Abstract
Background
The three main cardiac amyloidosis (CA) types have different progression and prognosis. Little is known about the mode of death (MOD) which is commonly attributed to cardiovascular causes in CA. Improving MOD’s knowledge could allow to adapt patient care.
Objective
This retrospective study describes the MOD that occurred during long-term follow-up in CA patients in light-chain (AL), transthyretin hereditary (ATTRv) or wild-type (ATTRwt).
Material and methods
Patients referred to and cared for, at the French referral centre for CA, Henri Mondor Hospital, Créteil between 2010 and 2016 were included. Clinical information surrounding patient deaths were investigated and centrally evaluated by two blinded clinical committees which classified MOD as cardiovascular, non-cardiovascular or unknown and sub-classified it depending on its subtype.
Results
From the 566 patients included, 187 had AL, 206 ATTRv and 173 ATTRwt. During the 864 patient-year follow-up, 160 (28%) deaths occurred, with median survival time of 17.3 months (interquartile range 5.1–35.4). The most frequent MOD was cardiovascular (64%) of which worsening heart failure occurred most frequently and for which, 69% were of AL subtype, 79% ATTRv and 76% ATTRwt. Sudden death also occurred more frequently in AL subtype accounting for 29% of AL deaths. Non-cardiovascular MOD occurred in 26% of patients overall. Among these, infection was the most common non-cardiovascular MOD in any type of CA (80%).
Conclusions
Mortality is high during natural course of CA and differs between subtypes. The main MOD were worsening heart failure, sudden death and infection, opening room to optimise management.
Introduction
Cardiac amyloidosis (CA) is a rare form of restrictive cardiomyopathy, resulting from various systemic dysfunctions whereby misfolded proteins form amyloid fibrils that accumulate in multiple organs including the heart [Citation1,Citation2]. There are three main amyloidosis types, which account for 90% of all cases. Immunoglobulin light chain amyloidosis (AL) the most common form, due to misfolded monoclonal immunoglobulin light chains, hereditary transthyretin (TTR) amyloidosis (ATTRv) caused by mutated TTR deposits and wild-type (non-mutant) TTR amyloidosis (ATTRwt) also known as senile systemic amyloidosis due to older patient profile [Citation2–4].
Each amyloidosis subtype has a distinctive clinical presentation, progression and prognosis but when cardiac involvement is confirmed, in around 70% of patients [Citation5], the grievous outcome is the same. Once the amyloid fibrils infiltrate and accumulate in the extracellular myocardial wall space, restrictive cardiomyopathy and progressive heart failure occurs [Citation4,Citation6,Citation7].
However, little is known today about mode of death (MOD) during the natural course of the three main cardiac amyloidosis subtypes [Citation8,Citation9]. Some retrospective evidence suggests cardiovascular events were prominent. One longitudinal cohort study in 215 ATTRv following liver transplantation with mainly neuropathy reported cardiac events as the leading cause of death (38%) [Citation10]. More recently, Escher et al. retrospectively investigated the mode of death in a smaller study with 66 AL and 48 ATTRwt CA subtypes [Citation5]. They found that although the modes of death differed between the two CA types groups, cardiovascular events occurred predominantly.
Defining the (MOD) in patients with CA is important to further our understanding of the underlying pathophysiology in the three CA subtypes. This understanding will provide avenues to improve patient management and implement appropriate preventive measures that may increase survival time. Having a clear definition of MOD in each subtype is also valuable for clinical trial design for therapeutic target identification.
Furthermore, to our knowledge, there are no objective descriptions of MOD with an adjudication committee and prespecified endpoints in all three most common CA types (AL, ATTRwt or ATTRv) in a large cohort.
The aim of this retrospective study was to define and classify MOD during long-term follow-up in three main amyloidosis types.
Methods
Study design and setting
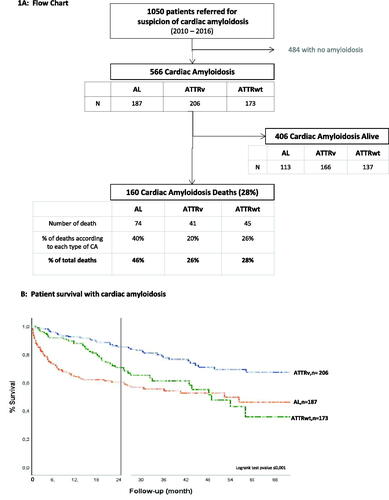
This retrospective, observational, monocentric study was conducted from January 2010 to December 2016 in the French referral centre for CA at Henri Mondor Hospital, Créteil, France. During this period, 1050 patients were referred to the centre with suspected CA and were registered in the Amyloidosis Network registry. Of those, 566 patients had a confirmed diagnosis of AL, ATTRv or ATTRwt amyloidosis and were considered for inclusion in our study. Those patients with confirmed CA, who were willing to participate, and died during the study period were included in the analysis. There were no exclusion criterion.
