Abstract
Objectives: The in vivo radio-protective effect of total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst was evaluated using Swiss albino mice, by pre-treatment with total triterpenes for 14 days, followed by a whole body exposure to γ-radiation.
Methods: The activities of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), and the level of reduced glutathione (GSH) were analysed in liver and brain homogenates. The extent of lipid and protein peroxidation was also estimated in liver and brain homogenates after irradiation. Protection of radiation-induced DNA strand breaks in peripheral blood lymphocytes and bone marrow cells was assessed using the comet assay.
Results: Total triterpenes were highly effective in reducing the levels of lipid peroxidation and protein oxidation to near normal values in both liver and brain tissues. Total triterpenes, when administered in vivo, were also found to be successful in restoring the antioxidant enzyme activities and GSH level in liver and brain of irradiated mice. Administration of total triterpenes, prior to radiation exposure, significantly decreased the DNA strand breaks.
Discussion: The results of the present study thus revealed the potential therapeutic use of Ganoderma total triterpenes as an adjuvant in radiation therapy.
Introduction
The fundamental equilibrium between life and death can be prejudiced by numerous environmental stressors. Among the various environmental stresses, free radicals, especially reactive oxygen species (ROS), are the most hazardous factors that influence living beings. In addition to the normal metabolic processes in which free radicals are produced as by-products, oxidative stress can be triggered by environmental influences such as UV light, ionizing radiation, and various chemical agents. Even though a majority of stresses can be overcome by the natural defence mechanisms of the cell, continuous disturbance of this balance will lead to either apoptotic or necrotic cell death. Oxidative stress-induced damage can also impair cellular proteins, lipids, and DNA, thereby causing alterations in various functions and physiological processes of the cell.
Ionizing radiations are an important source of oxidative stress. They can cause damage to cellular molecules either by direct transfer of energy or through the generation of oxygen-derived free radicals. Damage due to ionizing radiations mainly occurs through free radical-mediated mechanisms. For many years, there has been intensive research on radio-protective compounds due to their high importance in military, industrial, and clinical applications. Development of safe and effective radio-protective compounds for human applications is particularly significant in cancer therapy. Although radiotherapy is a common and effective tool for cancer treatment, the radio-sensitivity of normal tissues, especially those adjacent to the tumours, limits its therapeutic potential. Hence, protection of normal tissues against radiation-induced cellular injury is of immense importance in radiotherapy.
Dietary components, especially mushrooms, can serve as excellent pharmaceutical agents because of their low toxicity profile and ease of administration. Mushrooms might be considered as biological drug factories that possess a wide variety of therapeutically active compounds. Recent investigations have demonstrated that a number of mushrooms possess antioxidant and radio-protective activities.Citation1,Citation2 Ganoderma lucidum, commonly known as ‘reishi’ or ‘lingzhi’, is recognized as a superior medicinal mushroom that has been used to promote health and longevity in traditional Chinese medicine for many years. Fruiting bodies of G. lucidum contain numerous phytochemicals, among which the polysaccharides and triterpenoids have been recognized as the major active constituents. Aqueous extract and polysaccharides isolated from G. lucidum mushroom were found to possess radio-protective properties.Citation3,Citation4 Although more than 140 different triterpenes have been identified from G. lucidum, there is very little information available regarding the application of these triterpenes as successful therapeutic agents.Citation5–Citation8 Moreover, it has been reported that, based on the strain, origin, extraction process, and cultivation conditions of G. lucidum, there will be qualitative and quantitative differences in the chemical composition, physiological, and pharmacological properties of its isolated compounds.Citation9 In our previous study, we identified the antioxidative potential of ‘total triterpenes’ isolated from the fruiting bodies of G. lucidum occurring in South India.Citation10 It was also found to protect DNA and membranes from γ-radiation induced damages in vitro.Citation11,Citation12 The present study mainly focuses on the protective effect of the total triterpenes against γ-radiation induced oxidative stress in vivo. The activity was analysed using Swiss albino mice, with the pre-treatment of total triterpenes for 14 days followed by a whole body exposure to four Gy γ-radiation. To the best of our knowledge, this is the first report on the radio-protective effect of total triterpenes isolated from G. lucidum occurring in South India against γ-radiation-induced damage in vivo.
