ABSTRACT
Biological deterioration is a significant threat to wooden cultural heritage in polar regions. Based on a comprehensive review of previous and current research, we provide an overview of current knowledge of wood-decaying fungi and fungal decay in historic wooden structures in Antarctica and the High Arctic. Most available research focuses on degradation mechanisms, providing data on microbial biodiversity and factors influencing deterioration of the historic polar heritage. Less is reported on decay rates and consequences for cultural heritage, such as the type and severity of damage, causes, repair, and conservation methods. The effects of climate change improve conditions for fungal decay, and thus more severe damage must be expected. Consequently, further research and development should concentrate on these challenges to promote conservation of polar cultural heritage and sustainable heritage management. Greater cooperation between researchers across disciplines in polar regions may be of key importance to improve insights and treatments for polar wooden heritage.
Introduction
Polar cultural heritage is largely a wooden heritage as wood has been a primary building material throughout the Antarctic and High Arctic (see , and ). A long-held assumption has been that fungal decay in wooden structures in polar regions is uncommon due to the cold and dry climate (Jennings and Rayner Citation1984; Rayner and Boddy Citation1988). Consequently, until the early 2000s, fungal decay was not considered a serious threat to cultural heritage in the Antarctic and High Arctic.
Figure 1. A trapper’s cabin built in 1904 by Norwegian trappers at Kapp Lee, Edge Island in Svalbard. The picture was taken prior to restoration of the hut in 2009 by the Governor of Svalbard.

Figure 2. An emergency radio shack at Haudegen in Nordaustlandet, Svalbard. This was a manned German meteorological station built in 1944 during World War II.

Figure 3. Historic sites and monuments listed in the Scientific Committee on Antarctic Research (SCAR) database (ADD Citation2021) and historic stations (Headland Citation2009) in Antarctica. Map by Alma Thuestad, reproduced with permission.
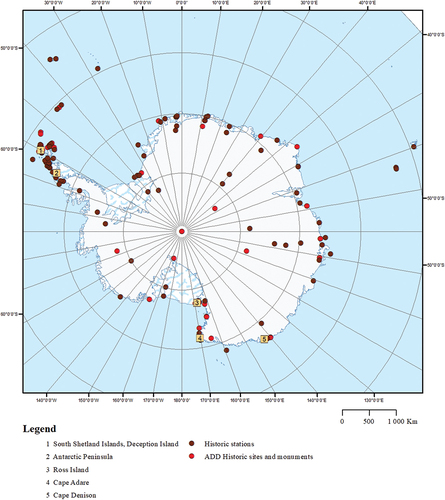
Figure 4. Cultural heritage sites in Svalbard listed in the Norwegian cultural heritage database, Askeladden. Map by Alma Thuestad, reproduced with permission.
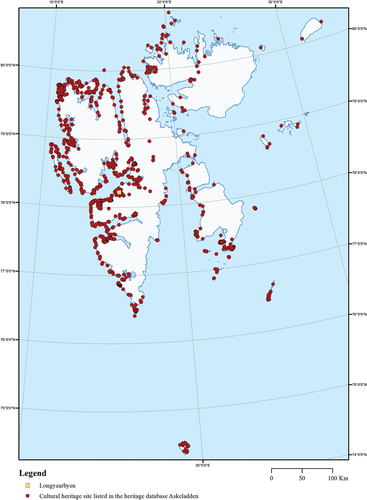
Figure 5. Men and dogs on the roof of a cabin at Camp Ridley at Cape Adare photographed by Carsten Borchgrevink, the leader of the 1898–1900 expedition which was based in this cabin.

Figure 6. The Carsten Borchgrevink cabin at Camp Ridley photographed in 2016–2017.

Despite the cold and dry climate, Mattsson and Flyen (Citation2011) found extensive fungal decay in wooden structures in the High Arctic Svalbard. The iconic cable car bucks in Longyearbyen (see and ), a highly visible remainder of the town's coal mining history, show how fungal decay can cause severe damage to cultural heritage (Flyen Citation2013). Held and Blanchette (Citation2017) and Held, Arenz and Blanchette (Citation2011) found extensive fungal decay in historic buildings on Deception Island in Antarctica’s South Shetland Islands. Blanchette et al. (Citation2021), Pedersen et al. (Citation2020), Matthiesen et al. (Citation2014) and Jurgens, Blanchette, and Filley (Citation2009) showed that wooden heritage in Greenland and the Canadian High Arctic is affected by enhanced microbial degradation due to climate change. Fungal decay is a proven threat to wooden heritage in the High Arctic and the Antarctic. It is recognised as a key transformative process underlying both current and expected future conditions of wooden heritage. Fungal decay is thus a significant challenge for polar cultural heritage conservation and management.
We provide an overview of current knowledge and understanding of wood-decaying fungi and fungal decay in historic wooden structures in polar regions. The questions we seek to answer are as follows: (1) Which wood-decaying fungal species have been identified in polar wooden heritage? (2) Which methods are used to detect and identify species of decay fungi? (3) What damage do wood-decaying fungi cause in wooden heritage? and (4) Which repair methods and precautionary measures are applied? The review encompasses both the High Arctic and the Antarctic as wooden cultural heritage is found throughout (see , and ). Challenges linked to environmental conditions, climate change, and other impact factors are also comparable. On this basis, we will identify some knowledge-gaps and directions for further research efforts.
Figure 7. Stranded boat in Grytviken on South Georgia. This Norwegian whaling station was established in 1904 and in operation until 1966 when the whale stocks became too low to continue exploitation.

Figure 8. Detail of stranded boat in Grytviken on South Georgia affected by fungal decay.

Figure 9. The remains of the magistrate’s Villa in Whalers Bay on Deception Island. The Norwegian whaling station dating to 1906–1931 constitutes some of the most significant whaling remains in Antarctica. Base B, a British meteorology and geology station (1944–1969) reused this building. The site is designated as Historic Site No. 71 under the Antarctic Treaty.

Wooden Heritage in Polar Regions
The Arctic Council divides Arctic cultural heritage into two main categories: (1) indigenous heritage and (2) heritage originating in cultures further south, usually individuals or smaller groups engaged in natural resource exploitation, such as hunting, trapping, fishing, whaling, and mining, but also exploration, research, and social work (”Assessment of Cultural Heritage Monuments and Sites in the Arctic” Citation2013). Antarctica’s cultural heritage is solely derived from exploration, research, and resource exploitation.
A comprehensive overview of cultural heritage throughout polar regions is challenging due to differences in methodology, heritage definition, and access to information (Barr Citation2019). However, for some areas, such as Svalbard and Antarctica, comprehensive records are available covering prehistoric sites, historic structures, and buildings still in use as well as a wide range of ruins and abandoned structures.
Human history in Antarctica is short, but intense. James Cook’s explorations in 1773 can be considered a starting point even though he did not go ashore. The earliest known sites are sealing sites from the 1820s on the South Shetlands (Pearson and Stehberg Citation2006). Between 1897 and 1922, interest in Antarctica and Antarctic research was on the rise, and scientific expeditions were sent out. This era, known as the Heroic Age of Antarctic exploration, is recognised as having pre-eminent historical significance (Senatore Citation2019; Headland Citation1989). Heritage sites are mostly reminiscent of scientific exploration, but also include remnants of whaling. Later research efforts have to a lesser extent left permanent structures except for national stations which are not considered historic.
Decisions on listing and protecting Antarctic cultural heritage are made according to a process established in 1972 by the Antarctic Treaty Consultative Meeting (ATCM) (”The Antarctic Treaty” Citation1959). Currently, there are 95 heritage sites on the official list of Historic Sites and Monuments (HSMs) (”Area Protection and Management/Historic Sites and Monuments” Citation2022; “List of Historic Sites and Monuments approved by the ATCM” Citation2013). Of these, around 30 sites encompass wooden structures. Buildings and on-site infrastructure are the predominant physical heritage. This holds true not just for the expeditions and scientific activity, but also the whaling which went on from the early twentieth century until the 1960s. Remains of whaling stations and other infrastructure are found in South Georgia, the South Orkney Islands, and the Antarctic Peninsula (Hacquebord and Avango Citation2016). Apart from buildings and structures, other cultural heritage in Antarctica consists of graves and memorials.
Any trace of human activity predating 1946 is protected as cultural heritage in Svalbard (“Svalbard Environmental Protection Act”, § 39). The Norwegian National Cultural Heritage Database lists 2191 mapped sites in the Svalbard Archipelago and 55 on the island Jan Mayen (“Askeladden” Citation2020). Predominant heritage structures are buildings and on-site infrastructure reminiscent of resource exploitation, scientific exploration, and adventure expeditions. The earliest traces of human activity are associated with extensive whaling activities in the waters around the archipelago (from around 1600 to about 1750) (Arlov Citation2003). The 50 whaling stations and numerous graveyards encompassing around 760 graves are widely known (Sandodden Citation2013). Cabins and traps left behind by the Russian (Pomor) (1700–1850) and Norwegian hunting and trapping (from around 1800 until present day) are common. There are also a multitude of traces of mineral exploitation ongoing since the early 1900s. Remains of scientific exploration and adventure expeditions (from around 1760 until the present day) are found at 113 sites. Some of the best-known are reminiscent of various attempts to reach the North Pole by air (“Askeladden” Citation2020).
Bibliographic Review – Method and Data
This paper reviews literature on fungal decay affecting wooden historic structures located in Antarctic and High Arctic regions. The approach is essentially a bibliographic review of literature across multiple disciplines (meta-analysis). The review mainly encompasses scientific literature published in English, but also some so-called ‘grey literature’ of which literature on Svalbard is mainly in Norwegian. Svalbard is a key area of interest as the archipelago has a rich concentration of heritage sites.
A targeted search of Google Scholar and Web of Science was used to identify relevant literature. The ICOMOS Open Archive, the digital and physical archives of the Svalbard Environmental Fund, and the Norwegian Directorate for Cultural Heritage were searched according to the same parameters. A set of criteria or ‘key words’ (see ) was used to facilitate the search and secure the selection of thematically relevant literature on decay fungi in wooden heritage. Several key words and combinations of key words were tested to ensure that the searches yielded relevant literature and concurrently excluded irrelevant literature. Google Scholar and Web of Science were searched using both English and Norwegian key words. The same Norwegian key words were used for searching Norwegian sources.
Table 1. Combinations of key words used to search for relevant literature and results.
