Abstract
Background: There is strong evidence that hypertension and depression are comorbid and oxidative stress is implicated in both pathologies. We aimed to elucidate the relationship between biochemical markers of the antioxidant-pro-oxidant equilibrium and depression in hypertension.
Methods: Blood was collected from patients diagnosed with depression, hypertension, or comorbid depression and hypertension and healthy age- and sex-matched controls. Whole blood reduced glutathione, erythrocyte superoxide dismutase (SOD-1), glutathione peroxidase (GPx-1), glutathione reductase (GR), malondialdehyde (MDA), and plasma hydrogen peroxide (H2O2) were assayed using spectrophotometry, and heme oxygenase (HO-1) levels were determined immunoenzymatically.
Results: Both hypertension and depression were associated with altered antioxidant-pro-oxidant profiles. Decreased GPx-1 and SOD-1 activities, increased GR activity, increased levels of GSH, and increased concentrations of MDA and H2O2 were observed in patients compared to controls. Inducible HO-1 was specifically decreased in patients with depression and was significantly associated with both the prevalence and severity of depressive symptoms.
Conclusions: Heme oxygenase is a biological factor that might explain the relationship between inflammation, oxidative stress, and the biological and functional changes in brain activity in depression. HO-1 is a candidate depression biomarker and provides an avenue for novel preventative and diagnostic strategies against this disease.
Highlights
Hypertension, depression, and comorbidity with both diseases were associated with increased peroxidation and antioxidant markers.
Inducible heme oxygenase (HO-1) was a specific marker of the antioxidant-pro-oxidant equilibrium in depressed patients.
HO-1 levels were decreased in patients with depression and negatively correlated with depressive symptoms.
Decreased expression of HO-1 may contribute to the risk of depression in hypertension.
Introduction
Epidemiological studies have shown that hypertensive patients are at an increased risk of depression. This relationship is bidirectional; a number of cross-sectional and cohort studies have shown that depressed patients are also more likely to develop cardiovascular diseases than the general population.Citation1–Citation3 Studies on the biological factors underlying depression and somatic health have resulted in several plausible mechanistic explanations based on metabolic, immunological, autonomic, and hypothalamic–pituitary–adrenal axis dysregulation, which are recognized biological causes of clinical depression.Citation4 However, there is a lack of specific biomarkers associated with depression, not least due to the large clinical heterogeneity in depressed patients. This has hindered efforts to identify the biological, genetical, and environmental causes of depression, and it is crucial that depressive subtypes and comorbidities are taken into account in studies of biological correlates of depression.
The pathogenesis of depression and essential hypertension is multifactorial. Of the biological mechanisms thought to contribute to hypertension and depression, oxidative stress pathways have been shown to play a role in cardiovascular and neurological function. Specifically, reactive species such as superoxide (O2•−) and peroxynitrite (ONOO2212) affect vascular tone and are excessively produced in hypertension. Peroxynitrite is a reactive nitrogen species formed via radical–radical coupling reactions between O2•− and nitric oxide (NO), which react rapidly in the vascular wall. As a result, the relationship between increased ROS production and high blood pressure can be explained by enhanced inactivation of the vasodilator NO by O2•−.Citation5–Citation6 Extensive lipid peroxidation, overproduction of endothelin, proliferation of vascular smooth muscle cells, and increased renal sodium reabsorption in hypertension have also been attributed to oxidative stress.Citation5
Depression is also a complex multifactorial disease that involves numerous biological processes and pathways including inflammation, oxidative stress, and progressive neuropathological defects. Neural, endocrine, immune, and metabolic pathways are implicated in major depressive disorder and are comprehensively reviewed elsewhere.Citation7–Citation9 A relationship between oxidative and nitrosative stress and depression is supported by evidence of increased free radical levels, lowered antioxidant levels, and increased lipid peroxidation in depressed patients.Citation10–Citation12
Oxidative stress results from the increased production of free radicals, decreased antioxidant levels, diminished activities of antioxidant enzymes, and impaired repair of oxidative damage.Citation13 The response to oxidative stress also includes induction of some protective mechanisms, and heme oxygenases (HOs) are widely considered central components of the mammalian stress response that protect against oxidative stress.Citation14
Since oxidative stress is common to the etiopathology of depression and hypertension, we hypothesized that an antioxidant-pro-oxidant imbalance contributes to the high susceptibility of hypertensive patients to depression and that biomarkers of the pro- and antioxidant equilibrium are associated with the prevalence and severity of depression in hypertensive patients. Here, we aimed to elucidate the relationship between biomarkers of peroxidation and antioxidant defense in depression and hypertension.