Diagnosis of cardiac amyloidosis and its types
A diagnosis of CA was considered in patients with amyloidosis when there was an increase in wall thickness (>12 mm) measured at echocardiography in the absence of another known cause of cardiac hypertrophy, and increased plasma levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) above 332 ng/L (in the absence of renal failure or atrial fibrillation). The severity of cardiac amyloidosis was assessed using two biological markers, plasma levels of high-sensitivity troponin T (hs-TnT) and NT-proBNP [Citation11].
ATTR diagnosis was based on TTR typical Congo red staining, and positive staining with TTR antibodies on endomyocardial or extra-myocardiac biopsy [Citation12] and/or on bisphosphonate scintigraphy. Genetic sequencing of ATTR was performed to differentiate ATTRwt from ATTRv.
AL was diagnosed by histological analysis from an extracardiac or endomyocardial tissue biopsy showing amyloid deposits specifically Congo red stained and labelled by an antibody directed to kappa or lambda FLC, and negative with antibody directed to TTR. Monoclonal gammopathy was diagnosed by electrophoresis and immunofixation of serum and urine, and quantification of circulating kappa and lambda FLC.
Definition of mode of death
The primary endpoint of this study was to record the mode of death (MOD) as being either cardiovascular (CV), non-cardiovascular (NonCV) or unknown death for each CA type. The MOD was defined as the first clinically relevant event to occur in the period before death (e.g. infection). The secondary endpoint was to sub-classify the cardiovascular or non-cardiovascular deaths MOD (see below).
Definition of cardiovascular deaths
Cardiovascular mode of death (CV-MOD) were sub-classified as sudden death, heart failure death (worsening heart failure), due to myocardial death, cerebrovascular accident, stroke, complications of a cardiovascular procedure, pulmonary embolism, or another cardiovascular cause.
Sudden death was defined as an unexpected death in a previously clinically stable patient. Their death was witnessed by a care taker, or health professional. When sufficient information was available, sudden death was subcategorised as with or without preceding cardiovascular symptoms.
Worsening heart failure (WoHF) was defined as a death that occurred as a result of intractable heart failure and generally occurred in the hospital, at a nursing home facility, at home, or in a hospice. WoHF was confirmed by reported heart failure symptoms, signs on physical examination, and diagnostic evidence including significant increase in brain natriuretic peptide (BNP) or N-terminal prohormone BNP (NT-proBNP). When possible, heart failure was further categorised according to the presence or absence of low output and/or congestion. Low output was indicated by fatigue, signs of vasoconstriction, prerenal azotaemia, need for vasopressors, low cardiac output, or hypotension. Congestion was confirmed according to presenting signs and symptoms, physical examination and usual non-invasive and invasive measurements. If a terminal arrhythmia was associated with heart failure, the death was classified as a heart failure death. If heart failure occurred secondary to a recent myocardial infarction, the death was classified as a myocardial infarction death. Otherwise, the heart failure event was subcategorised as unknown.
Myocardial infarction (MI): If death occurred outside the hospital death was confirmed on autopsy findings that showed a recent MI or coronary thrombus. Otherwise, MI was defined as a combination of increased biomarker and clinical symptoms (chest pain) and altered ECG and evidence of acute myocardial injury or thrombosis.
Stroke (cerebrovascular accident): If death occurred outside the hospital, it was classified as a stroke death if autopsy findings were positive. Otherwise, stroke was defined as a persistent (>24 h) disturbance of focal, neurological function resulting in symptoms indicative of atherothrombotic or thrombotic cerebral infarction, embolus, or haemorrhage for which there was no certain cause. Diagnosis required characteristic history, physical examination, imaging techniques, and/or autopsy data.
Cardiovascular procedure was defined as a death that occurred during the operative or perioperative period that could be directly attributed to the procedure itself.
Other cardiac death was defined as a death that could be attributed to a cardiac reason but was not one of the other modes listed above. For example, deaths resulting from valvular heart disease were considered other cardiac deaths.
Vascular death was defined as a death that could be attributed to a vascular reason such as pulmonary embolism, aortic dissection, or aortic rupture.
Definition of non-cardiovascular death
The final endpoint was to define the non-cardiovascular mode of death (NonCV-MOD) as due to infection/sepsis, haemorrhage, cancer, pulmonary, gastrointestinal, renal, accidental, suicide, or other causes. Patients who had infection first and died in the same hospitalisation of shock (septic or cardiogenic) or worsening heart failure were classified as infection. This MOD was classified according to the primary sepsis site (lung, gut, urinary tract, soft tissue, heart).
Definition of unknown death
An unknown death was defined as a death, in which, no specific morbid event could be assigned and when only the date of death was known without any other information.
Data collection and ethical considerations
Information about death were meticulously obtained from patient medical files, case report forms, investigator summaries, hospital death reports, discharge summaries from previous hospitalisations, and the death certificates. If necessary, further medical information about the events that occurred at the time of death was obtained from interviews with the patient’s general practitioner, family member or witness.