The results were examined for relevance based on title and abstract. Papers and reports thought relevant were read in their entirety. Key findings from the selected literature were extracted, compiled, and compared in order to summarise current knowledge regarding advances and discoveries of decay fungi and consequences for Antarctic and High Arctic wooden heritage.
Results and Findings
Literature on Fungal Decay in Antarctic and High Arctic Wooden Heritage
The initial searches using Google Scholar yielded both relevant and irrelevant publications on fungi in Antarctic and High Arctic wooden heritage. As shown in , the first search yielded over 9000 hits.
Google Scholar yielded the highest number of potentially relevant publications. After closer examination, a total of 62 and 12 publications focused on fungal decay in Antarctic and High Arctic wooden heritage were found through Google Scholar and Web of Science, respectively (see ). When looking closer at relevance, 3.9% of the potentially relevant publications from the final search of Google Scholar were actually relevant, while the corresponding percentage for Web of Science was 13.1%. Eighty percent of the hits using Norwegian key words when searching Google Scholar resulted in relevant publications, while the corresponding percentage for Web of Science was zero.
Table 2. Relevant publications on fungal decay in polar wooden heritage located through Google Scholar and Web of Science.
The Google Scholar search encompassed all relevant publications identified through the Web of Science. In addition, Google Scholar encompassed several Norwegian publications. The ICOMOS Open Archive mostly holds books, of which few were found relevant (see ). Twelve of 18 publications were also listed on Google Scholar, including all the publications found to be relevant. The search at the Svalbard Environmental Protection Fund revealed several relevant reports (see ). Publications located at the Norwegian Directorate for Cultural Heritage mainly encompass guides and instruction books on maintaining sites and buildings (see ). These reports, guides, and instruction books are, with very few exceptions, in Norwegian and would be considered grey literature.
Table 3. Relevant publications on fungal decay in polar wooden heritage located through ICOMOS open archive.
Table 4. Relevant publications on fungal decay in polar wooden heritage located in the digital archives of the Svalbard environmental protection fund and the Norwegian directorate for cultural heritage.
Research on Biodeterioration of Wooden Heritage
Biodeterioration is defined by Allsopp as ‘any undesirable change in a material brought about by the vital activities of organisms’ (Allsopp Citation2011, 151). Bacteria, archaea, fungi, lichens, and insects are generally a major problem for preservation of cultural heritage due to their biological degradation potential (Sterflinger and Pinar Citation2013), but in Antarctica and the High Arctic wood-decaying insects are not a known issue. Of the over 250 species of insects recorded in Svalbard (Coulson Citation2015), none are wood-decaying. Mould is a known wood-decaying mechanism; however, mould fungi do not cause severe damage to buildings and other structures (Mattsson et al. Citation2014).
The number of known fungal species worldwide is uncertain. Current estimates range from 1.5 to 12 million (Bhunjun, Niskanen, and Suwannarach Citation2022; Wu et al. Citation2019; Hawksworth et al. Citation2017). Approximately 150,000 species are named and classified (Bhunjun, Niskanen, and Suwannarach Citation2022). A wood-decaying fungus is any species of fungus able to utilise wood as a substrate, gradually depolymerising the wood resulting in strength loss, i.e. causing it to rot. Wood decay is classified as either white rot, brown rot, or soft rot (Goodell, Qian, and Jellison Citation2008), which often co-exist in nature. The decay mechanisms used for brown, white, and soft rot differ (Blanchette Citation2000). According to Otjen et al. (Citation1987), white rot fungi can be divided into simultaneous/non-selective white rot which cause the removal of cellulose and lignin, and selective white rot which cause the removal of lignin while leaving quantities of cellulose intact. Selective and non-selective white rot species both degrade components in cell walls, resulting in soft and stringy white or yellow looking wood. White rot mostly affects deciduous tree species but can also be found in coniferous woods. Brown rot also degrades cell walls, but leaves lignin largely intact, causing wood to shrink, cracking into cubical pieces and showing brown discolorations as it dries. Brown rot mostly occurs in conifers. Soft rot fungi can be found in wet environments, but also in areas with reduced amounts of moisture and in extreme environments. In wet environments, soft rot fungi leave the wood surface soft and easy to pick off. When dehydrating, the surface layers shrink, splitting the wood into small square blocks which often look like the effects of brown rot; however, the decay process is different and does not weaken the wood significantly until advanced stages of decay. In general, white and brown rot are classified in the Basidiomycota and soft rot is in the Ascomycota. Brown rot degradation is normally considered more problematic for wooden structural elements than white rot because of the way these fungi attack wood cell walls and promote rapid strength loss (Arantes and Goodell Citation2014). Brown rot fungi commonly affect wooden structures in temperate climates (Held, Arenz and Blanchette Citation2011).
Correct identification of the fungal organism at work is important as not all fungi are equally destructive (Singh Citation2000). The type of decay caused by fungi may be differentiated by microscopy and sequencing of DNA. Riley et al. (Citation2014) and Schilling et al. (Citation2020) indicate that the prevailing paradigm of classifying fungal decay caused by white or brown rot does not capture the diversity of fungal decay mechanisms. This review does not consider these questions, instead focusing on traditional characterisation when classifying rot fungus (i.e. brown rot, white rot, and soft rot).
Fungal organisms have some minimal requirements for growth, mainly sufficient moisture, and favourable temperature (Domsch, Gams, and Anderson Citation1980). Consequently, the extent of biodeterioration was long expected to decrease in colder climates. There has thus far been a long-held and widespread belief that biodeterioration is absent or minimal in Antarctica and the High Arctic. Little was reported on wood-decaying fungi in extreme cold climates until the early 2000s (Blanchette, Held, and Jurgens Citation2008; Frisvad Citation2008).
Decay Fungi in the Antarctic
Around 30 of the listed Antarctic heritage sites contain wooden buildings, structures, or objects. Several sites have been subject to thorough investigations focusing on fungi, particularly the appearance and diversity of wood-decaying fungi in such environments. A review of early Antarctic microbiological studies undertaken by Fogg (Citation1992) includes the Deutsche Sudpolar-Expedition (1901–1903), Charcot’s two French expeditions (1904–1907, 1908–1910), and Nordenskjold’s Swedish Antarctic Expedition (1901–1904). Kerry (Citation1990) listed fungal species with the potential to degrade wood, concluding that at least one species, Phialophora fastigiata, now called Cadophora fastigiata, survives on wood. Despite these findings, there was still a widespread belief that biodeterioration was absent or minimal in Antarctica (Hughes Citation2011). Research providing detailed information on biodeterioration, such as Duncan (Citation2007), include measurements of growth requirements and cold tolerances. Evidence of significant deterioration of wooden structures is provided in Held, Arenz and Blanchette (Citation2011), Arenz and Blanchette (Citation2009), Arenz and Blanchette (Citation2008), Held et al. (Citation2006), Blanchette et al. (Citation2004) and Farrell et al. (Citation2004). This research mainly pertains to land-based sites, but Björdal and Dayton (Citation2020) identified soft rot fungi and tunnelling bacteria in wood exposed to long-term anthropogenic activity collected from the seafloor close to McMurdo station. Having demonstrated the presence of wood-decaying fungi, evidence of adaptation to the extreme cold environment followed (Björdal and Dayton Citation2020; Duarte et al. Citation2018; Godinho et al. Citation2015; Henriquez et al. Citation2014; Godinho et al. Citation2013). Blanchette et al. (Citation2004) found strong evidence of seasonal growth ability in Antarctica. According to Duncan (Citation2007), fungi frozen during parts of the year go dormant and are viable upon thawing and able to re-establish each summer.
Reviewing the numerous research publications, it is evident that the research primarily involves fungal diversity and ecology (Gaiser et al. Citation2021; Held and Blanchette Citation2017; Farrell et al. Citation2011; Blanchette et al. Citation2010; Farrell et al. Citation2008; Duncan et al. Citation2006; Held et al. Citation2005) or soil fungal species (Albores et al. Citation2018; Arenz, Blanchette, and Farrell Citation2014; Arenz and Blanchette Citation2011; Arenz et al. Citation2011; Connell et al. Citation2010; Arenz and Blanchette Citation2009; Connell et al. Citation2008; Arenz et al. Citation2006). Research on biodeterioration has largely focussed on microlevel biology, species identification of fungus, and baseline data describing microbial diversity (Gaiser et al. Citation2021; Albores et al. Citation2018; Arenz, Blanchette, and Farrell Citation2014; Arenz and Blanchette Citation2011; Arenz et al. Citation2011; Farrell et al. Citation2011; Blanchette et al. Citation2010; Duncan et al. Citation2010; Duncan Citation2007; Arenz et al. Citation2006; Held et al. Citation2006; Blanchette et al. Citation2004, Citation2004; Blanchette and Charola Citation2003; Blanchette, Held, and Farrel Citation2002).
The most common methodology for species detection and identification (and as a result of the species identification, implicitly also type of decay) has been classic microbiology aimed at isolating monocultures of viable organisms, culture-independent DNA analyses, and electron microscopy utilising samples from wooden structures and artefacts as well as samples of sterile wood and cellulose, with and without nutrients added, left on-site, and later removed and evaluated for fungal colonisation (Held and Blanchette Citation2017; Arenz et al. Citation2011; Farrell et al. Citation2011). As early as 2004, molecular biology techniques were applied to improve the identification of microbial species, resulting in the identification of several previously unknown species, many unique to Antarctica (Blanchette et al. Citation2004).
Biodeterioration of wood in Antarctica is caused by a few species of soft rot fungi (Arenz and Blanchette Citation2009; Arenz et al. Citation2006; Blanchette et al. Citation2004). The commonly reported Cadophora species (Gaiser et al. Citation2021; Rusman et al. Citation2018; Blanchette et al. Citation2010; Arenz and Blanchette Citation2009) is a proven cause of soft rot decay. Key species of concern are Cadophora malorum, Cadophora luteo-olivacea, Cadophora fastigata, and some previously undescribed Cadophora species which are widespread. These have been found to infest soils and buildings at Ross Island sites (Arenz et al. Citation2006) and Antarctic Peninsula sites (Arenz and Blanchette Citation2009). These fungi are widely found in wood in historic huts and have the capacity to cause extensive soft rot if conditions conducive to such decay are present. Soft rot commonly occurs in wood exposed to extreme and adverse environmental conditions that inhibit other types of fungi from being established (Blanchette et al. Citation2004).
