Abstract
Objective: We propose some clues for classification of oxidative stresses based on their intensity and time-course.
Background: Oxidative stress is studied for more than three decades and it is clear that it may differ on the parameters of interest. But up to now there is no any system for formal discrimination between different types of the stress. Such approach can provide important benefits at description of experimental data.
Method: We briefly review information on oxidative stresses and show that the theoretical concept is actually poorly developed since introduction of the first definition in 1985 by H. Sies. We argue that the stresses can differ on their intensities and time-curses, but there was no theoretical basis for discrimination between them.
Results: On the basis of these analyses, we propose two systems of classifications of oxidative stresses enabling their description taking into account their intensity and time-course. We analyze essential biomarkers of oxidative stress to be used for classification such as levels of modified by reactive oxygen species proteins, lipids, nucleic acids, and low molecular mass compounds. Finally, we describe potential applications of the proposed classifications to biomedical, comparative and environmental research.
Conclusion: The proposed classifications of oxidative stress may facilitate description of experimental data and their comparison between different organisms and methods of induction of oxidative stresses. Additionally this work may provide some clues to develop quantitative approaches for formal categorization of oxidative stresses.
Application: Most applications of the classifications proposed are theoretical and applied studies where oxidative stress takes place.
Introduction
In 1985, Helmut Sies introduced the first definition of oxidative stress as ‘a disturbance in the prooxidant-antioxidant balance in favor of the former’.Citation1 Since that time, knowledge in the field has extended enormously and many details, particularly targets of attacks by reactive oxygen species (ROS) and molecular mechanisms of cellular response to ROS insults, have been disclosed. Therefore, that definition was updated and recently was stated as ‘Oxidative stress is a situation when steady-state ROS concentration is transiently or chronically enhanced, disturbing cellular metabolism and its regulation and damaging cellular constituents’.Citation2 Investigation of the literature and our own experience in the field demonstrate that in many cases it is difficult to identify the development of oxidative stress, mainly because of plural responses to ROS exposure. Therefore, development of some system(s) of classification(s) of oxidative stresses may be helpful in many cases, especially for newcomers in the field of ROS research. However, for some reason the literature lacks classifications of oxidative stresses. This paper aims to fill that gap and proposes two groups of classifications. The first one is based on the time-course of oxidative stress, and the second one is based on the intensity of oxidative stress. Obviously, in reality both are realized together, but currently it is difficult to describe them in one classification for technical and other reasons. That is due, to a large extent, of methodological complications in experimentation to carry out simultaneous three-dimensional mapping (response vs. time and dose/concentration of the inducer). Therefore, time-course and intensity-based classifications of oxidative stresses will be presented separately.
Time-course-based classifications
Figure A demonstrates schematically some potential time-course events of the system from the point of view of ROS levels under different conditions. When a biological system is in the normal state, ROS levels fluctuate in a certain steady-state (stationary) range. This level is dynamic and results from the balance between ROS appearance and disappearance. However, due to the action of an oxidative stress inducer this balance may be disturbed leading to an increase in stationary ROS level with certain physiological consequences that are described in detail elsewhere.Citation2–Citation9 After induction of oxidative stress, the system may respond according to several scenarios, which can be realized depending on the particular circumstances. Some of the critical factors are: (i) intensity of ROS generation if it is prolonged process, or dose applied if it is a single application of the inducer, (ii) efficiency of antioxidant and other defense systems, (iii) availability of energetic and plastic resources to provide adequate response for ROS level control, and finally, (iv) potential capability or need of the biological system either to maintain the initial ROS level or develop and adapt to a new ROS steady-state level.
Figure 1 Time-course of ROS level at induction of oxidative stress. (A) Under normal conditions ROS steady-state level fluctuates in a certain range, but when disturbed by an oxidant it may be increased. Further, it can either quickly return into the initial corridor (acute oxidative stress), or slowly return with stabilization at a slightly higher level than under normal conditions, with inclusion of all or part of the initial steady-state level, and finally it may stabilize at a higher quasi-stationary level. (B) Perturbations of steady-state ROS level may result in its increase followed by a return either to the initial or an even lower level (modified from Ristow and SchmeisserCitation13).