Methods
Isolation of total triterpenes
Total triterpenes were isolated from the fruiting bodies of G. lucidum as previously described.Citation10 Briefly, an ethanol extract of G. lucidum fruiting bodies was dissolved in chloroform and the soluble fraction was then concentrated. The concentrate was loaded on to a silica gel column and eluted with petroleum ether, chloroform, methanol, or various combinations of these solvents. The fractions that answered the tests for triterpenesCitation13 were combined and concentrated to give the total triterpenes.
Animal maintenance
Male Swiss albino mice (weighing 25 ± 2 g) were purchased from Small Animal Breeding Station, Mannuthy, Kerala, India and were housed in well-ventilated cages under controlled conditions of light and humidity. The mice were provided with normal mouse chow (Sai Durga Food and Feeds, Bangalore, India) and water ad libitum. All the experiments were carried out as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India and by the approval of the Institutional Animal Ethical Committee (149/99/CPCSEA dated 23-10-2009). Animals were divided into four groups of six animals. Group I animals were maintained as normal without any drug or radiation treatment. Group II animals served as positive controls that received only radiation treatment. Groups III and IV animals were administered with total triterpenes orally at doses of 50 and 100 mg/kg b.w.t., respectively.
Irradiation schedule
Animals were placed in perspex-covered boxes and were exposed to whole body irradiation, at a dose rate of 1.41 Gy/min, using a 60Co-Theratron Phoenix Teletherapy Unit.
Protection against oxidative stress induced by γ-radiation in vivo
Total triterpene treatment was given to Groups III and IV animals, once each day for 14 days. One hour after the last dose of triterpenes, the Groups II, III, and IV animals received 4 Gy γ-radiation, as a single whole body exposure. The animals were sacrificed 24 hours after the irradiation. Liver and brain tissues were removed and homogenized. The homogenate was then used to study the levels of antioxidant enzymes, lipids, and protein oxidation. The protein concentration was determined by Bradford's method.Citation14
Effect on radiation-induced lipid peroxidation
Lipid peroxidation levels in liver and brain homogenates were estimated by measuring the amount of thiobarbituric acid reactive substances (TBARS) and lipid hydroperoxides (LOOH) formed, using the TBARS assay and FOX II method, respectively.
In the TBARS assay,Citation15–Citation17 500 µl of liver and brain homogenates were heated with 500 µl of reagent containing 20% (w/v) trichloroacetic acid (TCA), 0.5% (w/v) thiobarbituric acid (TBA), 2.5 N HCl and 6 mM ethylene diamine tetraacetic acid (EDTA) for 20 minutes in a boiling water bath. After cooling, the solution was centrifuged at 2000g for 10 minutes and the absorbance of the supernatant was determined at 532 nm against a blank containing all the reagents except the biological sample. Concentration of TBARS formed was then calculated with the help of a standard graph using 1′,1′,3′,3′-tetra methoxy propane as malondialdehyde equivalent.
In the xylenol orange assay, or FOX II method,Citation18,Citation19 125 μl of liver and brain homogenates were incubated with 875 μl of FOX II reagent at 37°C for 30 minutes. The FOX II reagent contained solution A (98 mg ammonium ferrous sulphate and 79 mg xylenol orange dissolved in 100 ml of 250 mM H2SO4) and solution B (969 mg butylated hydroxyl toluene (BHT) dissolved in 900 ml of methanol) mixed in a 1:9 ratio. It was centrifuged at 10 000g for 15 minutes at 20°C and the absorbance of the supernatant was measured at 560 nm. Lipid peroxides formed were calculated from a standard graph of optical density plotted against the concentration of H2O2.
Effect on radiation-induced protein peroxidation
Protein peroxidation levels in liver and brain homogenates were estimated by measuring the amount of protein carbonyls formed and by assessing the extent of depletion of protein thiols.