In addition to the major group of wood decaying fungi found throughout Antarctica, brown rot fungi and filamentous basidiomycetes (Hypochniciellum spp. and Pholiota spp.) have been identified on Deception Island, an active volcanic island in the South Shetland Islands off the Antarctic Peninsula (Held and Blanchette Citation2017). This area has a warmer and more humid climate than continental Antarctica, thus allowing for soils to support more abundant and diverse fungal communities (Arenz, Blanchette, and Farrell Citation2014). Lawley et al. (Citation2004) report sites with three to four times higher fungal diversity than more southerly continental sites. Blanchette et al. (Citation2010) and Arenz and Blanchette (Citation2009) investigated filamentous fungi isolated in samples from coastal areas and historic expedition huts in the Ross Sea, historic structures on Deception Island, and the Antarctic Peninsula. They identified the genus Cadophora as one of the most abundant fungal groups, comprising more than 30% of culturable fungi at some locations.
Blanchette et al. (Citation2004) concluded that the early explorers probably did not bring along the soft rot fungi found to occur in historic huts. They based this conclusion on the fact that although the Cadophora species occur in temperate regions, they are uncommon and rarely found in wood used as building materials. The great diversity of Cadophora species, including several undescribed species, found in historic wood, and their presence in soils in remote areas with little human interference, as well as on dead moss and lichen thalli, strongly suggest that these fungi are endemic to Antarctica (Farrell et al. Citation2011; Blanchette et al. Citation2010; Duncan et al. Citation2008; Arenz et al. Citation2006; Blanchette et al. Citation2004; Farrell et al. Citation2004). However, there is evidence of fungal species introduced to Antarctica along with wood, including driftwood, and other organic artefacts the explorers brought with them and that later were spread by windblown debris and foot traffic or through infection from contaminated soil (Held and Blanchette Citation2017; Held, Arenz and Blanchette Citation2011; Arenz et al. Citation2011; Kerry Citation1990; Line Citation1988).
Fungi-Induced Damage to Wooden Heritage in the Antarctic
Fungi are notorious contaminants in areas with human activities such as the historic sites (Arenz, Blanchette, and Farrell Citation2014). Studies have shown that significant deterioration affecting Antarctica’s historic buildings takes place over time, albeit at a slow rate (Held and Blanchette Citation2017; Held, Arenz and Blanchette Citation2011, Citation2009; Held et al. Citation2005; Blanchette et al. Citation2004). According to Hughes (Citation2011), the most serious biodeterioration risks are decay of structural elements in direct contact with soil, under the floor, inside walls where ventilation is reduced, where water leakage occurs, and where temperatures are higher. Duncan (Citation2007) has shown that once a fungal attack is visible on the surface, damage within the timber is substantial and the structure is already weakened.
Moisture content is considered to be the most critical ‘limiting’ factor for fungal decay. Consequently, many studies have used moisture as an indicator of fungal growth ability (Brischke and Alfredsen Citation2020). Duncan (Citation2007), Held et al. (Citation2005), Mason (Citation1999), and Blunt (Citation1991) measured moisture content at the three Ross Island huts and Cape Denison, respectively. At all sites, in all buildings, and in many locations within the buildings, the moisture content was found sufficient (>20%) to support biodeterioration. Significant damage has also been reported at Antarctic Peninsula sites. Duncan (Citation2007) concludes that main factors affecting deterioration were related to structural design factors allowing moisture accumulation. Arenz and Blanchette (Citation2009) highlighted the importance of roof condition to prevent water ingress.
Held and Blanchette (Citation2017) documented two sites on Deception Island with buildings from 1955, where most of the wood in contact with soil was affected by fungi, leaving the buildings severely damaged. They underlined the favourable conditions caused by geothermal heating which allow the survival of diverse fungal species and consequently more severe damage to wooden heritage. Comparatively, Blanchette et al. (Citation2004) documented the presence of different Cadophora species in the Ross Island huts. However, detecting only limited decay at those sites, Blanchette et al. (Citation2004) conclude that there is no immediate threat to the huts.
Decay Fungi in the High Arctic
Looking at the High Arctic in general, the amount of research on fungi, fungal decay, and wooden heritage is limited. Research efforts have focussed on specific areas, such as Svalbard, Greenland, and Arctic Canada.
Biodeterioration in Svalbard was initially believed to follow the same pattern as in Antarctica, namely, a restricted decay caused by soft rot fungi of wood in direct soil contact (Mattsson and Flyen Citation2008). Research showed, however, that the decay of Svalbard’s wooden heritage is dominated by brown rot fungus (Mattsson, Flyen, and Nunez Citation2010; Mattsson and Flyen Citation2008). Leucogyrophana mollis was the dominant brown rot fungus in the investigated material, but a few other brown rot species were detected, as was evidence of soft rot decay. In 54 wooden samples, Leucogyrophana mollis were found in 34, Coniophora puteana in 2, and 1 species in the Corticiaceae family was identified (Mattsson and Flyen Citation2008). An earlier investigation of building materials revealed the occurrence of Antrodia serialis, Columnocystis abietina, Cylindrobasidium evolens, Dacryobolus sudans, Dacrymyces stillatus, Ditiola radicata, Gloeophyllum sepiarium, Hyphoderma setigerum, Sistotrema coroniferum, and Sterum sanguinolentum (Gulden and Torkelsen Citation1996), which include both brown and white rot fungi. Gulden and Torkelsen (Citation1996) focused on species identification. Research also encompasses materials from non-manmade structures. Kosonen and Huhtinen (Citation2008) found 24 species of wood-decaying basidiomycetes in 115 wooden samples from driftwood, of which the 5 most abundant species were brown rot fungi. At Jan Mayen, which has a somewhat similar nature and climate as Svalbard, Ryvarden and Høiland (Citation2009) found three species of Corticiaceae in driftwood. Driftwood can contribute to spread fungal spores and introduce new species.
Microbial degradation processes have been documented in Greenland (Hollesen, Matthiesen, and Elberling Citation2017; Hollesen et al. Citation2016, Citation2015; Matthiesen et al. Citation2014). Pedersen et al. (Citation2020) found extensive soft rot decay caused by Ascomycota fungi on wooden artefacts from archaeological sites in western Greenland. Although Basidiomycota fungi were found throughout many sites, regardless of climate and local environment, the actual decay at some sites was inhibited due to unfavourable growth conditions. In western Greenland, Pedersen et al. (Citation2020) used DNA sequencing which revealed a diverse group of Ascomycota as the dominant fungal type. Soft rot was found at archaeological sites in both buried and exposed wood. Cadophora species were the most common. Other Ascomycota fungi known to cause soft rot were also found, including Chaetomium, Cladosporium, Lecythophora, Leptodontidium, and Phialophora. Pedersen et al. (Citation2020) found a limited number of filamentous Basidiomycota causing white or brown rot, of which most were found only once, including Tremellales, Hohenbuehelia, Lentinellus, Dacrymyces, and an unknown Basidiomycota species. In a study of driftwood in Iceland, Greenland, and Siberia, Blanchette et al. (Citation2016) found Cadophora species to be the most frequent Ascomycota with soft rot as the most prevalent form of decay. Few Basidiomycota were found.
Blanchette, Held, and Jurgens (Citation2008) and Blanchette et al. (Citation2021) found no brown rot when investigating biodeterioration of wooden huts in the Canadian High Arctic, but soft rot fungi were prevalent. They also identified environmental factors and approaches to reduce future damage. However, Jurgens, Blanchette, and Filley (Citation2009) and Blanchette, Held, and Jurgens (Citation2008) reported finding Cadophora malorum, C. luteo-olivacea, C. fastigata and some previously undescribed Cadophora species. Blanchette et al. (Citation2021) reported finding species belonging to the genera Coniochaeta, Phoma, Cadophora, Graphium, and Penicillium and many other Ascomycota causing soft rot in wood in the Peary Huts located at Fort Conger in the Canadian High Arctic.
Figure 11. Microclimatic zones exemplified in-situ in a heritage trapper’s cabin in Kobbefjorden, Danes Island, Spitsbergen in Svalbard: (1) Permafrost area; freezing temperatures, no free water; (2) Thawing zone; low temperature, high water content; (3) Soil contact, relative humidity (RH); moderate temperature, high water content; (4) RH, precipitation; high temperature, high water content; and (5) RH, leakages; moderate temperature, high water content.

Fungi-Induced Damage to Wooden Heritage in the High Arctic
Flyen, Flyen, and Mattsson (Citation2020) consider biodeterioration caused by fungal decay as the most important factor causing deterioration of wooden heritage in Svalbard. Research shows that most historic wooden structures are affected and, furthermore, that many are heavily decayed (Mattsson et al. Citation2014; Mattsson and Flyen Citation2014, Citation2012, Citation2011, Citation2008; Flyen and Mattsson Citation2010; Mattsson, Flyen, and Nunez Citation2010). The main research focus has been on how fungal species appear and behave in wooden material and the types of damage affecting wooden heritage.
Wooden structures are often in direct contact with soil and exposed to constant moisture exposure when temperatures are above 0° Celsius. Mattsson and Flyen (Citation2011) describe areas vulnerable to biodeterioration, defining five main zones of risk based on the microclimate in structures. As ecological conditions favourable for fungal growth vary within a wooden structure, the microclimate is important for determining whether wood-decaying fungi will survive and their growth rate (Grobakken, Mattsson, and Alfredsen Citation2014; Flyen and Mattsson Citation2010). As shown in , in buildings and partially collapsed buildings, panel and load-bearing elements situated in microclimatic zone 2 are often heavily decayed, as are the floors which are often constructed directly on the ground (microclimatic zone 2), and the roof, especially where parts are open or collapsed (microclimatic zone 5) (Mattsson and Flyen Citation2011). Foundations are often composed of wooden poles penetrating the permafrost. These poles exhibit a specific pattern of fungal decay, starting in the core, spreading out in an area 20–30 centimetres below and above ground. Heavily degraded poles only hold a thin surface of unaffected wood. At Svalbard, these damages occur in building foundations and cable car poles (see and ). In quite a few cases the damage is not outwardly obvious as heavily decayed wood is found under the surface of what appears to be unharmed wood (Mattsson and Flyen Citation2014; Stokland Citation2012) (see ). Each construction has an individual decay pattern, but common to all is that most damages are developed over years and that the most severe damages are situated in contact with and close to the soil. In Alaska, Mills (Citation2011) describes heavily decayed wood located in the soil-covered lower parts of timber buildings. The lowermost rafters were replaced with new timber which was treated with preservatives to prevent future fungal decay and insect infestation.