Methods
Subjects
Four groups of patients aged 50 or above were recruited 15 patients diagnosed and currently treated for depression (recurrent depressive disorder); 20 patients diagnosed and currently treated for hypertension; 16 patients diagnosed and currently treated for hypertension with symptoms of comorbid depression; and 19 healthy age- and sex-matched controls. Subjects were recruited from the Psychoneurology of the Elderly Center Sue Ryder Home and the Department and Clinic of Geriatrics, Collegium Medicum UMK (Bydgoszcz, Poland). The 21-item Beck Depression Inventory (BDI) with a cut–off >13Citation15 was used to assess the severity of depressive symptoms a week prior to study enrollment.
Patients with stage one and two hypertension were included in the study according to Polish Society of Hypertension guidelines (2011). Hypertension stages were defined by the values of systolic blood pressure (SBP) and diastolic blood pressure (DBP): stage one was when the SBP and DBP values were 140–159 and 90–99 mmHg, respectively, and stage two was when the SBP and DBP values were 160–179 and 100–109 mmHg, respectively.
All subjects were evaluated by standard physical examination and routine clinical laboratory tests. Exclusion criteria were mental disorders coded on DSM axes I and II, diabetes, endocrine disorders, hepatitis, cancer, chronic infection, hematologic malignancies, hemolytic anemia, a diagnosed vitamin B12 or folic acid deficiency, and patients on oral iron therapy.
Written informed consent was obtained from all study participants. The Nicolaus Copernicus University in Torun Human Ethics Committee approved the study protocol and the study was conducted under the tenets of the Declaration of Helsinki.
Blood sample collection and biochemical analyses
Venous heparin and cloth-activated fasting blood samples were collected in the morning (08:00 am). Reduced glutathione (GSH) concentrations were determined spectrophotometrically in whole blood samples by the Beutler method (1975) using 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB). GSH reacts non-enzymatically with DTNB to generate GSSG and the highly colored 5-thio-2-nitrobenzoic acid (peak absorbance at 420 nm). The remaining blood was centrifuged and, after a careful removal of plasma and the buffy coat, red blood cells were washed with cold isotonic saline (0.89% NaCl, pH = 7.4). The sediment was then lysed by the addition of distilled water to obtain red blood cell hemolysate by three freeze-thaw cycles. Glutathione peroxidase (GPx-1), glutathione reductase (GR), and superoxide dismutase (SOD-1) activities were determined in erythrocytes from hemolyzed blood samples according to Paglia and ValentineCitation16, Flohe and GunzlerCitation17, and Misra and FridovichCitation18, respectively.
SOD-1 activity was determined in ethanol/chloroform-treated samples by measuring the inhibition of autoxidation of adrenaline at pH 10.2, 37°C. GR activity was determined by measuring the oxidation of NADPH via the decrease in absorbance at 340 nm at 37°C in the presence of GSSG. GPx activity was determined by measuring the oxidation of NADPH at 340 nm at 25°C in the coupled reaction with t-butylhydroperoxide, GSH, and GR. Hemolyzed erythrocyte samples were also assayed for malondialdehyde (MDA) using the 2-thiobarbituric acid method read at 532 nm.
Serum samples were aliquoted and immediately stored at −80°C for further analysis of heme oxygenase (HO-1). Fresh plasma samples were analyzed for hydrogen peroxide (H2O2) concentration using the PeroxiDetect Kit (Sigma) according to the manufacturer's instructions; sample absorbance was measured at 560 nm, normalized, and expressed as nmol/ml. HO-1 was assayed using a human enzyme-linked immunosorbent assay (ELISA; USCN Life Science Inc., Cloud-Clone Corp., Houston, USA) kit. The mean optical density of duplicate samples was analyzed in the same run by the same operator. The average coefficient of variation between duplicates was 4.93.
Statistical analyses
Multiple comparisons between groups were conducted using non-parametric statistics, since the sample sizes of the groups were not large enough to test for the assumption for normality.