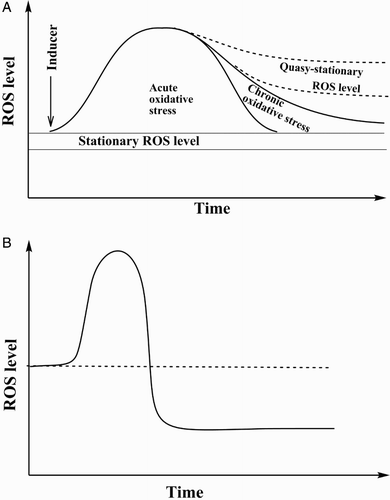
If the system possesses high enough antioxidant potential and enough resources, it can diminish the ROS level to return it into the initial range. That can be realized under two conditions: (i) the organism possesses a sufficient capability to combat ROS without synthesis of new molecules involved in ROS neutralization; and (ii) the organism possesses a high enough capability to stimulate specific biosynthetic processes to strength the mechanisms of the system that combat ROS. In the second case, usually expression of certain genes is stimulated and this process is tightly and delicately coordinated by specific signaling pathways.Citation4–Citation6 If operation of the system in both these scenarios results in a quick return (minutes or hours) of steady-state ROS level into the initial corridor, the situation is called ‘acute oxidative stress’.Citation6–Citation12 In other words, if oxidative stress lasts for minutes or hours after its induction, it is acute. However, in some cases the ROS level is not returned into the initial corridor quickly but is maintained for days, months or even longer at a higher steady-state. In this case, ROS level fluctuations may include either all the initial range in combination with the enhanced one, or include only some part of the initial range. But in any case, there is overlap with the initial stationary ROS level. This situation, with prolonged enhanced steady-state ROS level accompanied by certain physiological consequences, is called ‘chronic oxidative stress’.Citation6,Citation7,Citation9,Citation12 This frequently takes place either through enhanced permanent ROS generation or through attenuation of elimination systems, for example, in certain pathologies such as cancer, cardiovascular and neurodegenerative diseases, diabetes mellitus, or intoxication. Certainly, in some cases the system may adapt to the enhanced ROS level and will not deal with oxidative stress. Rather, this would be a new steady-state ROS level, but not a stress at all. However, if the ROS level is compared with the initial one (before induction of oxidative stress), one can claim the presence of oxidative stress. In the literature, the term ‘transient oxidative stress’ may be found.Citation13 In this case (Fig. B), after oxidative stress induction, the ROS level in the system may either return to the initial level or even reach a lower one due to stimulation of defense mechanisms. That is an important point, which demonstrates the necessity to study ROS homeostasis not only at a certain time point, but also investigate the time-course of the processes taking place. This situation is perfectly exemplified by the studies of the effects on living organisms of calorie restriction (CR) diets, defined usually as a 10–30% reduction of ad libitum calorie uptake in the absence of malnutrition, which is so far the most convincing intervention to delay aging in a number of different organisms.Citation13 Initially it was hypothesized that these biological effects of CR resulted from decreased oxidative stress due to reduced metabolic rate.Citation14,Citation15 A relationship in CR between a decrease in metabolic activity and a concomitant increase in longevity were found neither in yeastsCitation16 or flies,Citation17 nor mice and rats.Citation18 Rather, CR was associated with an increased metabolic rate in Caenorhabditis elegansCitation19,Citation20 and Drosophila.Citation21,Citation22 However, close inspection clearly demonstrated that in the initial phase of caloric limitation oxidative stress was developed and later on, probably due to activation of defense systems, ROS level decreased relative to normally fed counterparts.Citation23–Citation26 The association between CR, oxidative stress and hormesis is perfectly described and generalized in an excellent review from Ristow and Schmeisser.Citation13
Intensity-based classifications
Obviously, interaction between ROS and components of living organisms may result in development of different intensity oxidative stress. As usually in bicomponent systems, the resulting effect depends on the relative reactivities of both components and the physical possibility to encounter reaction partners. In order to analyze the integrated biological response, initially we have to use a reductionist approach to characterize certain individual parameters, which may give ideas about the response of components determining ROS homeostasis. Indeed, most of these indices demonstrate variable sensitivity to perturbations in ROS level. Therefore, we will select two groups of such markers: the first will be the amount of ROS-modified substances (ROMS), and the second one some ROS-inducible ROS-sensitive parameters (ROSISP).