Two separate, independent Clinical Endpoint Committees (CEC) were created to validate each MOD. Each CEC was composed of a senior physician, one senior register and one trained and experienced clinical research assistant. The MOD definitions were made according to a review of the heart failure literature and validated by both CECs [Citation13–15]. All CEC members were trained to assess the MOD endpoint by applying the MOD definition list to twenty random death reports. Details from each patient death were recorded in a report, which was blinded for both evaluating CECs. The results from both CECs were then merged. If there was a discrepancy about MOD classification between the two first CEC evaluations, a consensus was reached during a face-to-face meeting of both CECs.
The data collected for CA patients in the registry included demographic information (age and gender), body mass index (BMI), cardiovascular risk factors (diabetes, dyslipidemia or high blood pressure), average heart rate, average systolic and diastolic blood pression, New York Heart Association (NYHA) functional classification of heart failure and presence of pacemaker or implantable cardioverter-defibrillator (ICD). Cardiac and renal biomarkers (troponin (hs-TnT), NT-proBNP, and creatinine levels), natremia, kalaemia and haemoglobin were recorded. An electrocardiogram (ECG) and also a transthoracic echocardiography measurement of left ventricular ejection fraction (LVEF), interventricular septal thickness (IVST), left ventricular telediastolic diameter (LVTDD), left ventricular telediastolic volume (LVTDV), left ventricular telesystolic volume (LVTSV), and global longitudinal strain. Following registry entry, all clinical, biochemical information and imagery were entered directly into a specifically designed research database. Any additional imagery or information obtained during hospital visits was uploaded from the hospital database to the research database.
Written informed consent for participation in the Amyloidosis Network registry was obtained from each patient. The study was approved by the local ethics committee (authorization number #1431858). The study followed the ethical principles formulated in the Declaration of Helsinki. Data were recorded electronically according to the French CNIL (Commission Nationale de l’Informatique et des Libertés).
Statistical analysis
Continuous variables were expressed as median with the interquartile range (25th and 75th percentiles). Dichotomous data were expressed as absolute values and percentages. Differences in frequencies for quantitative variables were compared using the χ2 test with Pearson’s correction. Pearson correlation coefficient (r) was used to investigate relationships between parameters. p-values, less than .05 were considered significant. For continuous variables, the Mann–Whitney test was used to compare two groups, while the Kruskal–Wallis test was used to compare more than two groups. Survival was calculated from the diagnosis date to the date of death. For the survival analysis, patients required a follow up of at least 24 months to be coherent with the recent literature. Concerning patient characteristics, only patients who died with complete follow-up data were described.
The statistical analyses were performed using the SPSS software (v19.0 for Windows 2010 SPSS Inc., Chicago, IL). Any p-values below .05 would be considered statistically significant.
Results
Cardiac amyloidosis population characteristics
Of the 566 patients with confirmed CA, who were entered into the registry and treated at the centre, 160 deceased and were included in the analysis.
Although the three main amyloidosis types were similarly distributed in the registry, the proportion of AL-CA subtypes that died during the study period was nearly double the ATTR subtypes (). The baseline characteristics are described in . During the 864 patient-year follow-up, the median survival was 17.3 months (IQR 5.1–35.4). The Kaplan–Meier curves according to amyloidosis subtypes are shown in Figure 1(B). During the 5-year study period, overall, among those patients who died, relatively more were AL-CA (46%) than ATTRwt (28%) or ATTRv (26%) and predominately male (n = 386, 68%).
Table 1. Baseline characteristics.
At diagnosis, the median age was 71 years (range 60–80) and patients with ATTRwt-CA were slightly older with a median age of 82 years (77–86), which is to be expected for this subtype. Heart failure symptoms were severe in the three groups with 213 (38%) having NYHA heart failure stages III and IV, however as expected AL-CA 87 (48%) and ATTRwt 74 (43%) had more severe heart failure at baseline than those with ATTRv (25%). Similarly, the high cardiac biomarkers in the total population were typical of severe heart failure, but were lower in the ATTRv than in the ATTRwt and AL-CA subtypes, which is in line with less severe ATTRv disease ( ).
Similarly, the overall LVEF was typical for these three disease subtypes 55% (38–62). The NT-proBNP biomarker levels, that indicate cardiac amyloidosis severity, were significantly lower in the ATTRv group (744 pg/mL) than the AL-CA (3912 pg/mL) and ATTRwt (3716 pg/mL), which was to be expected for these more severe subtypes. The systolic blood pressure was well-controlled in each group: mean SBP 121 mmHg (108–136). Importantly, fewer patients with AL-CA had a pacemaker fitted 19 (10%) versus 39 (16%) in the ATTRv and 72 (42%) in the ATTRwt group. The LV global strain was the lowest in the ATTRwt type 9.0 (7.0-12) and low for the AL-CA type 12.0 (8.7–16.5).