The carbonyl content of the liver and brain of the control and treated animals was assayed by the method of Evans et al.Citation20 and Levine et al.Citation21 with some modifications. Briefly, 100 μl of homogenate (10%) was incubated with 20 μl streptomycin sulphate (10%, w/v) solution and the mixture was centrifuged at 2800g. The supernatant was collected, and the protein was precipitated by adding equal volumes of 20% (w/v) TCA. To the protein pellet, 1.5 ml of 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2 M HCl was added, mixed well and incubated for 1 hour at room temperature. To this, 1.5 ml of 20% TCA was added and kept for 15 minutes at room temperature. The mixture was then centrifuged at 3400g. The precipitates were washed three times with ethyl acetate: ethanol mixture (1:1) to remove the excess DNPH. The final pellet was dissolved in 1.25 ml of 6 M guanidine hydrochloride and the absorbance of both solutions (DNPH and HCl) was measured at 370 nm. The carbonyl content was calculated in terms of nmol/mg protein.
The total thiol and protein thiol contents of the liver and brain homogenates were assayed by the method of Sedlak and Lindsay.Citation22 For measuring total thiol content, aliquots of 250 μl of tissue homogenate were mixed with 750 μl of 0.2 M tris buffer (pH 8.2) and 50 μl of 0.01 M 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB). The mixture was made up to 5 ml with absolute methanol. A reagent blank (without sample) and a sample blank (without DTNB) were prepared in a similar manner. The test tubes were stoppered with rubber caps and the colour was developed for 15 minutes. The absorbance of the supernatant was read at 412 nm and the amount of total thiol (T-SH) was calculated.Citation23
For measuring non-protein thiols, 250 μl aliquots of tissue homogenate were mixed in 5 ml test tubes with 200 μl distilled water and 50 μl of 50% TCA. The tubes were shaken intermittently for 10–15 minutes and centrifuged at 3000g for 15 minutes. Supernatant (200 μl) was mixed with 400 μl of 0.4 M Tris buffer (pH 8.9) and 10 μl DTNB. The absorbance of the sample mixture was read within 5 minutes of the addition of DTNB at 412 nm against a reagent blank containing no tissue homogenate and the amount of non-protein thiol (P-SH) was estimated. The molar extinction coefficient at 412 nm was calculated as 13 100 L mol−1 cm−1 for both T-SH (total thiol) and Np-SH (non-protein thiol) procedures. The concentration of protein-bound thiol groups (P-SH) was calculated by subtracting the Np-SH from T-SH.Citation23
Effect on antioxidant status in liver and brain after irradiation
The activities of the antioxidant enzymes (SOD, CAT, and GPx) and the levels of GSH in liver and brain homogenate were analysed. The activity of SOD was estimated by the method of McCord and FridovichCitation24 based on the reduction of NBT. The activity of catalase was measured by the method of Beer and SizerCitation25 by measuring the rate of decomposition of hydrogen peroxide (H2O2) at 240 nm. The activity of GPx was assessed using the method of Hafemann et al.Citation26 based on the degradation of H2O2 in the presence of GSH. The level of reduced GSH was determined by the method of Moron et al.Citation27 based on the reaction with DTNB.
Effect of total triterpenes on γ-radiation induced DNA damage
Total triterpene treatment was given to Groups III and IV animals, once in a day for 14 days. One hour after the last dose of triterpenes, the Groups II, III, and IV animals received 6 Gy γ-radiation as a single whole body exposure. The animals were sacrificed immediately after the irradiation. Blood was collected and heparinized. Bone marrow cells were collected from the femurs in ice-cold phosphate buffered saline (PBS) (pH 7.4) containing 2% (w/v) fetal bovine serum (FBS).
