Abstract
We report a case of rocuronium-induced anaphylaxis in a previously healthy 7-year-old boy. The first presenting sign of anaphylaxis was bronchospasm, appearing 11 min after he received intravenous doses of rocuronium (1 mg/kg) (Eslax®, MSD Co. Ltd., Tokyo, Japan), propofol (2 mg/kg), and cefazolin sodium (25 mg/kg). After the administration of adrenalin and ephedrine hydrochloride, bronchospasm resolved, and the vital signs became stable. Percutaneous pinning of his left humeral supracondylar fracture was performed without problems. The next day, he was successfully liberated from the ventilator support and discharged on the fifth hospital day. On the 76th postoperative day, we performed intradermal tests of rocuronium, propofol, and cefazolin. It showed that diluted rocuronium alone induced 14 mm of flare and 8 mm of wheal within 5 min, both of which disappeared within 15 min after the intradermal injection. The reaction was too quick to mention the possible contribution of rocuronium-specific IgE. His rapid reaction at the rocuronium skin test and anaphylactic reaction upon the first exposure to this drug may highlight the association of rocuronium anaphylaxis with IgE independent mast cell stimulation through mas-related G-protein coupled receptor X2 (MRGPRX2 receptor).
1. Introduction
The incidence of anaphylactic reactions during general anesthesia has been reported to be between 1/3500 and 1/20,000, variable depending on authors [Citation1,Citation2]. Among the drugs which are used for anesthesia, neuromuscular blocking agents (NMBA) are implicated as the main cause and responsible for 30–70% of perioperative anaphylactic reactions [Citation1,Citation2]. In addition, initial administration of NMBA can induce anaphylactic reaction. We experienced a case of rocuronium anaphylaxis in a 7-year-old boy during the induction of initial anesthesia.
2. Case report
A 7-year-old healthy boy weighing 19 kg injured in his left arm by a fall from a horizontal bar. The next day, he was referred to our hospital and scheduled to undergo an open reduction in combination with percutaneous pinning of his light humeral supracondylar fracture under general anesthesia. He had no previous history of general anesthesia and had no known documented allergies. Pre-operative routine laboratory tests, chest radiography, and spirography were normal.
On arrival at the operating room, he looked a little nervous but well with heart rates 107 beats/min, respiratory rates 28 breaths/min, blood pressure 103/90 mmHg, and oxygen saturation 98% in room air. General anesthesia was induced with fentanyl citrate (50 µg/h), remifentanil hydrochloride (100 µg/h), and propofol (2 mg/kg). Rocuronium (1 mg/kg) provided muscle relaxation for tracheal intubation. Anesthesia was maintained with sevoflurane in air and oxygen (end-tidal concentration 1.5%). A few minutes later, drip infusion dose of cefazolin sodium (25 mg/kg) was started.
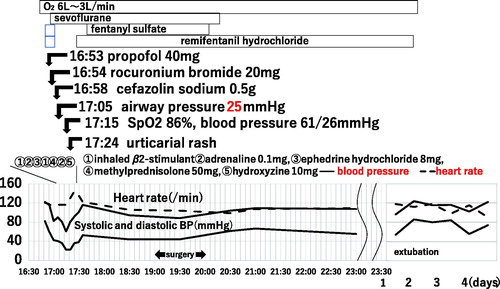
Eleven minutes after rocuronium administration, his lungs became difficult to ventilate with a bag and his airway pressure elevated to 25 mmHg. Bronchodilator procaterol hydrochloride (β2 stimulator) at 10 µg was inhalated. The patient’s lungs were hand-ventilated with 100% oxygen at 40 mmHg, and the fentanyl and remifentanil infusion were paused as the next arterial blood pressure reading was 61/26 mmHg. His oxygen saturation was noted to have decreased to 86%. Subcutaneous injection of 0.2 mg adrenaline with venous injection of 50 mg methylprednisolone was administered and signs of bronchospasm resolved. After intravenous injection of ephedrine at 0.4 mg/kg, his blood pressure normalized. By removing the sheets for surgery, urticarial eruptions on his trunk became apparent. A diagnosis of anaphylaxis was made. He received a bolus of hydroxyzine 10 mg and skin rash disappeared. Surgery was postponed about 1 h and the orthopedic surgeon and anesthetist reported the clinical course to his parent and the pediatric division. We proposed the plan to keep any pin tip over the patient’s skin (not internal) for the purpose of extracting pins without anesthesia next month. After reinitiation, surgery proceeded without incident. The next day, his pulse and blood pressure remained stable and he was successfully liberated from the ventilator support (). He was discharged home on the fifth hospital day. The orthopedist extracted all pins without anesthesia next month.