Mortality and mode of death
Among the 160 deaths analysed, information on MOD were available for 143 patients (89%) (). CV-MOD events occurred most frequently in 64% (102/160) of patients (). Among these, CV-MOD events occurred more often in AL-CA patients (66%), than ATTRv (59%) or ATTRwt (64%) CA where the distribution is homogeneous (p = .143) (). The most frequent CV-MOD was worsening heart failure, accounting for 74% (75/102) of CV-MOD deaths, of which 69% (34/49) occurred in patients with AL-CA, 79% (19/24) with ATTRv, and 76% (22/29) with ATTRwt subtype. However, 20% (20/102) of CV-MOD were sudden deaths of which more were observed in AL-CA subtype (29%) than the ATTRwt (14%) or ATTRv (8%) subtypes ().
Figure 2. (A) Prevalence of mode of death (cardiovascular, non-cardiovascular, unknown) in CA according to amyloidosis type. (B) Prevalence of cardiovascular mode of death in CA according to amyloidosis type. (C) Prevalence of non-cardiovascular mode of death in CA according to amyloidosis type.
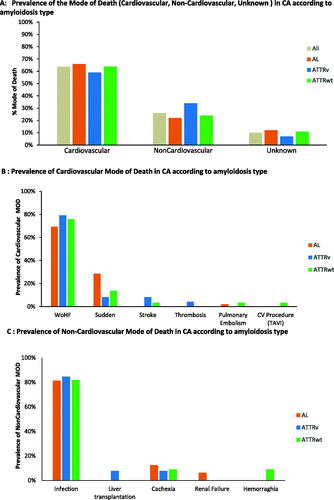
Table 2. Mode of death distribution according to amyloidosis type.
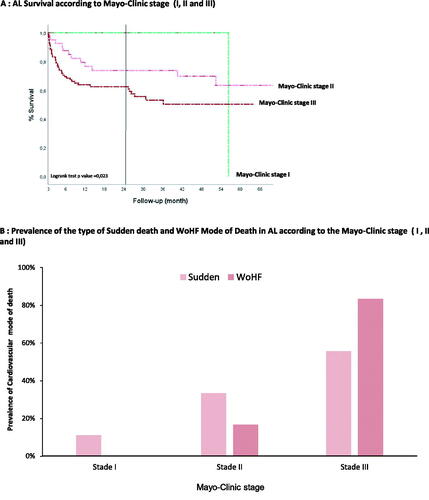
Importantly, illustrates that patient with AL-CA, Mayo-Clinic stage II have a significantly better chance of survival than those with Mayo-Clinic stage III. Concerning the MOD, those AL-CA, Mayo-Clinic stage II more died from sudden death as opposed to AL-CA Mayo-Clinic stage III, who died more often from WoHF ().
Figure 3. (A) AL survival according to Mayo-Clinic stage (I, II and III). (B) Prevalence of the type of Sudden death and WoHF mode of death in AL according to the Mayo-Clinic stage (I, II and III).
Concerning the three main type of ATTRv phenotype (cardiac, mixed and neurological), those patients with mixed (cardiac and neurologic) or neurological phenotype have a significantly better chance of survival compared to patients with cardiac phenotype alone (Supplementary Figure 2(A)). Among those patients with cardiac phenotype CVar MOD occurred more often (Supplementary Figure 2(B)), and from WoHF (Supplementary Figure 2(C)). Among the NonCVar deaths the most frequent MOD was infection (Supplementary Figure 2(D)).
Concerning ATTRv mutations, those with Val30Met or Ser77Tyr have a significantly better chance of survival compared to Val122Ile or other mutations (Supplementary Figure 3(A)).
Interestingly, ATTRv patients with Val122Ile died more often from CVar and those with Ser77Tyr died more often from NonCVar mode of death (Supplementary Figure 3(B)). Among those who died from CVar MOD, most died from WoHF (Supplementary Figure 3(C)). Among those who died from NonCVar MOD, the MOD was infection (Supplementary Figure 3(D)).
NonCV-MOD events occurred in 26% (41/160) of patients overall (). Among these, infection was the most common NonCV-MOD 80% (33/41), which was similarly distributed between the different types of CA (). More precisely, the primary causes of infection were lung 42% (14/33) and septicaemia 33% (11/33) (Supplementary Figure 1).