The effect of total triterpenes (10, 50, and 100 µg) on radiation-induced DNA strand breaks in peripheral blood lymphocytes and bone marrow cells were assessed using single cell gel electrophoresis or comet assay.Citation28,Citation29 To the frosted microscope slides, 200 µl of 1% normal melting agarose in PBS was added and immediately covered. The slides were then kept at 4°C for 10 minutes. A second layer of 200 µl of 0.5% (w/v) low melting agarose containing approximately 105 treated cells at 37°C was added. Cover slips were placed immediately and the slides were kept at 4°C. After solidification, the slides were placed in the chilled lysis solution (pH 10) containing 2.5 M NaCl, 100 mM Na2-EDTA, 10 mM Tris–HCl, 1% (v/v) DMSO, 1% (v/v) Triton X100 and 1% (w/v) sodium sarcosinate, for 1 hour at 4°C. The slides were removed from the lysis solution and placed in a horizontal electrophoresis tank filled with freshly prepared alkaline buffer (300 mM NaOH, 1 mM Na2-EDTA, and 0.2% (v/v) DMSO, pH ≥ 13.0). The slides were equilibrated in the same buffer for 20 minutes and electrophoresis was carried out at 25 V for 20 minutes. After electrophoresis, the slides were gently washed with 0.4 M Tris–HCl buffer, pH 7.4, to remove the alkali. The slides were stained with 50 μl of propidium iodide (20 μg/ml) and the images were captured using a Carl Zeiss Fluorescent microscope (Axioskop) with bright field phase-contrast and epi-fluorescence facility. The quantification of the DNA strand breaks was done by measuring % DNA in tail, tail length, tail moment, and olive tail moment with the aid of CASP software.
Statistical analysis
All values were expressed as the mean ± standard deviation (SD), n = 6. Statistical evaluation of the data was done by one-way analysis of variance followed by Bonferroni's test using InStat Graph Pad software. A P value less than 0.05 was considered as significant with respect to control group.
Results and discussion
Radiation-derived reactive oxygen intermediates can potentially produce damage to DNA, lipids, proteins, antioxidants, and other molecules. Products of oxidative cell damage are considered as biomarkers in the physiopathology of many diseases. In the current study, the extent of oxidative stress enforced by γ-radiation was assessed using independent parameters like DNA damage, lipid peroxidation, protein peroxidation, and levels of antioxidant systems in the tissues.
Lipid peroxidation is one of the major damages resulting from radiation-induced oxidative stress. Whole body irradiation leads to increased markers of lipid peroxidation including TBARS and LOOH. During irradiation, LOOH first originates from unsaturated fatty acids and subsequently degrades into different cytotoxic aldehydes such as 4-hydroxynonenal and malondialdehyde (MDA).Citation30–Citation32 Measurement of free MDA and LOOH levels are useful markers of oxidative stress induced by radiation. Table gives the result of TBARS and FOX II assays performed to gauge the level of lipid peroxidation in liver and brain homogenates. Lipid peroxidation levels were found to be elevated after irradiation with 4 Gy γ-radiations in liver and brain tissue of control animals. Treatment with total triterpenes effectively reduced the peroxidation of lipids in both liver and brain tissues. In the group treated with 100 mg/kg b.wt. total triterpenes, the increases in levels of lipid peroxides and TBARS were significantly attenuated in both liver and brain tissues.
Table 1 Effect of total triterpenes on lipid peroxidation levels in brain and liver of irradiated mice
Radiation-induced free radicals can oxidize proteins, thereby increasing their hydrophobicity and sensitivity to proteolysis. Free radicals may react with amino acids or sulphur groups, leading to cross-linking and aggregation of proteins. ROS oxidize proteins and thereby generate a series of stable as well as reactive intermediates such as protein hydroperoxides. These intermediates can further react with transition metal ions and generate numerous other reactive products.Citation33 The product of lipid peroxidation can also cause severe damage to the proteins present in the membranes, including formation of high molecular mass protein aggregates, inactivation of surface receptor molecules and enzymes, destruction of Ca2+, Na+, K+-ATPase, and damage of potassium channels. Protection against protein oxidation was assessed as formation of protein carbonyls and depletion of protein thiols. Whole body exposure to 4 Gy γ-radiations cause an increase in the peroxidation of proteins in the liver and brain of mice. Table gives the levels of protein peroxidation in liver and brain homogenates. Radiation treatment increased the formation of protein carbonyls and decreased the amount of protein thiols. From these results, it is clear that the treatment with total triterpenes could effectively prevent protein peroxidation as evident from decreased protein carbonyl and increased protein thiol levels in the liver and brain tissues of treated mice. The extent of radiation-induced lipid and protein peroxidation was higher in the case of the brain tissue than liver tissue. This may be because of the extreme susceptibility of the brain towards antioxidant damage owing to its high utilization of oxygen and poorly developed antioxidative defence mechanisms.Citation34 Administration of total triterpenes prior to radiation exposure significantly attenuated the increases in levels of lipid peroxidation and protein oxidation to near normal values in both liver and brain tissues.