Figure 1. Clinical course. The first symptoms of bronchospasm arose at 11 min after venous injection of propofol and rocuronium, followed by cefazolin sodium 4 min later. After the administration of inhaled β2-stimulant, adrenaline, ephedrine hydrochloride, methylprednisolone, and hydroxyzine, bronchospasm disappeared and vital signs became stable.
His family was with the hope of determining which drug was involved and identifying the culprit. On the 45th postoperative day, he received peripheral blood examination, which showed IgE against latex (CAP) was negative value of ≤0.10 UA/mL. Skin test for rocuronium, propofol, and cefazolin sodium was performed 76 d after operation, when his plaster cast had been removed. The scratch test with rocuronium in stock solution indicated 14 × 11 mm of flare that appeared within 5 min and diminished within 15 min after the start of stimulation. Because the reaction was too quick to ignore the suspicion of technical errors, we proceeded to the intradermal injection test. Rocuronium, propofol, and cefazolin sodium were diluted by 1:1000 to 1:200-fold, 1:1000 to 1:10-fold and 1:100 to 1:1-fold respectively as recommended [Citation1–4]. Within 5 min after the injection, flare diameter and wheal diameter reactive to rocuronium diluted by 1:500-fold reached 14 × 13 mm and 8 × 8 mm, respectively. Both of them disappeared within 15 min. The flare diameter of physiologic saline, propofol, and cefazolin sodium was 1 × 1 mm at any step (). We identified rocuronium as the culprit.
Table 1. Skin tests.
3. Discussion
In our case, the anaphylactic bronchospasm occurred at 11 min after the venous injection of propofol and rocuronium, followed by cefazolin sodium within several minutes. Subsequent symptoms of hypotension and urticaria on his skin suggested that one of these three drugs induced anaphylaxis. Sevoflurane, fentanyl, and remifentanil were thought to be irresponsible for anaphylaxis since they were safely continued for maintenance of anesthesia afterward.
Clinical signs and symptoms of anesthetic anaphylaxis usually start about 10 min after intravenous administration of the responsible agent [Citation1]. Among anesthetic drugs, NMBA has the highest frequency of inducing perioperative anaphylaxis [Citation2]. In contrast, anaphylaxis from latex and antiseptics exhibits a more delayed onset and generally occurs during maintenance of anesthesia or recovery. β-Lactams including cefazolin sodium are able to elicit perioperative anaphylactic reactions usually in an IgE dependent manner. Since, the patient had no known allergies to antibiotics, it was difficult to suspect that he was with IgE-mediated β-lactam allergy. In patients allergic to latex, bronchospasm may also be observed early following arrival in the operating theatre. Considering that latex allergy can readily be established by quantification of specific IgE in most patients [Citation1], it was less likely that our patient was hypersensitive to latex, as IgE against latex in his peripheral blood was negative. To determine the responsible drugs, we performed skin tests 76 d after surgery, and identified rocuronium as the culprit.
NMBA-induced anaphylaxis had been thought to be associated with NMBA-specific IgE mediating degranulation of mast cells. This had been considered to be due to common substituted ammonium ions (tertiary and/or quaternary NH4+) which are found in a variety of chemicals, including cosmetics, shampoo, toothpaste, and cough suppressant pholcodine. According to this concept, a possible explanation for an IgE-mediated reaction upon first exposure to NMBA could be cross-reactivity among NMBA and these chemicals. However, it has been shown that withdrawal of one such drug pholcodine was associated with decrease of total IgE levels, supporting the idea that such a compound might be a potent ‘polysensitizer’ and may elicit NMBA sensitization by another mechanism than by sharing a common epitope [Citation5]. IgE-dependency-based theory has not been able to fully explain the high rate of anaphylactic reactions upon the first exposure as well as the high rate of cross-sensitization demonstrated by various NMBA. In addition, many rocuronium IgE positive cases have negative skin test reactivity to rocuronium [Citation6] and some of these patients can safely receive rocuronium [Citation7].