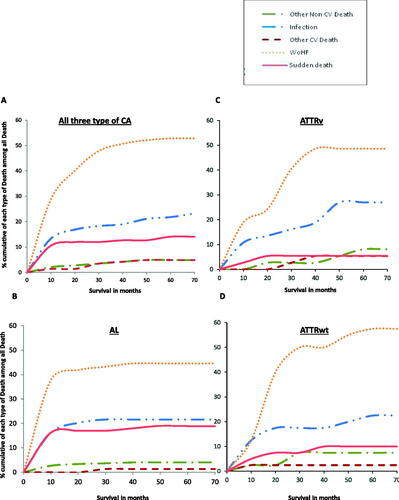
Over time, illustrates that among those CA patients who died, 55.6% passed away within 10 months post-diagnosis. Importantly, illustrates that of these, most 72% (64/89) were patients with AL-CA, who died mostly from worsening heart failure, sudden death and infection. At the end of the 5-year study period, overall, 72% (406/566) of the study population were alive, of which 60% (113/187) AL-CA, 80% (165/206) ATTRv 74% (128/173) ATTRwt ().
Figure 4. Cumulative cause of death type for all three cardiac amyloidosis types and per amyloidosis type.
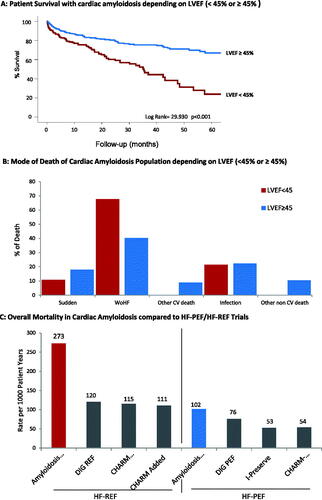
Concerning cardiac function depending on LVEF (<45% or ≥45%), indicates that CA patients with reduced ejection fraction deteriorate faster and die more frequently from worsening heart failure than those with preserved ejection fraction ().
Discussion
To our knowledge, this is the first, retrospective, registry study to objectively identify and define the MOD using an adjudication committee and prespecified endpoints for the three major cardiac amyloidosis types (AL-CA, ATTRv and ATTRwt). At the end of the 5-year study period, we observed 72% (406/566) of the study population survived, of which 60% were AL-CA, 74% ATTRwt, and 80% ATTRv. With a median survival of 14.6 months (IQR 3.7–33.9). This was similar to the 35% AL-CA and 75% ATTRwt reported by Escher et al. and the 2-year survival reported by Rapezzi et al. 63% AL, 98% ATTRv and 100% ATTRwt [Citation4,Citation5]. Also, we observed that the most frequent cause of death was CV-MOD of which most were due to worsening heart failure (74%) and occurred mostly in the AL-CA subtype (69%). The next most frequent CV-MOD was sudden death (20%). Notably, one third of AL-CA subtype (29%) had a sudden death. These results highlight the rapid disease progression and MOD for AL-CA subtype. Importantly, among those who died from NonCV-MOD, infection was the most frequent cause, and was similar for the three CA subtypes.
This study reflects the 6 years that the reference centre has been recording and managing a representative population of CA patients.
Mode of death in CA and heart failure trials
Among the cardiovascular event deaths, we observed, more than two-thirds were due to circulatory failure following cardiogenic shock, acute heart failure and multi-organ failure. This is similar to other heart failure (HF) trials, where most CV-MOD are sudden death and HF death [Citation16] and the proportion of deaths contributed by each MOD varies according to symptom severity [Citation17]. Specifically, concerning HFpEF’s trials, 30–40% of sudden deaths were CV-MOD [Citation15]. Notably, among the 1546 deaths in the PARADIGM-HF trials, CV-MOD occurred in 81%, of which 45% were considered sudden death and 26% HF [Citation18].
Furthermore, when comparing our data with key heart failure studies, mortality is clearly higher in CA patients, independent of whether ejection fraction is preserved or reduced (). This is important as CA patients might have been underdiagnosed and therefore included in trials confounding their outcome [Citation19]. In fact, 13–18% of HFpEF patients have been identified with ATTRwt CA [Citation20–22]. Notably, Oghina et al. found that among their CA cohort, between 16% and 65% would meet the standard inclusion criteria for HFPEF trials. This suggests that if not diagnosed, these patients could have been included in such trial [Citation23]. As expected, survival was lower in the CA cohort compared to those with other causes of HFpEF. This highlights the importance of diagnosing CA, and preventing their worse prognosis from limiting controlled trials testing the efficacy of medications for HFpEF [Citation24].
Overall mortality AL-CA, ATTRv, ATTRwt compared with other CA cohorts
During our 5-year follow-up, the overall mortality for all three CA subtypes was 160/566 (28%) which was relatively lower than the 52% Escher et al. recently reported for AL-CA and ATTRwt unadjusted 5-year follow-up [Citation5]. Also, we observed that among the deceased, 55.4% had died within 10 months following diagnosis, and proportionally more were patients with AL-CA (46%) compared to those with ATTRwt (28%) and ATTRv (26%). Interestingly, a part from confirming previous observations, these results highlight the rapid disease progression and MOD for AL-CA subtype, which reinforces the need for quick therapeutic action following diagnosis [Citation5,Citation25]. We also noticed that AL-CA subtypes had signs of worse baseline cardiac function; higher, NYHA class, heart rate, NT-proBNP, Troponin. This confirms previous reports of cardiomyopathies and conduction abnormalities in AL-CA patients presenting with HF [Citation25,Citation26]. This may possibly be due to a delayed diagnosis [Citation25].