The Kruskal–Wallis non-parametric ANOVA was used for multiple comparisons. Linear regression was used to assess the relationship between biochemical markers of pro-antioxidant equilibrium and severity of depressive symptoms expressed as BDI scores. The relationship between biochemical markers and the prevalence of depressive symptoms was analyzed by logistic regression. Clinical and biochemical characteristics of patients and controls did not show significant confounding effects in regression models. Statistica version 9 software was used for statistical analyses. The results were considered significant if the two-sided P-value was 0.05 or less.
Results
Antioxidant-pro-oxidant equilibrium in patient groups and controls
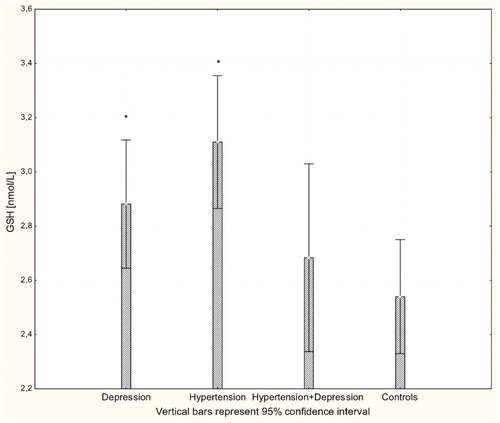
There were significant differences in the antioxidant-pro-oxidant equilibrium in all groups of patients. Multiple comparisons revealed significant differences in parameter values between patients and controls but not between different patient groups. The concentration of H2O2 was significantly increased in all patients groups compared to controls (P < 0.001; Fig. ). Likewise, there were significantly increased levels of the lipid peroxidation marker MDA in patients with hypertension, depression, and hypertension with comorbid depression compared to controls (P < 0.001; Fig. ). Concentrations of GSH were significantly increased in hypertensive and depressed patients (P < 0.01; Fig. ).
Figure 1 Concentration of H2O2 in plasma of patients with depression (n = 15), hypertension (n = 20), and hypertension with comorbid depression (n = 16) compared with controls (n = 19); *P < 0.001.
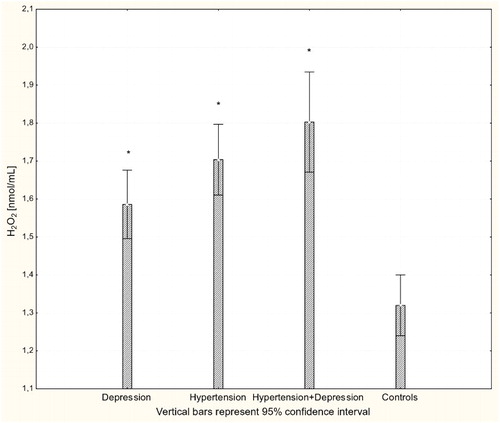
Figure 2 Concentration of MDA in erythrocytes of patients with depression (n = 15), hypertension (n = 20), and hypertension with comorbid depression (n = 16) compared with controls (n = 19); *P < 0.001.
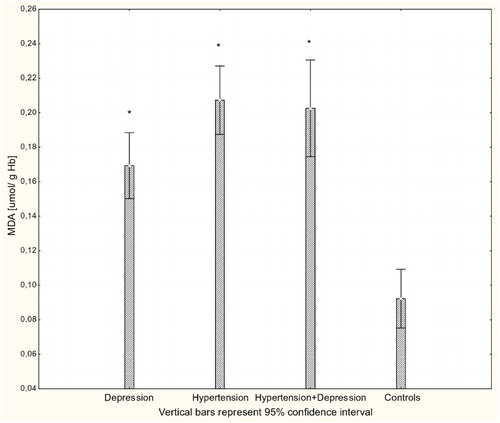
Figure 3 Concentration of GSH in whole blood of patients with depression (n = 15), hypertension (n = 20), and hypertension with comorbid depression (n = 16) compared with controls (n = 19); *P < 0.01.