Table 2 Effect of total triterpenes on protein peroxidation levels in brain and liver of irradiated mice
The antioxidant enzymes (SOD, CAT, and GPx) and GSH constitute the body's endogenous defence mechanisms to help protect against free radical-induced cell damage. SOD, CAT, and GPx establish a mutually supportive defence against ROS. GSH prevents the oxidation of protein thiol groups, either directly by reacting with reactive species or indirectly through glutathione transferases. The radiation-derived free radicals can in turn weaken antioxidant defence mechanisms leading to an increased membrane lipid peroxidation that results in the impairment of membrane structure and inactivation of membrane-bound enzymes.Citation35 Increased activities of these antioxidant enzymes and increased level of GSH in the living system can provide better protection against radiation-induced damage. The estimated antioxidant enzymes and GSH levels in the liver and brain homogenates of treated animals are summarized in Tables and , respectively. Total triterpenes, when administered in vivo, were found to be highly effective in restoring the antioxidant status in the liver and brain of irradiated mice. In the control group, there was depletion in the levels of antioxidant enzymes SOD, CAT, and GPx. The tissue GSH level also was decreased after the radiation treatment. However, total triterpenes when administered 14 days before the irradiation effectively restored the depleted levels of antioxidant systems in both liver and brain homogenates.
Table 3 Effect of total triterpenes on antioxidant enzymes and GSH levels in liver of irradiated mice
Table 4 Effect of total triterpenes on antioxidant enzymes and GSH levels in brain of irradiated mice
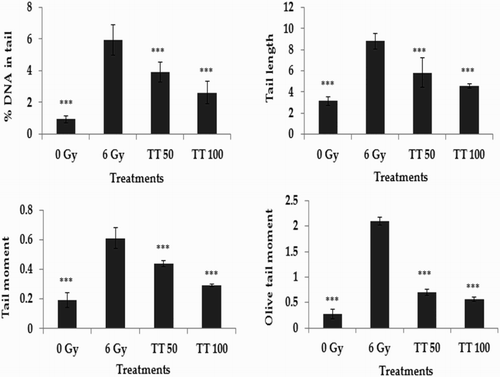
The alkaline comet assay is a widely used sensitive technique to monitor DNA lesions including single and double strand breaks and to study genotoxicity and apoptosis induced by toxic environmental agents.Citation36–Citation38 DNA strand breaks in peripheral blood lymphocytes, bone marrow cells, and spleen cells treated in vivo were assessed using the comet assay and the results are represented in Figs. –, respectively. Exposure to 6 Gy whole body irradiation induced strand breaks in the DNA of murine tissues such as blood, bone marrow, and spleen. The comet parameters %DNA in tail, tail length, tail moment, and olive tail moment were found to be elevated in the group treated with radiation alone, implying the formation of DNA strand breaks due to radiation exposure. However, administration of total triterpenes for 7 days before whole body irradiation attenuated the changes in comet parameters in peripheral blood lymphocytes (Fig. ), bone marrow cells (Fig. ), and spleen cells (Fig. ), indicating the protection against radiation-induced DNA damage. The results clearly indicate the ability of total triterpenes to protect cellular DNA against γ-radiation in vivo.