Barr (Citation1995) provided brief descriptions of deterioration at sites in Russia, such as the Nansen shelter at Franz Josef Land, where the walrus hide roof is rotting and wooden elements are scattered around. Research by Kravchenko, who found the remains of Barents’ ship in Novaya Zemlya, is discussed in Braat (Citation1984). Barents’ hut has been reported to be in poor condition (Hacquebord Citation1991) due to a lack of maintenance. It was not possible to find research on fungi in Russian wooden heritage published in English.
A range of factors working together contribute to degradation of wooden heritage. Indirect degrading parameters favour conditions for biological degradation, especially those related to increasing temperatures and the number of days above 0° Celsius. These conditions increase the amount of liquid water, thawing permafrost and an increased active layer, which is the upper part of the permafrost that thaws during the summer. An increase in active soil layer depths is significant because it exposes previously frozen soil layers to increased microbial activity (Flyen, Flyen, and Mattsson Citation2020; Hollesen et al. Citation2018; Hollesen, Matthiesen, and Elberling Citation2017; Mattsson Citation2017; Mattsson, Flyen, and Nunez Citation2010), thus influencing rates of decay (Mattsson and Flyen Citation2008). Wooden structures that penetrate the active layer hold the most serious damage within the structural elements embedded in the active layer (Flyen, Flyen, and Mattsson Citation2020; Flyen and Mattsson Citation2017; Mattsson, Flyen, and Nunez Citation2010). Permafrost preserves wood as cold temperatures and high saturation levels slow the decay of organic materials (Flyen, Flyen, and Mattsson Citation2020; Hollesen et al. Citation2018; Hollesen, Matthiesen, and Elberling Citation2017; Mattsson Citation2017; Mattsson, Flyen, and Nunez Citation2010) (see and ). Model predictions and on-site research show that a warmer climate will affect both the spatial extent of the permafrost and the depth of the active layer (Farquharson et al. Citation2019; Boike et al. Citation2018; Hollesen et al. Citation2015; Slater and Lawrence Citation2013; Etzemuller et al. Citation2011; Zhang, Chen, and Riseborough Citation2008).
Figure 12. The iconic cable car bucks in Longyearbyen, Svalbard were originally used to transport coal from the mines to the harbour. Longyearbyen, Svalbard’s largest settlement, was dominated by coal mining from around 1900 until the late 1990s.

Management of Polar Wooden Heritage
Cultural heritage laws and regulations vary throughout polar regions, as do management regimes and conservation practices. Antarctica’s cultural heritage is managed in accordance with the environmental protocol under the Antarctic Treaty (”The Protocol on Environmental Protection to the Antarctic Treaty” Citation1991) that sets the framework for management of a continent in which seven countries have active territorial claims, suspended under the Treaty. Fifty-four countries have acceded to the Treaty. Some countries, such as New Zealand and the United Kingdom, have a designated Antarctic Heritage Trust. At Australian Antarctic historic sites, heritage work is done by the National Antarctic Science Institution and the Mawson’s Hut Foundation (”Australian Antarctic Program” Citation2021). All conservation work is undertaken under the direction of the Australian Antarctic Division (AAD), a part of the Australian Government’s Department of Climate Change, Energy, the Environment, and Water. AAD leads the Australian Antarctic Program and administers all research and expedition activities within the Australian Antarctic Territory, including the sub-Antarctic islands.
The High Arctic is the domain of Russia, Denmark/Greenland, Canada, the USA and Norway, countries with distinctive legislation, policies, and managerial systems regarding cultural heritage. In Russia, the public authorities for cultural heritage are the Ministry of Culture of Russia and its regional offices (”Ministry of Culture of the Russian Federation” Citation2022). The legal framework for heritage protection, conservation, use, and promotion is based on Federation Law strengthened by federal regulations, state standards, and regional laws. In Greenland, all immovable monuments from before 1900 are automatically protected by law (”Inatsisartutlov nr. 11 af 19. maj 2010 om fredning og anden kulturarvsbeskyttelse af kulturminder” Citation2010). The Greenland National Museum and Archives, which is an independent cultural institution under the Ministry of Education, Culture, Church, and Foreign Affairs, Government of Greenland (”Nunatta Katersugaasivia Allagaateqarfialu” Citation2022), is responsible for cultural heritage management. In Canada, legislative heritage protection is split between federal and provincial-territorial governments (Pokotylo and Mason Citation2014). Each provincial government has its own distinct approach to heritage conservation. They may delegate authority to preserve historic buildings to municipalities and have a provincial heritage register. In the USA, heritage protection and management depend on the location and the ownership, including government, local, and indigenous stakeholders interacting within a federal political system with a strong tradition for private involvement (MacManamon Citation2000). Svalbard is a sovereign Norwegian territory and heritage management is the purview of the Ministry of the Environment, the Directorate for Cultural Heritage and the Governor of Svalbard. The archipelago’s physical cultural heritage is mostly state-owned and managed by the Governor in accordance with the Svalbard Environmental Protection Act (”Svalbard Environmental Protection Act” Citation2001). Management and conservation principles are further described in cultural development plans (Sandodden, Yri, and Solli Citation2013; Dahle, Bjerck, and Prestvold Citation2000) and local area plans for Svalbard’s settlements.
In keeping with Norwegian heritage laws, the cultural heritage in Svalbard encompasses ‘all traces of human activity in the physical environment, including localities associated with historical events’ (”Svalbard Environmental Protection Act”, § 3). Any moveable object, structure and site predating 1946 is automatically protected (”Svalbard Environmental Protection Act”, § 39). However, historic structures and buildings do not require statutory protection per se. As stated in the Burra Charter (”The Burra Charter” Citation2013), historic buildings can possess general heritage values that, with respect to past and future generations, should be maintained.
The publications cited above demonstrate that wood-decaying fungi and fungal decay are a proven threat to wooden heritage in both polar regions and at most sites. Decay-induced damage is thus a challenge that management and conservation strategies must account for insofar as conserving polar cultural heritage is an acknowledged management aim. The arising challenge is how to develop effective strategies.
There has been a debate in Australia and New Zealand on how to preserve historic buildings. In the 1980s, biocides were recommended to treat fungi-infected wooden artefacts in the main hut of the Australasian Antarctic Expedition (Blunt Citation1985). Different methods were considered for long-term preservation of the building, such as dismantling and shipping to a museum, re-cladding with new timber, insertion of vapour barriers inside walls to prevent ice ingress, covering buildings with a dome, as well as minimal intervention strategies. Despite being done in Antarctic historic buildings since the 1960s, Hughes (Citation2011, Citation2004) points to the removal of ice inside cabins as a controversial method due to uncertainty as to whether the accumulation of ice causes damage or contributes to protecting the structures. Discussing different methods for preservation is important, but Hughes (Citation2000) made it clear that understanding the causes of deterioration is key to proper treatment. While there are few publications concerning the treatment of affected materials in-situ, biodeterioration risk assessments have been carried out by several research groups. Hughes (Citation2011) underlines the need for a holistic approach when analysing a site’s status to assess the extent of existing decay, identify species, and measure growth rates and temperature to establish a baseline for long-term evaluation of biodeterioration risks. Practical methods are then needed to reduce moisture content, maintain low temperatures, and control nutrients to limit biodeterioration risks. A holistic approach is applicable to all polar heritage sites to support sustainable heritage management. It is difficult to predict the rate of degradation in a changing climate, but extended knowledge of fungal species, degradation rates, and types of damage will be beneficial. In 2004, Hughes concluded that close communication between heritage professionals in polar regions would benefit the cultural heritage protection and help improve conservation practices in both polar regions.
New Zealand’s Antarctic Heritage Trust has been working towards implementation of major conservation programmes since the mid-1990s. Since 2002, the Trust has managed the Ross Sea Heritage Restoration Project, which is an international heritage cold-climate conservation project to secure the five historic bases of Scott, Shackleton, Borchgrevink, and Hillary (Antarctic Heritage ”Ross Sea Heritage Restoration Project” Citation2022). Conservation and implementation plans are completed for all the Ross Island and Cape Adare huts (Ritchie Citation2006).
In Svalbard, it is a stated policy that it is not possible to preserve all cultural heritage (Sandodden, Yri, and Solli Citation2013; Dahle, Bjerck, and Prestvold Citation2000). A selection of especially valuable sites and monuments are therefore prioritised (Sandodden Citation2013). The remainder are mostly left to fall into decay and ruin at their own pace. The main considerations underlying this management strategy is heritage value, practical issues, and cost. Lack of infrastructure, remoteness and expensive logistics, and the sheer number of sites, combined with the fact that most monuments are already heavily degraded and out of use, require solutions that preserve remaining heritage values. Available grey literature on damage, categories of damage, and methods for repair constitute an important knowledge base supporting management strategies in Svalbard (Flyen Citation2020, Citation2016; Flyen and Mattsson Citation2018, Citation2017, Citation2010; Flyen Citation2013; Hagen et al. Citation2014; Mattsson et al. Citation2014; Sandodden, Yri, and Solli Citation2013; Sandodden Citation2013; Hagen et al. Citation2012; Knudsen and Yri Citation2010; Hoem and Paulsen Citation2008; Dahle, Bjerck, and Prestvold Citation2000).
Figure 14. A grave at Likneset, Spitsbergen in Svalbard. The graveyard, which was in use by whalers from the mid-1600s until the end of the 1700s, is the largest graveyard in Svalbard. The wooden coffin visible in the image has been pushed to the surface by active thawing processes.

Discussion and Knowledge Gaps: Decay Fungi in Polar Wooden Heritage
This review finds that most available research on decay fungi in Antarctica and the High Arctic is focused on establishing the fact that wood decay occurs in all polar environments. There is an almost unilateral focus on fungal diversity, i.e. on the identification of fungal species causing decay and on factors influencing biodeterioration of polar heritage. While there are many publications that cover particular aspects of fungi found in historic sites in both polar regions, relatively few papers compare between the two regions to test assumptions about treatments in severe and unfamiliar conditions.
