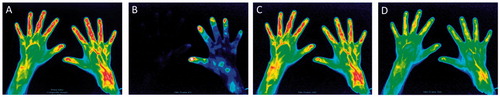
Table 3. Findings from FOI in three healthy volunteers.
3.4. Safety
None of the subjects experienced adverse events during FOI or MRI examinations.
4. Discussion
In this study, we assessed sensitivities and specificities of CE, US and FOI with 1.5-tesla MRI as a reference in the same subjects, and demonstrated that the performance profiles of Phase-1 and Phase-2 of FOI were comparable to those of GSUS or CDUS. To our knowledge, this is the first report that directly compared sensitivities and specificities of FOI, US and CE with MRI as a reference. Because MRI is generally considered as a gold standard for identifying synovitis [Citation9] and US is widely used as an on-site assessment modality of RA, the comparison is essential for evaluating the performance profile of FOI.
Werner et al. [Citation7] used a semi-quantitative method and reported sensitivities and specificities of FOI with 1.5-tesla MRI or US as a reference in patients with RA and other types of arthritides. A direct comparison of FOI and US with a single reference was not possible in their study because the MRI and US were performed in different groups of patients. Krohn et al. [Citation8] examined same subjects by FOI, US and MRI and reported sensitivity and specificity of FOI with either MRI or US as a reference. However, sensitivity and specificity of FOI were not compared with those of US using MRI as a reference. Schäfer et al. [Citation17] evaluated a method that quantifies signal intensity for each joint in RA patients. Sensitivity of 67% and specificity of 77% were reported only for FOI with MRI as a reference using a receiver operator characteristic analysis. These performance characteristics were not compared with those of other modalities using a single reference.
In the study by Werner et al. [Citation7], Phase-1 showed best specificity (94%), but low sensitivity (27%), while Phase-2 showed better sensitivity (72%) and moderate specificity (56%) with MRI as a reference. In their second report, sensitivities and specificities in patients with early and very early arthritis did not differ remarkably from the first report [Citation18]. The present study, in which Werner’s method for assessment of FOI was followed, showed better sensitivity for Phase-1 and better specificity for Phase-2 than their reports. There are three possible explanations for these differences. First, we recruited subjects with active RA who had distinct arthritis rather than intermediate or marginal one; this could result in fewer false negatives and better sensitivity. Meier et al. [Citation19] also showed better sensitivity in the subgroup of moderate-to-severely inflamed joints compared to mildly inflamed joints. Second, our study was confined to wrists and MCP 2–5 because the original RAMRIS evaluates only these joints. Exclusion of IP and PIP 2-5 joints from the calculation may explain the better sensitivity and specificity of FOI than previous studies where IP and PIP joints were included. The findings of Meier et al. [Citation19] that sensitivity and specificity differed by group of joints supports this possibility. Third, previous reports by Werner et al. [Citation7,Citation18] and Meier et al. [Citation19] enrolled patients with other types of arthritides, such as psoriatic arthritis, that have different distributions of synovitis or tendinitis from RA.
In three healthy volunteers, Phase-1 did not display false positive findings, agreeing with the findings of Werner et al. [Citation7,Citation18]. Combined with the results from active RA patients, Phase-1 appears to be the most reliable.
In our study, positive findings were found in one healthy subject whose fingers showed obviously different FOI findings than those from the other two subjects. PIP joints showed positivity as fingers were diffusely enhanced. Further investigations are needed to elucidate reasons for these false positive findings and how to discriminate from true positive findings. The higher false positive rate than found in previous studies is presumably due to the small number of healthy subjects in our study. Further, study of a larger number of healthy subjects would more precisely determine the false positive rate.