Cardiovascular mode of death
Our data further support previous reports of CV events occurring in approximately 60–70% of CA deaths, with no difference between subtypes (p = .143) [Citation5,Citation25].
Worsening heart failure
Among those patients who died with CV-MOD, those with ATTRv and ATTwt were more likely to die of worsening heart failure than those with AL-CA. This is important when choosing an appropriate treatment. Among cardiac-specific treatments, β-blockers and renin–angiotensin system inhibitors are not effective and may even be detrimental for CA patients. Patients with CA are non-optimal candidates for the LV assist device implants due to their small LV cavity and a dysfunctional right ventricle [Citation27]. According to the ISHLT recommendations, selected patients with heart failure due to AL-CA or ATTR amyloidosis may be considered for transplant in experienced centres [Citation28].
Since the end of our study, Tafamidis, a specific treatment designed for CA physiopathology became available for patients with pure neuropathy form of ATTRv. Then the Phase III ATTRACT trial demonstrated that Tafamidis reduced the relative risk of mortality by 30% and the rehospitalisation for acute heart failure by 32%.
For the AL subtype, chemotherapy: cyclophosphamide, bortezomib and dexamethasone (CyborD) is widely used but cardiac adverse events limit the benefit of this treatment in patients with CA [Citation29]. Also, Le Bras et al. demonstrated in a small cohort of patients with AL-CA that cardiac biomarkers, NT-proBNP, were affected after dexamethasone administration, suggesting that dexamethasone may disrupt the frail heart function in AL-CA patients and more recently is associated with early death in severe CA patients [Citation29].
Sudden death
Overall, sudden death occurred for 20% (20/160) of deaths in our study. Importantly, sudden death was markedly high in the AL-CA population 29% (14/49), despite appropriate chemotherapy, ICD or pacemaker placement. This is in line with previous reports that estimate sudden death with AL-CA to be between 15% and 23%. As well as two recent studies suggest that although the terminal cardiac event in AL-CA was associated with marked bradycardia [Citation30], progressive conduction disease [Citation31]. ICD therapy did not prevent death and sometime just delayed its occurrence [Citation32].
Non-cardiovascular mode of death
Infection is the principal non cardiovascular mode of death
For those patients who died of NonCV-MOD, infection was the main MOD for all three amyloidosis subtypes, independent of phenotype. This may be linked to extracardiac amyloid infiltration, which may alter organ function and decrease immune defences. Recently, Malka et al. using 99mTc-HMDP extracardiac scintigraphy showed that extracardiac fixation was observed in 29% of CA and 3% of controls. He observed that most frequent site was pleuropulmonary (16% of CA), followed by digestive tract and subcutaneous tissues and that the organs involved vary among the three CA types. Among those patients who died from infection, most were due to respiratory infection and septicaemia, originating in the intestine. In Supplementary Figure 1, we show that although the infection rate was generally high for all types, it was particularly high in ATTRwt.
Respiratory infection
A large number of epidemiological studies showed that both influenza and pneumococcal infections exacerbate pre-existing cardiac diseases and trigger new cardiovascular problems [Citation33–35]. Marra et al. identified the protective effect of pneumococcal vaccination for any cardiovascular-related event. The pneumococcal vaccination offers further protective benefit against all cause and cardiovascular related mortality [Citation36].
Our study was conducted before the COVID-19 pandemic. We can suggest that COVID-19 vaccination is fundamental in CA patients. Initiatives to monitor the vaccination status of vulnerable patients are needed, which is why we advocate for systematic vaccination registration and frequent communication about vaccination.
Gut infection
Chronic heart failure (CHF) is a multisystem disorder in which intestinal morphology, permeability, and absorption are modified [Citation37]. Sandeck et al. identified significant morphological and functional alterations of the intestine of CHF patients with a restricted intestinal perfusion, consequent increase in intestinal permeability, and a lack of immunological defence with an augmented bacterial biofilm. These two consequences may contribute to the origin of both chronic inflammation and malnutrition [Citation37]. In our CA patients, gut amyloidosis infiltration may have altered the intestinal function. Thus, our patients had also severe HF with liver dysfunction that might have also decreased the portal lipopolysaccharide clearing, as seen in CHF patients with oedematous decompensation, in whom plasma lipopolysaccharide levels are increased [Citation38]. Increased intestinal permeability due to barrier dysfunction is thought to cause microbial translocation which may induce low-grade inflammation in various diseases [Citation39]. Considering the close relationship of 'the leaky gut' and gut dysbiosis to the major diseases, meticulous dietetic and probiotic approaches to recover healthy microbiota could potentially have a positive influence in the future management of CA and improve their outcome.