Looking back at the research questions posed in the introduction, this review shows that (1) current knowledge of decay fungi and typologies in Antarctica is detailed, but less extensive in the High Arctic; (2) there are uncertainties concerning methodological approaches as choice of methods may influence findings, and methods for detecting and identifying fungal species used in Antarctica and the High Arctic differ in some regions. In Svalbard, microscopy has been the prevalent approach to identify fungal species while sequencing DNA is dominant in Antarctica and other parts of the High Arctic. As for the next questions posed in the introduction: (3) with a few exceptions, less is reported on the decay rate and potential consequences for cultural heritage including the type and severity of damage; and (4) even less information is available on how to repair this damage and prevent decay fungi-induced damage from occurring. This is especially apparent for Antarctica’s historic buildings where little has been reported on repair methods and precautionary measures. The situation is somewhat better for some High Arctic sites where findings on which repair methods are used and recommendations on possible methodological approaches are available. However, this is for the most part based on research in Svalbard and consists of grey literature published in Norwegian (Flyen and Boro Citation2021; Flyen and Mattsson Citation2018, Citation2017, Citation2010; Mattsson and Flyen Citation2014, Citation2012, Citation2011, Citation2008; Mattsson et al. Citation2014; Flyen Citation2013). Knowledge of decay fungi typology is inadequate in Svalbard.
Research efforts have resulted in a high number of published papers on microbiological growth and biodeterioration. The research on historical structures in Antarctica is thorough and detailed, focused on identifying species of fungi. Worth noting is that the number of sites and monuments in Antarctica is considerably lower than in the High Arctic. In Svalbard alone, more than 2000 sites are listed, which means it is unrealistic to expect the same level of detail as on the around 95 Antarctic sites. In the High Arctic, and especially Svalbard, published research on fungal decay in historic structures also discusses the type and extent of damage, the location of damages in structures, and, to some extent, measures and methods of repair.
Species identification is an important focus throughout polar regions (see ). Key species of concern in Antarctica are those causing soft rot: Cadophora malorum, Cadophora luteo-olivacea and Cadophora fastigata (Arenz and Blanchette Citation2009; Arenz et al. Citation2006). They have also been found at Canadian High Arctic sites (Jurgens, Blanchette, and Filley Citation2009; Blanchette, Held, and Jurgens Citation2008). The key species of concern in Svalbard is Leucogyrofana mollis (Flyen, Flyen, and Mattsson Citation2020; Mattsson Citation2017; Mattsson and Flyen Citation2014; Mattsson, Flyen, and Nunez Citation2010) which in Svalbard mainly cause brown rot. As for the High Arctic, there is not enough available research to provide a comprehensive overview of key species of decay fungi for the region as a whole.
Table 5. Overview of fungi causing decay mentioned in the reviewed papers. The fungi are listed according to region and the type of rot they cause.
The differing finds regarding key species of concern, mainly brown rot in Svalbard versus soft rot in other polar regions, may be influenced by the fact that heavily decomposed wood has predominantly been the subject of investigation in Svalbard. Thus, it is unclear whether species other than Leucogyrofana mollis had a significant role in the initial decay processes. It is not clear from the literature whether this is also the case in Antarctica. However, findings from Deception Island show brown rot fungi surviving and causing extensive decay. Different methods of typology may also have contributed to the differences in findings. To meet this knowledge gap, newly established decay fungi and developing damages should be investigated to establish an overview of which types of decay fungi are established first and second. This will make it easier to understand damage development and appropriate measures early in the decay process. Another potential challenge in Svalbard is linked to repair and conservation measures executed prior to current knowledge of fungi-induced damage, as little is known about the status of this wooden heritage.
A similarity between Antarctica and the High Arctic is that a few researchers are responsible for most research. Consequently, research on fungal decay in polar historic wooden structures is a narrow field, and the involved researchers strongly influence what is considered interesting and relevant. Another difference, particularly apparent in research on Svalbard, is that unlike Antarctica, the number of internationally published peer-reviewed articles is low. Research publications are generally grey literature, in Norwegian and, when digitally available, only through Norwegian websites. Consequently, the data are not readily available to the international research community.
Concluding Remarks
As stated initially, polar cultural heritage is largely a wooden heritage. Comprehensive knowledge and understanding concerning biological deterioration is thus an important challenge for management authorities and other stakeholders. Research cited in this meta-analysis shows the presence of wood-decaying fungi at all documented sites and, where documented, varying degrees of fungi-induced damage in both Antarctica and the High Arctic. The long-held assumption that fungal decay is uncommon in polar regions due to a cold and dry climate has been proven false. This is reflected both in scientific and grey literature.
Biodeterioration processes in wooden heritage cannot realistically be stopped. A better understanding of the conditions that cause fungal growth may allow for better management to reduce the rate of deterioration. Knowledge of degradation processes, decomposition rates, and the genuine technical condition of the historic structures is thus a basic premise. If Antarctic and High Arctic historic sites are to be conserved retaining their original historic context as much as possible, it is essential to further develop a repertoire of measures and methodologies that address causes as well as symptoms.
A consideration that is not readily apparent in the reviewed literature, and consequently not discussed in this paper, is the heritage itself. Polar heritage holds challenges when it comes to a comprehensive overview of sites and their conservation status. Due to remoteness and difficult access, it is not easy to document the condition of polar heritage and to investigate the temperature and moisture conditions that are key factors affecting fungal deterioration rates. Using Svalbard as an example, although a high number of sites are listed in the heritage database, condition reporting is generally lacking.
Further surveys and research are an obvious answer to improve knowledge and understanding of status and develop suitable management and conservation measures. Following this, it might be fruitful to delve deeper into the biology and ecology of polar fungi. In Antarctica, soft rot, which apparently are indigenous, are commonly found. The introduced brown and white rot fungi appear to only survive in areas with more moderate environments such as Deception Island. In Svalbard, brown rot appears to be predominant in wooden heritage. The major brown rot fungi in Svalbard are Leucogyrofana mollis which can be found in northern European countries and may have been introduced into Svalbard. Studies of decaying wood in the Canadian High Arctic and in Greenland did not reveal this taxon. In Greenland, soft rot has been found to dominate at a number of archaeological sites. Can different environmental conditions and the introduction of fungi from Northern Europe explain differing finds in Svalbard? Can different methodologies explain different species identification? Can Leucogyrofana mollis also be found in wood imported from northern Europe to Sub-Antarctica and Antarctica? Would sequencing of DNA identify different or new fungal species in Svalbard? Knowing the species and their decay mechanisms is important as different fungi cause different types of damage which again demand different measures.
Climate change is an ongoing challenge. The fact is that as the climate and microclimatic conditions become more favourable for fungal decay, more severe damage must be expected. Understanding the effects of changing abiotic conditions and decay fungi adaption to these conditions is important. Heritage sites throughout polar regions hold many similarities. Although not the focus of this review, the reviewed literature indicates that climate and climatic impact on sites are comparable, although impact varies. It is difficult to predict future climate and effects on biodegraders and cultural heritage because every site will have its own specific challenges linked to on-site microclimatic conditions. As the effects of climate change are cross-boundary, greater cooperation between researchers across disciplines in both polar regions may be of key importance to improve insights and treatments for these important historic sites. Stronger coordination and further development of common forums may facilitate cooperation aimed at improving approaches to and solutions for conservation and management of wooden polar heritage. This review summarises a large body of research and documents the range of information available in English and Norwegian and provides a comparison between High Arctic and Antarctic regions. Hopefully, this review will be useful not only for the scientific community but also for conservators and site managers faced with the problem of determining how best to preserve polar wooden heritage.
Acknowledgements
The two anonymous reviewers are kindly acknowledged for helpful comments which contributed to improving this paper. The authors would like to thank Research Professor Gry Alfredsen at the Norwegian Institute of Bioeconomy Research for constructive feedback. The authors would also like to thank the Norwegian Polar Institute, Tom Edvinsen, Carl Erik Kilander, Ann Kristin Balto, and Stine Barlindhaug for permitting the use of photographic material.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Anne-Cathrine Flyen
Anne-Cathrine Flyen is an architect specialising in cultural heritage preservation and management in Arctic areas and environmental monitoring of cultural heritage focusing on biological decay in wooden structures.
Alma Elizabeth Thuestad
Alma Elizabeth Thuestad is an archaeologist specialising in historic landscape and resource use in circumpolar areas, environmental monitoring of cultural heritage, and archaeological applications of GIS and remote sensing.
References
- ADD (Antarctic Digital Database). 2021. British Antarctic Survey (BAS). https://www.bas.ac.uk/project/add/
- Albores, S., P. Sanguinedo, B. H. Held, M. P. Cerdeiras, and R. A. Blanchette. 2018. “Biodiversity and Antimicrobial Activity of Antarctic Fungi from the Fildes Peninsula, King George Island.” Sydowia 70: 185–192. doi:10.12905/0380.sydowia70-2018-0185.
- Allsopp, D. 2011. “Worldwide Wastage: The Economics of Biodeterioration.” Microbiol Tod 38: 150–153.
- “The Antarctic Treaty.” 1959. Secretariat of the Antarctic Treaty. https://www.ats.aq/e/antarctictreaty.html
- Arantes, V., and B. Goodell. 2014. “Current Understanding of Brown-Rot Fungal Biodegradation Mechanisms: A Review.” Deterioration and Protection of Sustainable Biomaterials 1158: 1–21. doi:10.1021/bk-2014-1158.ch001.
- “Area Protection and Management/Historic Sites and Monuments.” 2022. Secretariat of the Antarctic Treaty. https://www.ats.aq/e/protected.html
- Arenz, B., and R. A. Blanchette. 2008. “East Base, SOS: Assessment of Deterioration and Recommendations for Conserving This Important Antarctic Site.” In Historical Polar Bases – Preservation and Management, edited by S. Barr and P. Chaplin, 79–84. Oslo: International Polar Heritage Committee.
- Arenz, B., and R. A. Blanchette. 2009. “Investigations of Fungal Diversity in Wooden Structures and Soils at Historic Sites on the Antarctic Peninsula.” Canadian Journal of Microbiology 55 (1): 46–56. doi:10.1139/W08-120.
- Arenz, B., and R. A. Blanchette. 2011. “Distribution and Abundance of Soil Fungi in Antarctica at Sites on the Peninsula, Ross Sea Region and McMurdo Dry Valleys.” Soil Biology & Biochemistry 43 (2): 308–315. doi:10.1016/j.soilbio.2010.10.016.
- Arenz, B., R. A. Blanchette, and R. L. Farrell. 2014. “Fungal Diversity in Antarctic Soils.” In Antarctic Terrestrial Microbiology, edited by Antarctic Terrestrial Microbiology, 35–53. New York: Springer.