This study has some limitations. We confined our study to subjects with active RA. As an on-site modality expected to identify subclinical or residual inflammation, FOI should be tested in a more realistic clinical setting with a larger number of subjects including marginal cases. Based on the better profile of Phase-1 in this pilot study, we would like to focus on Phase-1 in future studies. There are also limitations inherent to FOI. First, FOI is unable to discriminate anatomically the source of fluorescence signals as it only visualizes signals in a single plane. The source of the signal can be structures that are more superficial than synovia. This may be one of reasons for false positive findings of FOI. Second, FOI is virtually a two-dimensional modality evaluating joints only from the dorsal side. Although near-infrared light used in the device penetrates up to 4 cm of tissue in an experimental setting [Citation20], FOI failed to detect palmar tenosynovitis in the clinical study [Citation18]. This may be a cause of disagreement with other modalities that assess joints more comprehensively. Third, unlike MRI or US, morphological changes such as bone erosion or synovial thickening are not assessable. However, this is not problematic when the focus of imaging is to assess synovial inflammation.
5. Conclusions
Fluorescence optical imaging, using a three-phase, semi-quantitative assessment, was comparable to US in patients with active RA, at least in wrists and MCP 2–5 joints. FOI has a potential as an assessment modality of RA.
Ethics approval
The study was approved by the Institutional Review Board of Tokyo Medical and Dental University Hospital (#25-14) and carried out in compliance with the ethics guidelines for clinical studies in Japan and the Helsinki Declaration (revised in 2008). Written informed consent was obtained for each subject.
Authors’ contribution
FH and MH contributed to the study design, data acquisition and interpretation, statistical analysis, drafting and revising manuscript. WYK, HY, MT and RS contributed to data acquisition and interpretation. SS and TK contributed to application of OMERACT RAMRIS system, performing and assessment of MRI. NT contributed to preforming FOI and coordination of the study. HK contributed to interpretation of data and revised the manuscript critically for important intellectual content. All authors have read and approved the final manuscript.
Acknowledgments
The authors thank Stephanie G. Werner and Hans-Eckhard Langer from the RHIO (Rheumatology, Immunology, Osteology) Center Duesseldorf and RHIO Research Institute, Duesseldorf, Germany for teaching the three-phase, semi-quantitative assessment method of FOI image sequences. We also thank Eiko Aoyagi and Marie Kokido for their cooperation in FOI procedures, and thank Marie Kokido for her assistance as a clinical research coordinator.
Disclosure statement
Xiralite® was provided by Meditec Far East Inc. for a limited term under a contract with Tokyo Medical and Dental University (TMDU). TMDU, particularly the Department of Lifetime Clinical Immunology, has received unrestricted research grants from Chugai Pharmaceutical Co., Ltd.; Ono Pharmaceuticals; Mitsubishi Tanabe Pharma Co.; UCB Japan; CSL Behring; Towa Pharmaceutical Co., Ltd.; Abbvie Japan Co., Ltd.; Japan Blood Products Organization; Ayumi Pharmaceutical Co.; and Nippon Kayaku Co., Ltd., with which TMDU currently pays the salary of FH. The author has also received speaking fees from Ono Pharmaceuticals and Astellas Pharma Inc. MH received unrestricted research grants from Abbvie Japan Co., Ltd.; Astellas Pharma Inc.; Bristol Myers Squibb K.K.; Chugai Pharmaceutical Co., Ltd.; Eisai Co., Ltd.; Mitsubishi Tanabe Pharma Co.; Ono Pharmaceuticals; Pfizer Japan Inc.; Sanofi-Aventis KK.; Santen Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; and UCB Japan. TMDU paid salary for MH, HY, MT and RS from these research grants. HY is currently an employee of Chugai Pharmaceutical Co., Ltd. Tokyo Women’s Medical University (TWMU) received unrestricted research grants from Ayumi Pharmaceutical Co.; Chugai Pharmaceutical Co., Ltd.; Eisai Co., Ltd., Nippon Kayaku Co., Ltd.; Taisho Toyama Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Co.; and Teijin Pharma Ltd, with which TWMU paid salary for MH and RS. RS received a research grant from Bristol-Myers KK. HK has received honoraria (lecture fee) from Ono Pharmaceuticals; Chugai Pharmaceutical Co., Ltd.; and Mitsubishi Tanabe Pharma Co. and has received research grants/support from Ajinomoto Pharmaceuticals Co., Ltd.; Mitsubishi Tanabe Pharma Co.; Japan Science and Technology Agency; Takeda Pharmaceutical Co., Ltd.; Bristol-Myers Squibb KK; Eisai Co., Ltd.; Ono Pharmaceuticals; Pfizer Japan Inc; Chugai Pharmaceutical Co., Ltd.; and Astellas Pharma Inc. WYK, SS, TK, NT declare that they have no financial competing interests. No non-financial competing interest exists for any of the authors.