Study limitations
Our study was limited by its retrospective design. This study described MOD in a cohort of CA patient in the Amyloidosis registry but did not include a control cohort of patients with heart failure. Although the sample size for this study is large for a rare disease and larger than recent work in this field, it remains small for the retrospective design. Although meticulous effort was made to record all information at the time of death, less information was available for those patients who deceased with sudden death. We were unable to record cardiac activity or electrical events perimortem and so cannot describe clinical information about sudden death. However, the proportion of sudden deaths is similar to the reported literature [Citation5].
Also, we did not report on patient medication at the time of death and therefore analyse lack information about treatments that may have affected death or improved survival. There is a need for further data from patients treated with Tafamidis for ATTR or chemotherapy for the AL-CA.
Perspectives
Patient pathway in this population remains heterogeneous, non-regulated, and needs improvement for prompt diagnosis, management, and better trial design for new therapies. Considering the deleterious prognosis in the AL subtype early diagnosis would permit earlier treatment. The results from this study suggest that meticulous dietetic and probiotic approaches, antibiotic therapy and vaccination should be put in place for patients with CA to prevent death from infection.
Early treatment with Tafamidis may improve survival time, specifically for cardiac ATTR amyloidosis. It is still unknown if ICD or pacemakers improve survival, particularly in AL-CA patients that have a high rate of sudden death compare to the other type of CA. Further research may be required.
Furthermore, developing a better understanding of MOD in CA population may improve our understanding of their pathophysiology, and guide near-term drug and device development. Matching available in novel therapies with specific mechanisms of death may be more successful therapeutic strategy challenge in CA population.
Conclusion
This study further supports preliminary evidence that although mode of death in all three CA types is worsening heart failure, many also die of sudden death or infection. This is an opportunity to hasten diagnosis, implement treatment and improve management of these challenging patients.
| Abbreviations | ||
| AL | = | immunoglobulin light chain amyloidosis |
| ATTR | = | amyloidosis transthyretin |
| ATTRv | = | hereditary transthyretin amyloidosis |
| ATTRwt | = | wild-type transthyretin amyloidosis |
| BMI | = | body mass index |
| BNP | = | brain natriuretic peptide |
| CA | = | cardiac amyloidosis |
| CEC | = | Clinical Endpoint Committees |
| CV | = | cardiovascular |
| CV-MOD | = | cardiovascular mode of death |
| ECG | = | electrocardiogram |
| HF | = | heart failure |
| HFpEF | = | heart failure preserved ejection fraction |
| HFrEF | = | heart failure reduced ejection fraction |
| Hs-TnT | = | high-sensitivity troponin T |
| ICD | = | cardioverter-defibrillator |
| IVST | = | interventricular septal thickness |
| LVEF | = | left ventricular ejection fraction |
| LVTDD | = | left ventricular telediastolic diameter |
| LVTDV | = | left ventricular telediastolic volume |
| LVTSV | = | left ventricular telesystolic volume |
| MI | = | myocardial infarction |
| MOD | = | mode of death |
| NonCV | = | non-cardiovascular |
| NonCV-MOD | = | non-cardiovascular mode of death |
| NT-proBNP | = | N-terminal pro-B-type natriuretic peptide |
| NYHA | = | New York Heart Association |
| TTR | = | transthyretin |
| WoHF | = | worsening heart failure |
Supplemental Material
Download MS Word (49.4 KB)Acknowledgements
The authors thank all the members of the Mondor Amyloidosis Network for their continued collaboration and dedication to care and management of amyloidosis. The authors also thank Amanda Whereat B.Sc. UNSW, Australia (Speak the Speech Consulting) for drafting, editing and polishing the manuscript.
Disclosure statement
D. B. has received honoraria/financial support from Alnylam Pharmaceuticals and Pfizer. S. O. has received honoraria from Pfizer. T. D. has received research grants, consultant fees, and/or honoraria from Pfizer, Alnylam Pharmaceuticals, Ionis, Neurimmune, Prothena, GlaxoSmithKline. T. G. received consultant fees and/or financial support from Alnylam Pharmaceuticals, Elivie. The remaining authors have nothing to declare.
Data availability statement
The authors are willing to share data that support the findings of this study please contact: [email protected].
References
- Grogan M, Dispenzieri A, Gertz MA. Light-chain cardiac amyloidosis: strategies to promote early diagnosis and cardiac response. Heart. 2017;103(14):1065–1072.
- Damy T, Kristen AV, Suhr OB, et al. Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS). Eur Heart J. 2019:ehz173. doi:https://doi.org/10.1093/eurheartj/ehz173
- Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412.
- Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203–1212.
- Escher F, Senoner M, Doerler J, et al. When and how do patients with cardiac amyloidosis die? Clin Res Cardiol. 2020;109(1):78–88.
- Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286–1300.