- Arenz, B., B. W. Held, J. A. Jurgens, and R. A. Blanchette. 2011. “Fungal Colonization of Exotic Substrates in Antarctica.” Fungal Diversity 49 (1): 13–22. doi:10.1007/s13225-010-0079-4.
- Arenz, B., B. W. Held, J. A. Jurgens, R. L. Farrel, and R. A. Blanchette. 2006. “Fungal Diversity in Soils and Historic Wood from the Ross Sea Region of Antarctica.” Soil Biology & Biochemistry 38 (10): 3057–3064. doi:10.1016/j.soilbio.2006.01.016.
- Arlov, T. B. 2003. Svalbards Historie. Trondheim: Tapir Akademisk Forl.
- “Askeladden.” 2020. Directorate for Cultural Heritage. https://askeladden.ra.no
- “Assessment of Cultural Heritage Monuments and Sites in the Arctic.” 2013. Reports and Assessments on the Topic of Arctic Cultures and Languages. Arctic Council.
- “Australian Antarctic Program.” 2021. Australian Government. Department of Climate Change, Energy, the Environment and Water. Australian Antarctic Division. https://www.antarctica.gov.au/
- Barr, S. (Ed.) 1995. “The history of western activity in Franz Josef Land.” In Polarhåndbok nr. 8. Franz Josef Land, 59–106. Oslo: Norwegian Polar Institute, University of Salzburg.
- Barr, S. 2019. “Cultural Heritage, or How Bad News Can Also Be Good.” In Arctic Triumph, edited by N. Sellheim, Y. Zaika, and I. Kelman, 43–57. New York: Springer.
- Bhunjun, C. S., T. Niskanen, and N. Suwannarach. 2022. “The Numbers of Fungi: Are the Most Speciose Genera Truly Diverse?” Fungal Diversity 114: 387–462. doi:10.1007/s13225-022-00501-4.
- Björdal, C. G., and P. K. Dayton. 2020. “First Evidence of Microbial Wood Degradation in the Coastal Waters of the Antarctic.” Scientific Reports 10 (1): 12774. doi:10.1038/s41598-020-68613-y.
- Blanchette, R. A. 2000. “A Review of Microbial Deterioration Found in Archaeological Wood from Different Environments.” International Biodeterioration & Biodegradation 46 (3): 189–204. doi:10.1016/S0964-8305(00)00077-9.
- Blanchette, R. A., and A. E. Charola. 2003. “Deterioration in Historic and Archaeological Woods from Terrestrial Sites.” In Art, Biology, and Conservation: Biodeterioration of Works of Art, edited by R. J. Koestler, V. R. Kosestler, and F. E. Nieto-Fernandez, 328–347. New York: The Metropolitan Museum of Art.
- Blanchette, R. A., B. W. Held, B. Arenz, J. A. Jurgens, N. Baltes, S. Duncan, and R. L. Farrel. 2010. “An Antarctic Hot Spot for Fungi at Shackleton’s Historic Hut on Cape Royds.” Microbial Ecology 60 (1): 29–38. doi:10.1007/s00248-010-9664-z.
- Blanchette, R. A., B. W. Held, and R. L. Farrel. 2002. “Defibration of Wood in the Expedition Huts of Antarctica: An Unusual Deterioration Process Occurring in the Polar Environment.” The Polar Record 38 (207): 313–322. doi:10.1017/S0032247400018003.
- Blanchette, R. A., B. W. Held, L. Hellmann, L. Millman, and U. Büntgen. 2016. “Artic Driftwood Reveals Unexpectedly Rich Fungal Diversity.” Fungal Ecology 23: 58–65. doi:10.1016/j.funeco.2016.06.001.
- Blanchette, R. A., B. W. Held, and J. A. Jurgens. 2008. “Northumberland House, Fort Conger and the Peary Huts in the Canadian High Arctic: Current Condition and Assessment of Wood Deterioration Taking Place.” In Historical Polar Bases – Preservation and Management, edited by S. Barr and P. Chaplin, 30–37. Oslo: International Polar Heritage Committee ICOMOS.
- Blanchette, R. A., B. W. Held, J. A. Jurgens, S. Aislabie, S. Duncan, and R. L. Farrel. 2004. “Environmental Pollutants from the Scott and Shackleton Expeditions During the ‘Heroic Age’ of Antarctic Exploration.” The Polar Record 40 (2): 143–151. doi:10.1017/S0032247403003334.
- Blanchette, R. A., B. W. Held, J. A. Jurgens, D. L. McNew, T. Harrington, S. Duncan, and R. L. Farrel. 2004. “Wood-Destroying Soft Rot Fungi in the Historic Expedition Huts of Antarctica.” Applied and Environmental Microbiology 70 (3): 1328–1335. doi:10.1128/AEM.70.3.1328-1335.2004.
- Blanchette, R. A., B. W. Held, J. Jurgens, A. Stear, C. Dupont, and A. Csank. 2021. “Fungi Attacking Historic Wood of Fort Conger and the Peary Huts in the High Arctic.” Plos One 16 (1): e0246049. doi:10.1371/journal.pone.0246049.
- Blunt, W. 1985. Mawson’s Huts Interim Conservation Report. Sydney: University of New South Wales.
- Blunt, W. 1991. Mawson’s Huts, Antarctica: A Conservation Proposal. Sydney: University of New South Wales.
- Boike, J., I. Juszak, S. Lange, S. Chadburn, E. Burke, P. P. Overduin, K. Roth, et al. 2018. ”A 20-Year Record (1998–2017) of Permafrost, Active Layer and Meteorological Conditions at a High Arctic Permafrost Research Site (Bayelva, Spitsbergen).” Earth System Science Data 10 (1): 355–390. doi:10.5194/essd-10-355-2018.
- Braat, J. 1984. “Dutch Activities in the North and the Arctic During the Sixteenth and Seventeenth Centuries.” Arctic 37 (4): 473–480. doi:10.14430/arctic2229.
- Brischke, C., and G. Alfredsen. 2020. “Wood-Water Relationships and Their Role for Wood Susceptibility to Fungal Decay.” Applied Microbiology and Biotechnology 104 (9): 3781–3795. doi:10.1007/s00253-020-10479-1.
- “The Burra Charter: The Australia ICOMOS Charter for Places of Cultural Significance.” 2013. Australia ICOMOS.
- Connell, L., R. Redman, S. Craig, G. Scorzetti, M. Iszard, and R. Rodriguez. 2008. “Diversity of Soil Yeasts Isolated from South Victoria Land, Antarctica.” Microbial Ecology 56 (3): 448–459. doi:10.1007/s00248-008-9363-1.
- Connell, L., R. Redman, R. Rodriguez, A. Barrett, M. Iszard, and A. Fonseca. 2010. “Dioszegia Antarctica sp. nov. and Dioszegia Cryoxerica sp. nov., Psychrophilic Basidiomycetous Yeasts from Polar Desert Soils in Antarctica.” International Journal of Systematic and Evolutionary Microbiology 60 (6): 1466–1472. doi:10.1099/ijs.0.015412-0.
- Coulson, S. J. 2015. “The Alien Terrestrial Invertebrate Fauna of the High Arctic Archipelago of Svalbard: Potential Implications for the Native Flora and Fauna.” Polar Research 34 (1): 27364. doi:10.3402/polar.v34.27364.
- Dahle, K., H. B. Bjerck, and K. Prestvold. 2000. Kulturminneplan for Svalbard: 2000-2010. Longyearbyen: Sysselmannen på Svalbard.
- Domsch, K. H., W. Gams, and T. Anderson. 1980. Compendium of Soil Fungi. London: Academic Press.
- Duarte, A. W. F., J. A. Dos Santos, M. V. Vianna, J. M. F. Vieira, V. H. Mallagutti, F. J. Inforsato, L. C. P. Wentzel, et al. 2018. ”Cold-Adapted Enzymes Produced by Fungi from Terrestrial and Marine Antarctic Environments.” Critical Reviews in Biotechnology 38 (4): 600–619. doi:10.1080/07388551.2017.1379468.
- Duncan, S. 2007. “Fungal Diversity and Cellulolytic Activity in the Historic Huts, Ross Island, Antarctica.” PhD diss., University of Waikato.
- Duncan, S., R. L. Farrel, N. Jordan, J. A. Jurgens, and R. A. Blanchette. 2010. “Monitoring and Identification of Airborne Fungi at Historic Locations on Ross Island, Antarctica.” Polar Science 4 (2): 275–283. doi:10.1016/j.polar.2010.03.008.
- Duncan, S., R. L. Farrel, J. M. Thwaites, B. Arenz, J. A. Jurgens, and R. A. Blanchette. 2006. “Endoglucanase-Producing Fungi Isolated from Cape Evans Historic Expedition Hut on Ross Island, Antartica.” Environmental Microbiology 8 (7): 1212–1219. doi:10.1111/j.1462-2920.2006.01013.x.
- Duncan, S., R. Minasaki, R. L. Farrel, J. M. Thaites, B. W. Held, B. Arenz, J. A. Jurgens, and R. A. Blanchette. 2008. “Screening Fungi Isolated from Historic Discovery Hut on Ross Island, Antarctica for Cellulose Degradation.” Antarctic Science 20 (5): 463–470. doi:10.1017/S0954102008001314.
- Etzemuller, T. V., K. Schuler, K. Isaksen, H. H. Christiansen, H. Farbrot, and R. Benestad. 2011. “Attribution 3.0 License. The Cryosphere Modeling the Temperature Evolution of Svalbard Permafrost During the 20th and 21st Century.” The Cryosphere 5 (1): 67–79. doi:10.5194/tc-5-67-2011.
- Farquharson, L. M., V. E. Romanovsky, W. L. Cable, D. A. Walker, S. V. Kokelj, and D. Nicolsky. 2019. “Climate Change Drives Widespread and Rapid Thermokarst Development in Very Cold Permafrost in the Canadian High Arctic.” Geophysical Research Letters 46 (12): 6681–6689. doi:10.1029/2019gl082187.
- Farrell, R. L., B. E. Arenz, S. M. Duncan, B. W. Held, J. A. Jurgens, and R. A. Blanchette. 2011. “Introduced and Indigenous Fungi of the Ross Island Historic Huts and Pristine Areas of Antarctica.” Polar Biology 34 (11): 1669–1677. doi:10.1007/s00300-011-1060-8.