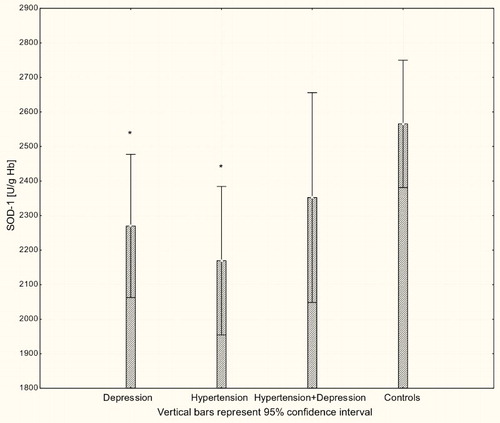
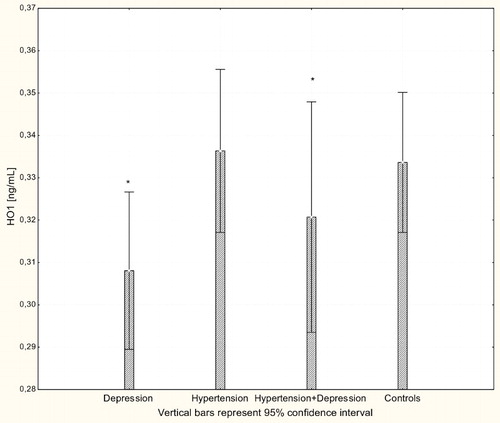
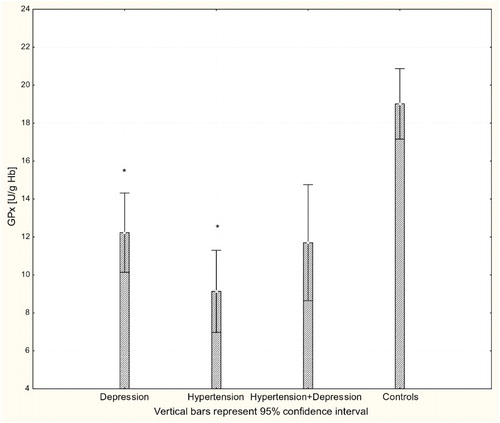
With respect to antioxidant enzyme activities, GR activity was significantly increased in patients with hypertension and patients with depression (P < 0.001) compared to controls (Fig. ), while GPx activity was significantly lower in hypertensive, depressive, and comorbid patients compared to controls (P < 0.001; Fig. ). The activity of SOD-1 in hypertensive patients and depressed patients was significantly lower compared to healthy subjects (P < 0.001; Fig. ), while lower levels of HOs were seen in the plasma of patients with depression and patients with comorbid depression and hypertension (P < 0.05; Fig. ).
Figure 4 Activity of GR in erythrocytes of patients with depression (n = 15), hypertension (n = 20), and hypertension with comorbid depression (n = 16) compared with controls (n = 19); *P < 0.01.
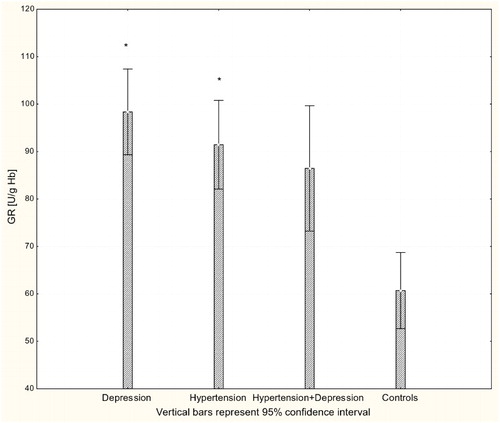
Figure 5 Activity of GPx-1 in erythrocytes of patients with depression (n = 15), hypertension (n = 20), and hypertension with comorbid depression (n = 16) compared with controls (n = 19); *P < 0.001.
The relationship between antioxidant-pro-oxidant equilibrium and depression
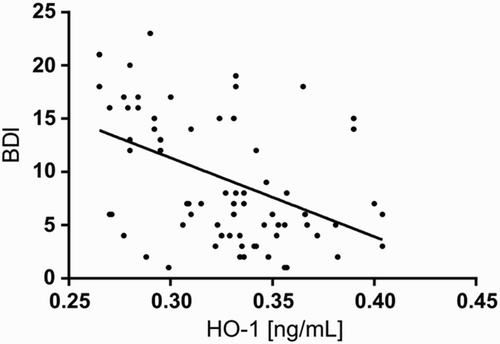
We next assessed the relationship between biochemical markers of the antioxidant-pro-oxidant equilibrium and the severity of depression symptoms expressed by BDI scores. There was a significant negative relationship between BDI scores and HO-1 levels; decreased enzyme expression was correlated with increased severity of depressive symptoms in depressed subjects (P < 0.05) (described by BDI = 17.0259–14.6551*HO1; see Fig. ).
Figure 8 Scatter plot of BDI against HO-1 in study population. BDI – Beck Depression Inventory; HO-1 – heme oxygenase.