Figure 1 Protection against radiation-induced DNA damage in blood lymphocytes. TT: Ganoderma triterpenes (μg/ml). Data represented as mean ± SD, from three individual experiments, ***P < 0.001, nsP > 0.05 (Bonferroni test) with respect to damage group.
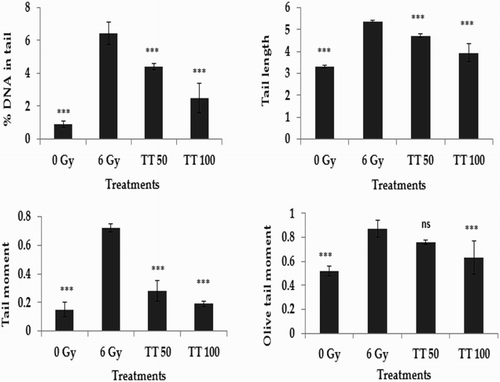
Figure 2 Protection against radiation-induced DNA damage in bone marrow cells. TT: Ganoderma triterpenes (μg/ml). Data represented as mean ± SD, from three individual experiments, ***P < 0.001, nsP > 0.05 (Bonferroni test) with respect to damage group.
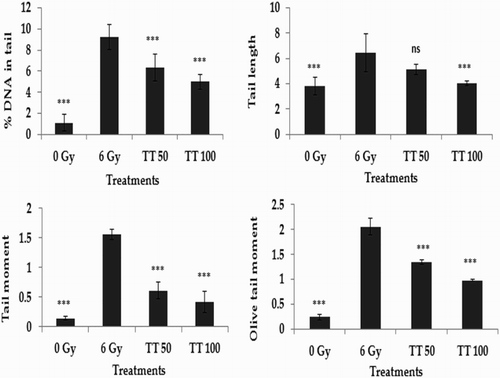
Figure 3 Protection against radiation-induced DNA damage in spleen cells. TT: Ganoderma triterpenes (μg/ml). Data represented as mean ± SD, from three individual experiments, ***P < 0.001 (Bonferroni test) with respect to damage group.
An earlier study conducted in our laboratory also revealed in vitro and in vivo antioxidant activities of Ganoderma total triterpenes.Citation10 The activities of antioxidant enzyme systems were boosted by the oral treatment of total triterpenes for 30 days.Citation10 The antioxidant effect of total triterpenes in terms of inhibition of lipid and protein oxidation and its ability to enhance antioxidant enzyme activities could be the possible mechanisms behind its exhibited radio-protective activity. Antioxidant enzymes could successively remove the free radicals and their reactive products induced by the ionizing radiation. Total triterpenes also proved to be highly effective in scavenging or neutralizing the free radicals produced in various in vitro experiments.Citation10 Radical scavenging or the neutralizing ability of total triterpenes could be the possible reason behind its observed DNA protective effect in whole body irradiation. Our earlier studies also confirmed its ability to prevent radiation-induced DNA strand breaks in pBR 322 plasmid DNA in vitro,Citation12 human peripheral blood lymphocytes ex vivo,Citation12 and splenic lymphocytes in vitro.Citation11 Ganoderma total triterpenes also protected against mitochondrial and microsomal membrane damage in vitro and prevented micronuclei formation in mice bone marrow cells in vivo.Citation12 These observations also support the findings of the current study.
Conclusions
Total triterpenes isolated from the fruiting bodies of G. lucidum protected Swiss albino mice from oxidative stress induced by γ-radiation in vivo. Total triterpenes administered for 14 consecutive days before whole body exposure to γ-radiation was found to reduce lipid and protein oxidation in both liver and brain tissues of irradiated mice. It also restored the antioxidant enzyme activities and the GSH level in liver and brain of irradiated mice. Total triterpenes were also very effective in preventing the single and double strand DNA breaks induced by γ-radiation. The present study thus revealed the potential therapeutic use of Ganoderma total triterpenes as a natural radio-protector to prevent hazardous effects of accidental radiation exposures. Further investigations and human trials are necessary to establish the use of Ganoderma total triterpenes in clinical applications.