Additional information
Funding
References
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–637.
- McQueen FM, Stewart N, Crabbe J, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis. 1998;57:350–356.
- Backhaus M, Burmester GR, Sandrock D, et al. Prospective two year follow up study comparing novel and conventional imaging procedures in patients with arthritic finger joints. Ann Rheum Dis. 2002;61:895–904.
- Dougados M, Devauchelle-Pensec V, Ferlet JF, et al. SAT0408 Both clinical and ultrasonographic evaluation of synovitis are relevant to predict subsequent radiological deterioration in rheumatoid arthritis. Ann Rheum Dis. 2013;71:610–671.
- Wakefield RJ, D'Agostino MA, Naredo E, et al. After treat-to-target: can a targeted ultrasound initiative improve RA outcomes? Ann Rheum Dis. 2012;71:799–803.
- Werner SG, Langer HE, Ohrndorf S, et al. Inflammation assessment in patients with arthritis using a novel in vivo fluorescence optical imaging technology. Ann Rheum Dis. 2012;71:504–510.
- Krohn M, Ohrndorf S, Werner SG, et al. Near-infrared fluorescence optical imaging in early rheumatoid arthritis: a comparison to magnetic resonance imaging and ultrasonography. J Rheumatol. 2015;42:1112–1118.
- Farrant JM, O'Connor PJ, Grainger AJ. Advanced imaging in rheumatoid arthritis. Part 1: synovitis. Skeletal Radiol. 2007;36:269–279.
- Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101:529–533.
- Szkudlarek M, Court-Payen M, Jacobsen S, et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48:955–962.
- Bird P, Conaghan P, Ejbjerg B, et al. The development of the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis. 2005;64:i8–i10.
- Conaghan P, Bird P, Ejbjerg B, et al. The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: the metacarpophalangeal joints. Ann Rheum Dis. 2005;64:i11–i21.
- Ejbjerg B, McQueen F, Lassere M, et al. The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: the wrist joint. Ann Rheum Dis. 2005;64:i23–i47.
- Østergaard M, Edmonds J, McQueen F, et al. An introduction to the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis. 2005;64:i3–i7.
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872.
- Schäfer VS, Hartung W, Hoffstetter P, et al. Quantitative assessment of synovitis in patients with rheumatoid arthritis using fluorescence optical imaging. Arthritis Res Ther. 2013;15:R124.
- Werner SG, Langer HE, Schott P, et al. Indocyanine green-enhanced fluorescence optical imaging in patients with early and very early arthritis: a comparative study with magnetic resonance imaging. Arthritis Rheum. 2013;65:3036–3044.
- Meier R, Thürmel K, Moog P, et al. Detection of synovitis in the hands of patients with rheumatologic disorders: diagnostic performance of optical imaging in comparison with magnetic resonance imaging. Arthritis Rheum. 2012;64:2489–2498.
- Houston JP, Thompson AB, Gurfinkel M, et al. Sensitivity and depth penetration of continuous wave versus frequency-domain photon migration near-infrared fluorescence contrast-enhanced imaging. Photochem Photobiol. 2003;77:420–430.