The relationship between HO and the prevalence of depressive symptoms was confirmed by logistic regression, which allowed classification of hypertensive patients as depressed based on HO concentrations significantly better than by chance alone (P < 0.01).
Discussion
Here, we show that there are significant disturbances in the antioxidant-pro-oxidant equilibrium in patients with hypertension and depression. Both diseases were associated with increased levels of peroxidation and altered antioxidant enzyme activities. Interestingly, the antioxidant-pro-oxidant profiles for hypertension and depression were very similar. However, the expression of inducible HO-1 was specifically altered in depression and was significantly associated with both the prevalence and severity of depressive symptoms.
Our results on altered antioxidant enzyme activities (GR, GPx-1, and SOD-1) in depression are concordant with the existing body of evidence. Decreased activities of SOD-1 have been reported in depression;Citation19,Citation20 however, increased activities of erythrocyte and serum SOD-1 have also been reported;Citation21,Citation22 with antidepressant medication shown to reduce SOD-1 activity to control levels.Citation23 The latter study also reported increased activities of erythrocyte GPx and plasma GR and, likewise, antidepressants significantly affected these parameters. In our study, GR activity was also increased but GPx was decreased. Nevertheless, there is recent very strong evidence that whole blood GPx activity is lower in depressed patients,Citation24 in line with the evidence from Stefanescu and CiobicaCitation20 and further evidence that antidepressants have limited or no effect on enzyme activity.Citation25 There is also a substantial body of evidence that antioxidant defense systems are impaired in hypertension due to decreased activity of SOD-1 and increased, decreased, or unchanged activity of GP-x.Citation26–Citation28 The imbalance in antioxidant enzyme activities may result in increased susceptibility of red blood cells to lipid peroxidation.Citation22 Indeed, increased levels of MDA have been found in both hypertensionCitation29 and depression, and antidepressant treatment has been shown to lower lipid peroxidation.Citation21,Citation23,Citation30
Our analysis of the antioxidant-pro-oxidant equilibrium in hypertension and depression reveals similarities between hypertension and depression, with non-specific changes in SOD-1, GR, and GPx-1 activity or peroxide concentration. Inducible HO-1 was specifically altered in depression, with enzyme expression significantly associated with both prevalence and severity of depressive symptoms. The biological effects of the enzyme have been ascribed to its immunomodulatory, anti-inflammatory, and antioxidant functions.Citation31,Citation32 HO catalyzes degradation of heme to biliverdin, iron, and carbon monoxide, and degradation of free heme is important to prevent its cytotoxic and pro-oxidant effects.Citation33 Inducible HO-1 is known to be upregulated in response to pro-oxidant and pro-inflammatory factors including free heme, H2O2, prostaglandins, nitric oxide, peroxynitrite, and pro-inflammatory cytokines.Citation34 The increased levels of H2O2 and MDA in our study indicate a pro-oxidant milieu in depressed and hypertensive patients. The cellular response to excess ROS involves the induction of antioxidant response element (ARE) genes including glutathione-synthesizing glutamate– cysteine ligase and HO-1.Citation35 Here, we observed increased levels of glutathione but decreased expression of HO-1 in depressed patients compared to controls. This observation may be explained by another, ROS-independent mechanism of ARE gene regulation, namely via Bach1. Bach1 is usually bound by its ligand heme, which displaces it from the ARE for export from the nucleus and subsequent degradation. It has been previously been shown that Bach1 de-repression is required prior to Nrf2-dependent HO-1 gene expression; therefore, the Bach1-heme-HO-1 pathway allows coordination of overall intracellular levels of heme and iron with antioxidant protection.Citation36 We have previously reported a strong link between immune function, oxidative stress, and iron homeostasis in the blood of depressed patients;Citation37 however, the molecular mechanism was not explored. Our current results point toward a plausible role for HO-1 in depression, and we hypothesize that deregulation of the Bach1-heme-HO-1 pathway represents a molecular mechanism that might further explain the interplay between immune function, oxidative stress, and iron homeostasis in depression.
The products of heme catabolism also have cytoprotective, anti-apoptotic, antioxidant, and anti-inflammatory properties, suggesting an important role for HO-1 in the resolution of inflammation.Citation38–Citation40 The activity of HO is directly related to cardiovascular function and has a recognized vasoprotective role;Citation14,Citation41 however, little is known about the role of the enzyme in clinical hypertension. Here, no difference in the concentration of HO-1 protein in hypertensive patients and normotensive controls was observed. In contrast, depression was associated with significant down-regulation in both depressed patients and patients with comorbid depression and hypertension.