It had also been believed that positive skin test reactivity had indicated mast cell degranulation risen by specific IgE bound to mast cells. Recently, rocuronium was described to elicit hypersensitive reaction through activation of the Mas-related G-protein coupled receptor member X2 (MRGPRX2) receptor on mast cells [Citation8]. This MRGPRX2-mediated mast cell stimulation rather than IgE-mediated pathway is equipped to explain why skin tests with rocuronium may be positive in the absence of rocuronium-specific IgE [Citation9].
Additionally, an IgE-dependent reaction is associated with a slow response, leading the initial mast cell degranulation at 9 min (formation of large and heterogeneously shaped granule structures that undergo prolonged exteriorization) and highest levels of secretion about 30 min after induction of the reaction. By contrast, MRGPRX2 pathway triggers a much faster response that induces human mast cells rapidly to secrete smaller and relatively spherical granule structures within 3 min and reaches nearly maximum within 10–15 min after stimulation [Citation10]. This quick MRGPRX2 pathway mechanism theory would explain rapid onset of anaphylactic symptoms and rapid skin test reaction after induction of rocuronium in our case. In addition, although before finding of MRGPRX2, it was revealed that skin testing with NMBA in humans indicated rapid response (10–15 min) and their weal punch biopsy specimens demonstrated mast cells full of small spherical granules ongoing degranulation [Citation11]. MRGPRX2 theory is more rational concerning these facts.
Readministration of responsible drug with MRGPRX2 dependent hypersensitivity is possible with reduced speed or low doses theoretically [Citation9]. However, only a few years have passed since the identification of MRGPRX2. We should make efforts to accumulate data for IgE-independent mechanism of rocuronium anaphylaxis. In January 2018, Japan Medical Safety Research Organization announced that rocuronium had induced two out of 12 anaphylaxis deaths after injection of drugs in adult cases. Given the current situation, rocuronium is contraindicated and we should plan skin tests and if possible, in combination with basophil activation test to several NMBA [Citation12] before an elective surgery in our case. Additionally, under the necessity of using propofol and cefazolin sodium, we need to consider indication, drip speed, and doses. For the prevention of rocuronium anaphylaxis, we expect the development of agents to target a rocuronium-MRGPRX2 pathway and evaluations of ketotifen fumarate that inhibits a mouse orthologue of MRGPRX2 stimulation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ebo DG, Fisher MM, Hagendorens MM, et al. Anaphylaxis during anaesthesia: diagnostic approach. Allergy. 2007;62:471–487.
- Masuda K. Anaphylaxis to neuromuscular blocking agents. Clin Dermatol. 2016;70:161–163.
- Hagau N, Gherman N, Cocis M, et al. Antibiotic-induced immediate type hypersensitivity is a risk factor for positive allergy skin tests for neuromuscular blocking agents. Allergol Int. 2016;65:52–55.
- Mitsuhata Y. Anaphylaxis during general anesthesia. J Jpn Soc Clin Anesth. 2012;32:479–487.
- Florvaag E, Johansson SG, Oman H, et al. Pholcodine stimulates a dramatic increase of IgE in IgE-sensitized individuals: a pilot study. Allergy. 2006;61:49–55.
- Aalberse RC, Kleine Budde I, Mulder M, et al. Differentiating the cellular and humoral components of neuromuscular blocking agent-induced anaphylactic reactions in patients undergoing anaesthesia. Br J Anaesth. 2011;106:665–674.
- Leysen J, Uyttebroek A, Sabato V, et al. Predictive value of allergy tests for neuromuscular blocking agents: tackling an unmet need. Clin Exp Allergy. 2014;44:1069–1075.
- McNeil BD, Pundir P, Meeker S, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–241.
- Spoerl D. Reclassifying anaphylaxis to neuromuscular blocking agents based on the presumed patho-mechanism: IgE-mediated, pharmacological adverse reaction or “innate hypersensitivity”? Int J Mol Sci. 2017;18:1223.
- Gaudenzio N, Sibilano R, Marichal T, et al. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest. 2016;126:3981–3998.
- Levy JH, Gottge M, Szlam F, et al. Weal and flare responses to intradermal rocuronium and cisatracurium in humans. Br J Anaesth. 2000;85:844–849.
- Decuyper II, Mangodt EA, Van Gasse AL, et al. In vitro diagnosis of immediate drug hypersensitivity Anno 2017: potentials and limitations. Drugs R D. 2017;17:265–278.