- Sperry BW, Vranian MN, Hachamovitch R, et al. Subtype-specific interactions and prognosis in cardiac amyloidosis. J Am Heart Assoc. 2016;5(3):e002877.
- Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641–2654.
- Gertz MA, Benson MD, Dyck PJ, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol. 2015;66(21):2451–2466.
- Algalarrondo V, Antonini T, Theaudin M, et al. Cause of death analysis and temporal trends in survival after liver transplantation for transthyretin familial amyloid polyneuropathy. Amyloid. 2018;25(4):253–260.
- Gillmore JD, Damy T, Fontana M, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39(30):2799–2806.
- Béquignon E, Guellich A, Bartier S, et al. How your ears can tell what is hidden in your heart: wild-type transthyretin amyloidosis as potential cause of sensorineural hearing loss in elderly-AmyloDEAFNESS pilot study. Amyloid. 2017;24(2):96–100.
- Zile MR, I-Preserve Investigators, Gaasch WH, Anand IS, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in heart failure with preserved ejection fraction study (I-Preserve) trial. Circulation. 2010;121(12):1393–1405.
- Hamaguchi S, JCARE-CARD Investigators, Kinugawa S, Sobirin MA, et al. Mode of death in patients with heart failure and reduced vs. preserved ejection fraction: report from the registry of hospitalized heart failure patients. Circ J. 2012;76(7):1662–1669.
- Vaduganathan M, Patel RB, Michel A, et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;69(5):556–569.
- O'Connor CM, Carson PE, Miller AB, et al. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective randomized amlodipine survival evaluation. Am J Cardiol. 1998;82(7):881–887.
- Group M-HS. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet. 1999;353(9169):2001–2007.
- Desai AS, for the EVALUATE-HF Investigators, Solomon SD, Shah AM, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322(11):1077–1010.
- Oghina S, Bougouin W, Bézard M, et al. The impact of patients with cardiac amyloidosis in HFpEF trials. JACC Heart Fail. 2021;9(3):169–178.
- Desai AS, McMurray JJ, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36(30):1990–1997.
- González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–2594.
- Bennani Smires Y, Victor G, Ribes D, et al. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging. 2016;32(9):1403–1413.
- D'Errico S, Mazzanti A, Baldari B, et al. Sudden death in lambda light chain AL cardiac amyloidosis: a review of literature and update for clinicians and pathologists. Int J Clin Exp Pathol. 2020;13(7):1474–1482.
- Sattar Y, Ruiz Maya T, Zafrullah F, et al. Diagnosis and management of a cardiac amyloidosis case mimicking hypertrophic cardiomyopathy. Cureus. 2018;10(12):e3749.
- Grupper A, Park SJ, Pereira NL, et al. Role of ventricular assist therapy for patients with heart failure and restrictive physiology: improving outcomes for a lethal disease. J Heart Lung Transplant. 2015;34(8):1042–1049.
- Mehra MR, International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases, Pediatric and Heart Failure and Transplantation Councils, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016;35(1):1–23.
- Bézard M, Kharoubi M, Galat A, et al. Natural history and impact of treatment with tafamidis on major cardiovascular outcome-free survival time in a cohort of patients with transthyretin amyloidosis. Eur J Heart Fail. 2021;23(2):264–274.
- Bézard M, Oghina S, Vitiello D, et al. Dexamethasone is associated with early deaths in light chain amyloidosis patients with severe cardiac involvement. PLoS One. 2021;16(9):e0257189.
- Le Bras F, Molinier-Frenkel V, Guellich A, et al. Sequential cyclophosphamide-bortezomib-dexamethasone unmasks the harmful cardiac effect of dexamethasone in primary light-chain cardiac amyloidosis. Eur J Cancer. 2017;76:183–187.
- Sayed RH, Rogers D, Khan F, et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J. 2015;36(18):1098–1105.
- Rehorn MR, Loungani RS, Black-Maier E, et al. Cardiac implantable electronic devices: a window into the evolution of conduction disease in cardiac amyloidosis. JACC Clin Electrophysiol. 2020;6(9):1144–1154.
- Hamon D, Algalarrondo V, Gandjbakhch E, et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. 2016;222:562–568.
- Vardeny O, Solomon SD. Influenza vaccination: a one-shot deal to reduce cardiovascular events. Eur Heart J. 2017;38(5):334–337.
- Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264–274.
- Musher DM, Rueda AM, Kaka AS, et al. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis. 2007;45(2):158–165.
- Marra F, Zhang A, Gillman E, et al. The protective effect of pneumococcal vaccination on cardiovascular disease in adults: a systematic review and meta-analysis. Int J Infect Dis. 2020;99:204–213.
- Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50(16):1561–1569.
- Niebauer J, Volk HD, Kemp M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353(9167):1838–1842.
- Fukui H. Increased intestinal permeability and decreased barrier function: does it really influence the risk of inflammation? Inflamm Intest Dis. 2016;1(3):135–145.