The relationship between HO and hypertension has been extensively studied but mainly in animal models. HO-1 overexpression is protective of renovascular hypertension in rats, most likely via interference with the action of angiotensin II (AngII).Citation42 The interaction between AngII and HO-1 was further confirmed in a human study in which HO-1 mRNA and protein synthesis and activity was down-regulated in neutrophils from healthy subjects treated with AngII and in cells from hypertensive patients.Citation43 Moreover, HO-1 inhibition was reversed in neutrophils from hypertensive patients treated with anti-hypertensive drugs. The effect of anti-hypertensive treatment might have accounted for the lack of significant changes in HO-1 levels in treated hypertensive patients in our study.
There is little evidence about HO-1 expression and activity in depression; however, the neuroprotective role of the enzyme in neurodegenerative disorders such as Alzheimer's disease has been established.Citation44 Furthermore, recent findings have shown that the neuroprotective effect of the tricyclic antidepressant desipramine was related to increased HO-1 expression,Citation45 and the neuroprotective and anti-neuroinflammatory effects of some herbal medications on hippocampal and microglial cells have been also ascribed to HO activity.Citation46 Taken together, the literature suggests that HO has a neuroprotective role, and since neurodegenerative process might play an important role in depressionCitation10 the association between decreased levels of HO and depression reported here can be indirectly linked to neural cell damage. However, HO-1 induction can be associated with both beneficial and deleterious effects depending on the experimental conditions and the neurodegenerative rodent models used.Citation47 Neuroinflammation is a well-known contributor to brain disorders and depression and neurodegenerative diseases in particular. Inflammatory cascades that activate microglia are triggered in response to brain damage such as oxidative stress, and activated microglia are a source of the factors that induce apoptosis and disrupt the blood–brain barrier (BBB). For instance, Zhao et al.Citation48 showed that enhanced expression of HO protected aganist BBB damage in rats.
Conclusions
Here, we present evidence on a potential role for HO as a biological factor that links inflammation, oxidative stress, and the biological and functional changes in brain activity in depression. The identification of decreased HO-1 expression as a potential risk factor for depression in patients with essential hypertension may provide an avenue for preventive strategies against depression in this group of patients. For instance, there may be a rationale for monitoring HO-1 as a biomarker for depression in patients with hypertension. Furthermore, HO in comorbid depression may represent a more universal biological mechanism mediating the development and progression of depressive disorder in general, which is further supported by the fact that psychological stress induces inflammatory and pro-oxidant pathways is often an antecedent to depression. However, neither inflammation nor oxidative stress is sufficient to induce depression; therefore, identification of biological factors specifically related to depression may have important implications for the diagnosis, treatment, and prevention of the disease.
Further research
Our results support the growing body of evidence that, while HO-1 is a critically important cytoprotective molecule, the degree of upregulation in response to an insult may serve as a marker of injury. Further studies should aim to determine whether HO-1 expression plays an antecedent, possibly causal, role in the psychiatric disorder or is solely related to the clinical manifestations in patients, i.e., chronicity and/or the impact of therapy. Whether HO-1 expression can predict the occurrence of depressive symptoms has yet to be validated in epidemiological studies, and a large-scale prospective trial in which the enzyme is measured would be of benefit.
Limitations
Larger, independent studies in medication-free subjects are required in order to translate our results into clinical practice.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest There are no conflicts-of-interest.
Ethics approval Written informed consent was obtained from all the participants of the study. The study was approved by the Nicolaus Copernicus University in Torun Human Ethics Committee and was conducted under the tenets of the Declaration of Helsinki.
Acknowledgement
This study was funded by the research grant awarded to Joanna Rybka from the Ministry of Science and Higher Education.