Disclaimer statements
Contributors The author T.P.S. designed the studies, carried out the experiments and drafted the manuscript. J.J. participated in the design of the study and assisted in performing the surgical procedures. K.K.J. participated in the coordination of the study, critically evaluated and corrected the manuscript. All authors read and approved the final manuscript.
Funding None.
Conflict of interest The authors declare that there are no conflicts of interest in publishing the manuscript. All the authors are associated and contributed to the work and there are no competing financial interests.
Ethics approval All the experiments were carried out as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India, and by the approval of Institutional Animal Ethical Committee (149/99/CPCSEA dated 23-10-2009).
Acknowledgements
Authors express their deep gratitude to Dr T.A. Ajith, Amala Institute of Medical Sciences and Dr D.K. Maurya, Baba Atomic Research Centre, for their valuable suggestions during the study. The authors sincerely thank Dr Taryn Hogan, Biomedical Research Section 16, UT Health Science Center at Tyler, for her critical corrections in the manuscript.
References
- Chang R. Functional properties of edible mushrooms. Nutr Rev 1996;54(11):91–3. doi: 10.1111/j.1753-4887.1996.tb03825.x
- Lakshmi B, Tilak JC, Adhikari S, Devasgayam TPA, Janardhanan KK. Evaluation of antioxidant activity of Indian Mushrooms. Pharma Biol 2004;42(3):179–85. doi: 10.1080/13880200490514023
- Pillai TG, Salvi VP, Maurya DK, Nair CKK, Janardhanan KK. Prevention of radiation-induced damages by aqueous extract of Ganoderma lucidum occurring in southern parts of India. Curr Sci 2006;91:341–4.
- Pillai TG, Nair CKK, Janardhanan KK. Polysaccharides isolated from Ganoderma lucidum occurring in Southern parts of India, protects radiation induced damages both in vitro and in vivo. Environ Toxicol Pharmacol 2008;26:80–5. doi: 10.1016/j.etap.2008.02.004
- Hobbs CH. Medicinal mushrooms: an exploration of tradition, healing and culture. Santa Cruz: Botanica Press; 1995. p. 251.
- Mizuno T, Sakai T, Chihara G. Health foods and medicinal usages of mushrooms. Food Rev Int 1995;11:69–81. doi: 10.1080/87559129509541020
- Wasser SP, Weis AL. Reishi mushroom (Ganoderma lucidum (Curtis: Fr.) P. Karst). In: Nevo E, (ed.) Medicinal mushrooms. Haifa: Peledfus Publ House; 1997: p. 39.
- Stamets P. Growing gourmet and medicinal mushrooms. 3rd ed. Berkeley, CA: Ten Speed Press; 2000.
- Nishitoba T, Sato H, Shirasu S, Sakamura S. Evidence on the strain specific terpenoid pattern of Ganoderma lucidum. Agric Biol Chem 1986;50(8):2151–4.
- Smina TP, Mathew J, Janardhanan KK, Devasagayam TPA. Antioxidant activity and toxicity profile of total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst occurring in South India. Environ Toxicol Pharmacol 2011;32:438–46. doi: 10.1016/j.etap.2011.08.011
- Smina TP, Strayo De., Devasagayam TPA, Adhikari S, Janardhanan KK. Ganoderma lucidum total triterpenes prevent radiation – induced DNA damage and apoptosis in splenic lymphocytes in vitro. Mutat Res Genet Toxicol Environ Mutagen 2011;726:188–94. doi: 10.1016/j.mrgentox.2011.09.005
- Smina TP, Maurya DK, Devasagayam TPA, Janardhanan KK. Protection of radiation induced DNA and membrane damages by total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst. Chem Biol Interact. 2015;233:1–7. doi: 10.1016/j.cbi.2015.03.019
- Harborne JB, ed. Phytochemical methods: a guide to modern techniques of plant analysis. 2nd ed. London: Chapman and Hall Press; 1973: p. 270–9.
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. doi:10.1016/0003-2697(76)90527-3
- Sinnhuber RO, Yu TC. 2-Thiobarbituric acid for the measurement of rancidity in fishery products II – The qualitative measurement of malondialdehyde. Food Technol 1958;12:9–12.