- Farrell, R. L., R. A. Blanchette, M. Auger, S. Duncan, B. H. Held, J. Jurgens, and R. Minasaki. 2004. “Scientific Evaluation of Deterioration in Historic Huts of Ross Island, Antarctica.” In Cultural Heritage in the Arctic and Antarctic Regions, edited by S. Barr and P. Chaplin, 33–37. Lørenskog, Norway: International Polar Heritage Committee of ICOMOS.
- Farrell, R. L., S. Duncan, R. A. Blanchette, B. W. Held, J. A. Jurgens, and B. Arenz. 2008. “Scientific Evaluation of Deterioration of Historic Huts of Ross Island, Antarctica.” In Historical Polar Bases – Preservation and Management, edited by S. Barr and P. Chaplin, 59–64. Oslo: International Polar Heritage Committee.
- Flyen, A. C. 2013. “Gruveminner i Longyearbyen og Hiorthhamn. Fredete Taubanebukker: Tilstand og Bevaring.” NIKU Rapport. Oslo: Norsk Institutt for Kulturminneforskning.
- Flyen, A. C. 2016. “Miljøovervåking av Kulturminner på Svalbard. Eksisterende og Tidligere Overvåkingssystemer på Svalbard.” NIKU Oppdragsrapport, 44. Oslo: Norsk Institutt for Kulturminneforskning.
- Flyen, A. C. 2020. “Bevaring av Krigsminner. Tilstands- og Sårbarhetsvurdering av Krigsminnene i og Omkring Longyearbyen på Svalbard.” NIKU Oppdragsrapport, 92. Oslo: Norsk Institutt for Kulturminneforskning.
- Flyen, A. C., and M. Boro. 2021. Svalbard: Hiorthhamn Kulturmiljø. Kulturminner og Klima - Risikovurdering og Planlegging av Tiltak. En del av Prosjekt Adapt Northern Heritage 2020. Oslo: Riksantikvaren.
- Flyen, A. C., C. Flyen, and J. Mattsson. 2020. “Climate Change Impacts and Fungal Decay in Vulnerable Historic Buildings at Svalbard.” Paper presented at the 12th Nordic Symposium on Building Physics (NSB 2020), Tallin, Estonia.
- Flyen, A. C., and J. Mattsson. 2010. “Håndtering av Råteskader i Kulturminner på Svalbard. Skadeårsaker og Løsningsmetoder.” Rapport NIKU/Mycoteam. Oslo: Norsk Institutt for Kulturminneforskning.
- Flyen, A. C., and J. Mattsson. 2017. “Permafrost og Fundamenteringsforhold for Kulturminner i Longyearbyen.” In NIKU Oppdragsrapport, edited by Flyen, A.C, 29. Oslo: Norsk Institutt for Kulturminneforskning.
- Flyen, A. C., and J. Mattsson. 2018. “Bevaring av Teknisk Industrielle Kulturminner. Reparasjon av Taubanebukker i Longyearbyen.” NIKU Oppdragsrapport, 36. Oslo: Norsk Institutt for Kulturminneforskning.
- Fogg, G. E. 1992. A History of Antarctic Science. G.E. Fogg 1992. Cambridge: Cambridge University Press.
- Frisvad, J. C. 2008. “Fungi in Cold Ecosystems.” In Psychrophiles: From Biodiversity to Biotechnology, edited by R. Margesin, F. Schinner, J. Marx, and C. Gerday, 137–156. New York: Springer.
- Gaiser, R. F., C. A. Robles, J. M. Kobashigawa, S. Pereira, N. Skronski, and C. C. Carmarán. 2021. “Mycobiota Associated to Casa Moneta Museum Wood, South Orkney Islands, Antarctica.” Polar Biology 44 (9): 1817–1831. doi:10.1007/s00300-021-02916-2.
- Godinho, V. M., L. E. Furbino, I. F. Santiago, F. M. Pellizzari, N. S. Yokoya, D. Pupo, T. M. Alves, et al. 2013. ”Diversity and Bioprospecting of Fungal Communities Associated with Endemic and Cold-Adapted Macroalgae in Antarctica.” The ISME Journal 7 (7): 1434–1451. doi:10.1038/ismej.2013.77.
- Godinho, V. M., V. N. Goncalves, I. F. Santiago, H. M. Figueredo, G. A. Vitoreli, C. E. Schaefer, E. C. Barbosa, et al. 2015. ”Diversity and Bioprospection of Fungal Community Present in Oligotrophic Soil of Continental Antarctica.” Extremophiles 19 (3): 585–596. doi:10.1007/s00792-015-0741-6.
- Goodell, B., Y. Qian, and J. Jellison. 2008. “Fungal Decay of Wood: Soft Rot—Brown Rot—White Rot.” In Development of Commercial Wood Preservatives, edited by T. P. Schultz, H. Militz, M. H. Freeman, B. Goodell, and D. D. Nicholas, 9–31. Washington, DC: American Chemical Society.
- Grobakken, L. R., J. Mattsson, and G. Alfredsen. 2014. “The Importance of Critical in-Situ Conditions for In-Service Performance of Wood.” Agarica 34: 29–36.
- Gulden, G., and A. -E. Torkelsen. 1996. “Fungi I. Basidiomycota: Agaricales, Gasteromycetales, Aphyllophorales, Exobasidiales, Dacrymycetales and Tremellales.” In A Catalogue of Svalbard Plants, Fungi, Algae and Cyanobacteria, edited by A. Elvebakk and P. Prestrud, 173–206. Oslo: Norsk Polarinstitutt.
- Hacquebord, L. 1991. “Five Early European Winterings in the Atlantic Arctic (1596–1635): A Comparison.” Arctic 44 (2): 146–155. doi:10.14430/arctic1531.
- Hacquebord, L., and D. Avango. 2016. “Industrial Heritage Sites in Spitsbergen (Svalbard), South Georgia and the Antarctic Peninsula: Sources of Historical Information.” Polar Science 10 (3): 433–440. doi:10.1016/j.polar.2016.06.005.
- Hagen, D., N. E. Eide, K. Fangel, and O. I. Vistad. 2012. Sårbarhetsvurdering og Bruk av Lokaliteter på Svalbard : Sluttrapport fra Forskningsprosjektet Miljøeffekter av Ferdsel. Trondheim: Norsk Institutt for Naturforskning.
- Hagen, D., N. E. Eide, A. C. Flyen, K. Fangel, and O. I. Vistad. 2014. Håndbok for Sårbarhetsvurdering av Ilandstigningslokaliteter på Svalbard. Trondheim: Norsk Institutt for Naturforskning.
- Hawksworth, D. L., R. Lucking, J. Heitman, and T. Y. James. 2017. “Fungal Diversity Revisited: 2.2 to 3.8 Million Species.” Microbiol Spectrum 5 (4): 28752818. doi:10.1128/microbiolspec.FUNK-0052-2016.
- Headland, R. K. 1989. Chronological List of Antarctic Expeditions and Related Historical Events. Cambridge: Cambridge University Press.
- Headland, R. K. 2009. “Antarctic Winter Scientific Stations to the International Polar Year, 2007–2009.” The Polar Record 45 (1): 9–24. doi:10.1017/s0032247408007663.
- Held, B. W., B. E. Arenz, and R. A. Blanchette. 2011. “Factors Influencing Deterioration of Historic Structures at Deception Island, Antarctica.” In Polar Settlements - Location, Techniques and Conservation, edited by S. Barr and P. Chaplin, 35–43. Oslo: International Polar Heritage Committee ICOMOS.
- Held, B. W., and R. A. Blanchette. 2017. “Deception Island, Antarctica, Harbors a Diverse Assemblage of Wood Decay Fungi.” Fungal Biology 121 (2): 145–157. doi:10.1016/j.funbio.2016.11.009.
- Held, B. W., J. A. Jurgens, B. E. Arenz, S. M. Duncan, R. L. Farrell, and R. A. Blanchette. 2005. “Environmental Factors Influencing Microbial Growth Inside the Historic Expedition Huts of Ross Island, Antarctica.” International Biodeterioration & Biodegradation 55 (1): 45–53. doi:10.1016/j.ibiod.2004.06.011.
- Held, B. W., J. A. Jurgens, S. M. Duncan, R. L. Farrell, and R. A. Blanchette. 2006. “Assessment of Fungal Diversity and Deterioration in a Wooden Structure at New Harbor, Antarctica.” Polar Biology 29 (6): 526–531. doi:10.1007/s00300-005-0084-3.
- Henriquez, M., K. Vergara, J. Norambuena, A. Beiza, F. Maza, P. Ubilla, I. Araya, et al. 2014. ”Diversity of Cultivable Fungi Associated with Antarctic Marine Sponges and Screening for Their Antimicrobial, Antitumoral and Antioxidant Potential.” World Journal of Microbiology & Biotechnology 30 (1): 65–76. doi:10.1007/s11274-013-1418-x.
- Hoem, S., and B. Paulsen. 2008. “Ny-Ålesund. Forvaltningsplan for de Fredete Bygningene i Tettstedet.” Rapport. Longyearbyen: Sysselmannen på Svalbard.
- Hollesen, J., M. Callanan, T. Dawson, R. Fenger-Nielsen, T. M. Friesen, A. M. Jensen, A. Markham, V. V. Martens, V. V. Pitulko, and M. Rockman. 2018. “Climate Change and the Deterioating Archaeological and Environmental Archives of the Arctic.” Antiquity 92 (363): 573–586. doi:10.15184/aqy.2018.8.
- Hollesen, J., H. Matthiesen, and B. Elberling. 2017. “The Impact of Climate Change on an Archaeological Site in the Arctic.” Archaeometry 59 (6): 1175–1189. doi:10.1111/arcm.12319.
- Hollesen, J., H. Matthiesen, A. B. Moller, and B. Elberling. 2015. “Permafrost Thawing in Organic Arctic Soils Accelerated by Ground Heat Production.” Nature Climate Change 5 (6): 574–578. doi:10.1038/nclimate2590.
- Hollesen, J., H. Matthiesen, A. B. Moller, A. Westergaard-Nielsen, and B. Elberling. 2016. “Climate Change and the Loss of Organic Archaeological Deposits in the Arctic.” Scientific Reports 6 (1): 28690. doi:10.1038/srep28690.
- Hughes, J. D. 2000. “Ten Myths About the Preservation of Historic Sites in Antarctica and Some Implications for Mawson’s Huts at Cape Denison.” The Polar Record 36 (197): 117–130. doi:10.1017/S0032247400016223.