References
- Burgic-Radmanovic M, Trkulja D, Banjac V, Burgic S, Dragojevic S, Nikolic A. P01–17 - Hypertension in comorbidity with depression. Eur Psychiatry 2010;25:237. doi: 10.1016/S0924-9338(10)70236-4
- Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens 2012;30(5):842–51. doi: 10.1097/HJH.0b013e32835080b7
- Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the national health and nutrition examination survey I epidemiologic follow-up study. Arch Fam Med 1997;6(1):43–9. doi: 10.1001/archfami.6.1.43
- Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 2013;11(1):129. doi: 10.1186/1741-7015-11-129
- Grossman E. Does increased oxidative stress cause hypertension? Diabetes Care 2008;31(Suppl. 2):S185–9. doi: 10.2337/dc08-s246
- Herrera MD, Mingorance C, Rodríguez-Rodríguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev 2010;9(2):142–52. doi: 10.1016/j.arr.2009.07.002
- Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 2013;18(5):595–606. doi: 10.1038/mp.2012.33
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 2011;35(3):804–17. doi: 10.1016/j.neubiorev.2010.10.001
- Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012;36(2):764–85. doi: 10.1016/j.neubiorev.2011.12.005
- Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression's multiple comorbidities explained by (neuro)inflammatory and oxidative and nitrosative stress pathways. Neuro Endocrinol Lett 2011a;32(1):7–24.
- Maes M. The cytokine hypothesis of depression: inflammation, oxidative and nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett 2008;29(3):287–91.
- Palta P, Samuel L, Miller ER, Szanton SL. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom Med 2014;76(1):12–19. doi: 10.1097/PSY.0000000000000009
- Matés JM, Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem 1999;32(8):595–603. doi: 10.1016/S0009-9120(99)00075-2
- Stec DE, Ishikawa K, Sacerdoti D, Abraham NG. The emerging role of heme oxygenase and its metabolites in the regulation of cardiovascular function. Int J Hypertens 2012;2012:593530. doi: 10.1155/2012/593530
- Smarr KL, Keefer AL. Measures of depression and depressive symptoms: beck depression inventory-II (BDI-II), center for epidemiologic studies depression scale (CES-D), geriatric depression scale (GDS), hospital anxiety and depression scale (HADS), and patient health questionnaire. Arthritis Care Res 2011;63(S11):S454–66. doi: 10.1002/acr.20556
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70(1):158–69.
- Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol 1984;105:114–21. doi: 10.1016/S0076-6879(84)05015-1
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170–5.
- Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res 2007;38(2):247–52. doi: 10.1016/j.arcmed.2006.10.005
- Stefanescu C, Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord 2012;143(1–3):34–8. doi: 10.1016/j.jad.2012.05.022
- Bilici M, Efe H, Köroğlu MA, Uydu HA, Bekaroğlu M, Değer O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 2001;64(1):43–51. doi: 10.1016/S0165-0327(00)00199-3
- Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol 2007;22(2):67–73. doi: 10.1002/hup.829
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep Commun Free Radic Res 2003a;8(6):365–70. doi: 10.1179/135100003225003393
- Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Lower whole blood glutathione peroxidase (GPX) activity in depression, but not in myalgic encephalomyelitis/chronic fatigue syndrome: another pathway that may be associated with coronary artery disease and neuroprogression in depression. Neuro Endocrinol Lett 2011b;32(2):133–40.
- Behr GA, Moreira JCF, Frey BN. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxid Med Cell Longev 2012;2012:1–13. doi: 10.1155/2012/609421
- Simic DV, Mimic-Oka J, Pljesa-Ercegovac M, Savic-Radojevic A, Opacic M, Matic D, et al. Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. J Hum Hypertens 2006;20(2):149–55. doi: 10.1038/sj.jhh.1001945
- Pedro-Botet J, Covas MI, Martín S, Rubiés-Prat J. Decreased endogenous antioxidant enzymatic status in essential hypertension. J Hum Hypertens 2000;14(6):343–5. doi: 10.1038/sj.jhh.1001034