- Hunter FE, Gebicki JM, Hoffsten PE, Weinstein J, Scott A. Swelling and lysis of rat liver mitochondria induced by ferrous ions. J Biol Chem 1963;238:828–35.
- Devasagayam TPA, Pushpendran CK, Eapen J. Differences in lipid peroxidation in rat liver rough and smooth microsomes. Biochim Biophys Acta 1983;750:91–7. doi: 10.1016/0005-2760(83)90207-2
- Jiang ZY, Hunt JV, Wolf SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 1992;202:384–9. doi: 10.1016/0003-2697(92)90122-N
- Nourooz-Zadeh J, Sarmadi J, Wolff SP. Measurement of plasma hydroperoxide concentration by the ferrous oxidation. Anal Biochem 1994;220:403–9. doi: 10.1006/abio.1994.1357
- Evans P, Lyras L, Halliwell B. Measurement of protein carbonyls in human brain tissue. Methods Enzymol 1999;300:145–56. doi: 10.1016/S0076-6879(99)00122-6
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186: 464–78. doi: 10.1016/0076-6879(90)86141-H
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968;25:192–205. doi:10.1016/0003-2697(68)90092-4
- Kayali R, Cakatay U, Akcay T, Altug T. Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem Func 2006;24:79–85. doi:10.1002/cbf.1190
- McCord JM, Fridovich I. Superoxide dismutase enzyme function for erythrocaprein. J Biochem 1969;244:6049–56.
- Beer RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 1951;95:133–40.
- Hafeman DG, Sundae RA, Houestra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 1974;104:580–7.
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979;582:67–8. doi: 10.1016/0304-4165(79)90289-7
- Maurya DK, Adhikari S, Nair CKK, Devasagayam TPA. DNA protective properties of vanillin against γ-radiation under different conditions: possible mechanisms. Mutat Res 2007;634(1–2):69–80. doi: 10.1016/j.mrgentox.2007.06.003
- Singh NP. Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat Res 2000;455:111–27. doi:10.1016/S0027-5107(00)00075-0
- Arab K, Steghens J. Plasma lipid hydroperoxides measurement by an automated xylenol orange method. Anal Biochem 2004;325:158–63. doi:10.1016/j.ab.2003.10.022
- Choudhary S, Zhang W, Zhou F, Campbell L, Chan L, Thompson EB, et al. Cellular lipid peroxidation end products induce apoptosis in human lens epithelial cells. Free Radic Biol Med 2002;32:360–9.
- Refsgaard HHF, Tsai L, Stadtman ER. Modification of proteins by polyunsaturated fatty acid peroxidation products. Proc Natl Acad Sci 2000;97:611–6. doi: 10.1073/pnas.97.2.611
- Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J 1997;324:1–18. doi: 10.1042/bj3240001
- Manda K, Ueno M, Moritake T, Anzai KΑ. Lipoic acid attenuates X-irradiation – induced oxidative stress in mice. Cell Biol Toxicol 2007;23:129–37. doi:10.1007/s10565-006-0137-6
- Halliwell B, Gutteridge JMC, eds. Production of hydroxyl radicals in living systems. In: Free radicals biology and medicine. 2nd ed. Oxford: Clarendon Press; 1989: p. 31.
- Maurya DK, Balakrishnan S, Salvi VP, Nair CKK. Protection of cellular DNA from γ-radiation-induced damages and enhancement in DNA repair by troxerutin. Mol Cell Biochem 2005;280:57–68. doi: 10.1007/s11010-005-8052-3
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, et al. Single cell gel/comet assay: guidelines for the in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 2000;35(3):206–21. doi: 10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J
- Hook GJ, Zhang P, Lagroye I, Li L, Higashikubo R, Moros EG, et al. Measurement of DNA damage and apoptosis in Molt-4 cells after in vitro exposure to radiofrequency radiation. Radiat Res 2004;161(2):193–200. doi: 10.1667/RR3127