- Hughes, J. D. 2004. “Deterioration of Antarctic Historic Sites - Effects of Antarctic Climates on Materials and Implications for Preservation.” In Cultural Heritage in the Arctic and Antarctic Regions, edited by S. Barr and P. Chaplin, 29–32. Lørenskog, Norway: International Polar Heritage Committee ICOMOS.
- Hughes, J. D. 2011. “Deterioration Processes Affecting Historic Sites in Antarctica and the Conservation Implications.” PhD diss., University of Canberra.
- “Inatsisartutlov nr. 11 af 19. maj 2010 om Fredning og anden Kulturarvsbeskyttelse af Kulturminder.” 2010. Grønlands Selvstyre.
- Jennings, D. H., and A. D. M. Rayner. 1984. The Ecology and Physiology of the Fungal Mycelium. Cambridge: Cambridge University Press.
- Jurgens, J. A., R. A. Blanchette, and T. R. Filley. 2009. “Fungal Diversity and Deterioration in Mummified Woods from the Ad Astra Ice Cap Region in the Canadian High Arctic.” Polar Biology 32 (5): 751–758. doi:10.1007/s00300-008-0578-x.
- Kerry, E. 1990. “Effects of Temperature on Growth Rates of Fungi from Subantarctic Macquarie Island and Casey, Antarctica.” Polar Biology 10 (4): 293–299. doi:10.1007/BF00238428.
- Knudsen, E., and H. T. Yri. 2010. “Teknisk Industrielle kulturminner i Longyearbyen og omegn. Verneverdi og Forvaltning.” Rapportserie 1/2010. Sysselmannen på Svalbard.
- Kosonen, T., and S. Huhtinen. 2008. “Wood-Rotting Basidiomycetes of Svalbard (Norway).” Karstenia 48 (1): 21–28. doi:10.29203/ka.2008.425.
- Lawley, B., S. Ripley, P. Bridge, and P. Convey. 2004. “Molecular Analysis of Geographic Patterns of Eukaryotic Diversity in Antarctic Soils.” Applied and Environmental Microbiology 70 (10): 5963–5972. doi:10.1128/AEM.70.10.5963-5972.2004.
- Line, M. 1988. “Microbial Flora of Some Soils of Mawson Base and the Vestfold Hills, Antarctica.” Polar Biology 8 (6): 421–427. doi:10.1007/BF00264718.
- “List of Historic Sites and Monuments Approved by the ATCM.” 2013. Antarctic Treaty Consultative Meeting (ATCM).
- MacManamon, F. P. 2000. “The Protection of Archaeological Resources in the United States: Reconciling Preservation with Contemporary Society.” In Cultural Resource Management in Contemporary Society, edited by F. P. McManamon and A. Hatton, 40–54. London: Routledge.
- Mason, G. 1999. “Modelling Mass Transfer Inside Scott’s Hut, Cape Evans, Antarctica.” PhD diss., University of Canterbury.
- Matthiesen, H., J. B. Jensen, D. Gregory, J. Hollesen, and B. Elberling. 2014. “Degradation of Archaeological Wood Under Freezing and Thawing Conditions-Effects of Permafrost and Climate Change.” Archaeometry 56 (3): 479–495. doi:10.1111/arcm.12023.
- Mattsson, J. 2017. “The Impact of Microclimate on Biodeterioration of Wood in Historic Buildings.” PhD diss., NTNU: Norwegian University of Science and Technology.
- Mattsson, J., and A. C. Flyen. 2008. “Bio-deterioation in Buildings in Svalbard.” In Historical Polar Bases - Preservation and Management, 23–29. International Polar Heritage Committee of ICOMOS.
- Mattsson, J., and A. C. Flyen. 2011. “Preventive Methods Against Biodeterioration of Protected Building Materials in Svalbard.” In Polar Settlements: Location, Techniques and Conservation, edited by S. Barr and P. Chaplin, 44–50. Oslo: ICOMOS International Polar Heritage Committee.
- Mattsson, J., and A. C. Flyen. 2012. “How Can Signs of Biodeterioration Save Culturasl Heritage?” Paper presented at Cultural Heritage Preservation, EWCHP-2012, Kjeller, Norway.
- Mattsson, J., and A. C. Flyen. 2014. “The Importance of Microclimate in Bbiodetoriation in Historic Wooden Structures.” In 10th Nordic Symposium on Building Physics (NSB 2014), edited by J. Arfvidsson, L. Harderup, A. Kumlin, and B. Rosencrantz, 632–639. Lund: Sweden.
- Mattsson, J., A. C. Flyen, I. Gronli, and C. M. Whist. 2014. “Muggsoppskader i Bygninger på Svalbard.” Agarica 34: 101–110.
- Mattsson, J., A. C. Flyen, and M. Nunez. 2010. “Wood-Decaying Fungi in Protected Buildings and Structures on Svalbard.” Agarica 29: 5–14.
- Mills, R. O. 2011. “Log-Building Rehabilitation at the Historic Steel Creel Town Site, Alaska.” In Polar Settlements: Location, Techniques and Conservation, edited by S. Barr and P. Chaplin, 94–100. Oslo: ICOMOS International Polar Heritage Committee.
- “Ministry of Culture of the Russian Federation.” 2022. Russian Government. http://government.ru/en/department/27
- “Nunatta Katersugaasivia Allagaateqarfialu.” 2022. Nunatta Katersugaasivia Allagaateqarfialu. https://en.nka.gl/heritage/
- Otjen, L., R. A. Blanchette, M. Effland, and G. Leatham. 1987. “Assessment of 30 White Rot Basidiomycetes for Selective Lignin Degradation.” Holzforschung 41 (6): 343–349. doi:10.1515/hfsg.1987.41.6.343.
- Pearson, M., and R. Stehberg. 2006. “Nineteenth Century Sealing Sites on Rugged Island, South Shetland Islands.” The Polar Record 42 (4): 335–347. doi:10.1017/s003224740600564x.
- Pedersen, N. B., H. Matthiesen, R. A. Blanchette, G. Alfredsen, B. W. Held, A. Westergaard-Nielsen, and J. Hollesen. 2020. “Fungal Attack on Archaeological Wooden Artefacts in the Arctic-Implications in a Changing Climate.” Scientific Reports 10 (1): 14577. doi:10.1038/s41598-020-71518-5.
- Pokotylo, D., and A. R. Mason. 2014. “Canada: Cultural Heritage Management.” In Encyclopedia of Global Archaeology, edited by C. Smith, 1103–1107. New York: Springer.
- “The Protocol on Environmental Protection to the Antarctic Treaty.” 1991. Secretariat of the Antarctic Treaty.
- Rayner, A. D. M., and L. Boddy. 1988. Fungal Decomposition of Wood. Its Biology and Ecology. Chichester: John Wiley & Sons.
- Riley, R., A. A. Salamov, D. W. Brown, D. G. Nagy, D. Floudas, B. W. Held, A. Levasseur, et al. 2014. ”Extensive Sampling of Basidiomycete Genomes Demonstrates Inadequacy of the White-Rot/brown-Rot Paradigm for Wood Decay Fungi.” PNAS 111 (27): 9923–9928. doi:10.1073/pnas.1400592111.
- Ritchie, N. A. 2006. “Frozen Solid: Recent Archaeological Work at Shackleton’s Cape Royd’s Hut Site (1907–1909), Ross Island, Ross Dependency, Antarctica.” Australasian Historical Archaeology 24: 39–46.
- “Ross Sea Heritage Restoration Project.” 2022. Antarctic Heritage Trust. https://nzaht.org/conserve/ross-sea-heritage-restoration-project/
- Rusman, Y., B. W. Held, R. A. Blanchette, Y. He, and C. E. Salomon. 2018. “Cadopherone and Colomitide Polyketides from Cadophora Wood-Rot Fungi Associated with Historic Expedition Huts in Antarctica.” Phytochemistry 148: 1–10. doi:10.1016/j.phytochem.2017.12.019.
- Ryvarden, L., and K. Høiland. 2009. “Some Higher Basidiomycota from Jan Mayen, Norway.” Agarica 28: 50–52.
- Sandodden, I. S. 2013. Katalog Prioriterte Kulturminner og Kulturmiljøer på Svalbard. Versjon 1.1 (2013). Longyearbyen: Sysselmannen på Svalbard.
- Sandodden, I. S., H. T. Yri, and H. Solli. 2013. “Kulturminneplan for Svalbard 2013–2023.” Rapportserie, 112. Longyearbyen: Sysselmannen på Svalbard.
- Schilling, J. S., J. T. Kaffenberger, B. W. Held, R. Ortiz, and R. A. Blanchette. 2020. “Using Wood Rot Phenotypes to Illuminate the ‘Gray’ Among Decomposer Fungi.” Frontiers in Microbiology 11: 1288. doi:10.3389/fmicb.2020.01288.
- Senatore, M. X. 2019. “Archaeologies in Antarctica from Nostalgia to Capitalism: A Review.” International Journal of Historical Archaeology 23 (3): 755–771. doi:10.1007/s10761-019-00499-7.
- Singh, J. 2000. “Fungal Problems in Historic Buildings.” Journal of Architectural Conservation 6 (1): 17–37. doi:10.1080/13556207.2000.10785259.
- Slater, A. G., and D. M. Lawrence. 2013. “Diagnosing Present and Future Permafrost from Climate Models.” Journal of Climate 26 (15): 5608–5623. doi:10.1175/Jcli-D-12-00341.1.
- Sterflinger, K., and G. Pinar. 2013. “Microbial Deterioration of Cultural Heritage and Works of Art—Tilting at Windmills?” Applied Microbiology and Biotechnology 97 (22): 9637–9646. doi:10.1007/s00253-013-5283-1.
- Stokland, J. N. 2012. “Wood Decomposition.” In Biodiversity in Dead-Wood, edited by J. N. Stokland, J. Siitonen, and B. G. Jonsson, 10–28. London: Cambridge University Press.
- “Svalbard Environmental Protection Act.” 2001. Ministry of Climate and Environment. Norway.
- Wu, B., M. Hussain, W. Zhang, M. Stadler, X. Liu, and M. Xiang. 2019. “Current Insights into Fungal Species Diversity and Perspective on Naming the Environmental DNA Sequences of Fungi.” Mycology 10 (3): 127–140. doi:10.1080/21501203.2019.1614106.
- Zhang, Y., W. Chen, and D. W. Riseborough. 2008. “Disequilibrium Response of Permafrost Thaw to Climate Warming in Canada Over 1850–2100.” Geophysical Research Letters 35 (2): 2. doi:10.1029/2007gl032117.