- Russo C, Olivieri O, Girelli D, Faccini G, Zenari ML, Lombardi S, et al. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens 1998;16(9):1267–71. doi: 10.1097/00004872-199816090-00007
- Kedziora-Kornatowska K, Kornatowski T, Bartosz G, Pawluk H, Czuczejko J, Kedziora J, et al. Production of nitric oxide, lipid peroxidation and oxidase activity of ceruloplasmin in blood of elderly patients with primary hypertension. Effects of perindopril treatment. Aging Clin Exp Res 2006;18(1):1–6. doi: 10.1007/BF03324634
- Kotan VO, Sarandol E, Kirhan E, Ozkaya G, Kirli S. Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: a 24-week follow-up study. Prog Neuropsychopharmacol Biol Psychiatry 2011;35(5):1284–90. doi: 10.1016/j.pnpbp.2011.03.021
- Ke B, Shen X-D, Zhai Y, Gao F, Busuttil RW, Volk H-D, et al. Heme oxygenase 1 mediates the immunomodulatory and antiapoptotic effects of interleukin 13 gene therapy in vivo and in vitro. Hum Gene Ther 2002;13(15):1845–57. doi: 10.1089/104303402760372945
- Tsuchihashi S, Livhits M, Zhai Y, Busuttil RW, Araujo JA, Kupiec-Weglinski JW. Basal rather than induced heme oxygenase-1 levels are crucial in the antioxidant cytoprotection. J Immunol 2006;177(7):4749–57. doi: 10.4049/jimmunol.177.7.4749
- Pae H-O, Kim E-C, Chung H-T. Heme oxygenase and carbon monoxide: medicine chemistry and biological effects guest editor: Yuji Naito integrative survival response evoked by heme oxygenase-1 and heme metabolites. J Clin Biochem Nutr 2008;42(3):197–203. doi: 10.3164/jcbn.2008029
- Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 2009;110(2):469–85. doi: 10.1111/j.1471-4159.2009.06160.x
- Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 2010;38(17):5718–34. doi: 10.1093/nar/gkq212
- Attucks OC, Jasmer KJ, Hannink M, Kassis J, Zhong Z, Gupta S, et al. Induction of heme oxygenase I (HMOX1) by HPP-4382: a novel modulator of bach1 activity. PLoS ONE 2014;9(7):e101044. doi: 10.1371/journal.pone.0101044
- Rybka J, Kędziora-Kornatowska K, Banaś-Leżańska P, Majsterek I, Carvalho LA, Cattaneo A, et al. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med 2013;63:187–94. doi: 10.1016/j.freeradbiomed.2013.05.019
- Belcher JD, Nath KA, Vercellotti GM. Vasculotoxic and proinflammatory effects of plasma heme: cell signaling and cytoprotective responses. ISRN Oxidative Med 2013;2013:1–9. doi: 10.1155/2013/831596
- Cozzi A, Levi S, Corsi B, Santambrogio P, Campanella A, Gerardi G, et al. Role of iron and ferritin in TNFalpha-induced apoptosis in HeLa cells. FEBS Lett 2003;537(1–3):187–92. doi: 10.1016/S0014-5793(03)00114-5
- Lee T-S, Chau L-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 2002;8(3):240–6. doi: 10.1038/nm0302-240
- Marcantoni E, Di Francesco L, Dovizio M, Bruno A, Patrignani P. Novel insights into the vasoprotective role of heme oxygenase-1. Int J Hypertens 2012;2012:1–12. doi: 10.1155/2012/127910
- Botros FT, Schwartzman ML, Stier CT, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int 2005;68(6):2745–55. doi: 10.1111/j.1523-1755.2005.00745.x
- Alba G, El Bekay R, Chacón P, Reyes ME, Ramos E, Oliván J, et al. Heme oxygenase-1 expression is down-regulated by angiotensin II and under hypertension in human neutrophils. J Leukoc Biol 2008;84(2):397–405. doi: 10.1189/jlb.0108035
- Mancuso C, Santangelo R, Calabrese V. The heme oxygenase/biliverdin reductase system: a potential drug target in Alzheimer's disease. J Biol Regul Homeost Agents 2013;27(2 Suppl):75–87.
- Lin H-Y, Yeh W-L, Huang B-R, Lin C, Lai C-H, Lin H, et al. Desipramine protects neuronal cell death and induces heme oxygenase-1 expression in Mes23.5 dopaminergic neurons. PLoS ONE 2012;7(11):e50138. doi: 10.1371/journal.pone.0050138
- Eom HW, Park SY, Kim YH, Seong SJ, Jin ML, Ryu EY, et al. Bambusae caulis in Taeniam modulates neuroprotective and anti-neuroinflammatory effects in hippocampal and microglial cells via HO-1- and Nrf-2-mediated pathways. Int J Mol Med 2012;30(6):1512–20.
- Tronel C, Rochefort GY, Arlicot N, Bodard S, Chalon S, Antier D. Oxidative stress is related to the deleterious effects of heme oxygenase-1 in an in vivo neuroinflammatory rat model. Oxid Med Cell Longev 2013;2013:1–10. doi: 10.1155/2013/264935
- Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci Off J Soc Neurosci 2007;27(38):10240–8. doi: 10.1523/JNEUROSCI.1683-07.2007