Fig. A schematically shows theoretical relationships between dose/concentration of oxidative stress inducer and certain end points, namely ROSISP (curve 1) and ROMS (curve 2). Oxidatively modified components may be represented by any cellular substances that usually are evaluated as an amount of ROS-oxidized lipids, proteins, nucleic acids and their components, as well as oxidized low molecular mass thiols like glutathione, cysteine, etc. ROSISP can be represented by cell viability, stress resistance, or activity of antioxidant and associated/detoxification enzymes. These two groups of parameters demonstrate different patterns at varying doses/concentrations of the inducer. The level of ROMS shows a sigmoidal relationship with inducer dose/concentration that approaches a plateau and reflects oxidation of available substrate. ROSISP depends on dose/concentration in complicated manner. At low levels of the inducer it is not changed, but an increase in inducer level enhances ROSISP to a maximum, which then declines and reaches the value found in the absence of the inducer (no observable effect point – NOE). Finally it goes lower than the initial level, approximating either to zero or to some asymptote. Two curves given in Fig. A reflect variation of the parameters of interest over a broad range of inducer doses/concentrations. In order to simplify the analysis, the time point will be fixed due so that the time parameter will be excluded from further analysis.
Figure 2 Schematic presentation of classification of oxidative stress based on its intensity. All diapason of relationship ‘Endpoint’ and ‘Dose/concentration of inducer’ is divided for: (A) four zones, namely I – basal oxidative stress (BOS), II – low intensity oxidative stress (LOS), III – intermediate intensity oxidative stress (IOS), and IV – high intensity oxidative stress (HOS); (B) for three zones, namely mild oxidative stress (MOS), temperate oxidative stress (TOS), and severe (or strong) oxidative stress (SOS); and (С) for two zones, namely mild oxidative stress (MOS) and strong oxidative stress (SOS). NOE = no observable effect point.
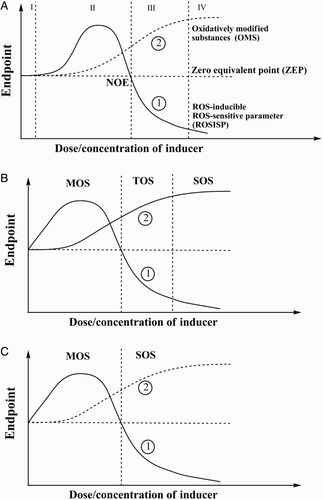
Recently we proposed classification of oxidative stress based on its intensity.Citation8,Citation9 According to this idea, depending on dose/concentration of oxidative stress inducer we may analyze the behavior of two curves and differentiate oxidative stress in four zones (Fig. A). Zone I, where oxidative stress cannot be registered by conventional methods, is called basal oxidative stress. In zone II, termed low intensity oxidative stress, both parameters increase, but with different patterns: ROMS (Fig. A, curve 2) gradually climbs, whereas ROSISP (Fig. A, curve 1) grows to a certain maximum value and later, at higher ROS dose/concentrations, returns to the initial level. At the intersection of ROSISP and ZEP, ROSISP has the same value as without oxidative stress induction (zero point) or in zone I. However, at transition to zone III ROSISP goes down, whereas ROMS continues to grow this zone III is termed intermediate intensity oxidative stress (IOS). Finally, in zone IV, called high intensity oxidative stress (HOS), ROMS approaches a maximum, whereas ROSISP tends to zero or a certain asymptote. Discrimination between zones III and IV is a complicated task. However, some formal tools may be applied. It is possible to use ideas on cooperative interaction between ligand and some binding substances. In this case, the so-called Hill equation may be applied to curve 2.Citation27,Citation28 Maximum ‘binding’ may be calculated using experimental data. After that, one may suggest that the border between zones III and IV would be at the point where ROMS is 90% of its maximum value. The proposed approach is formal, but it can help to discriminate between the IOS and HOS zones.
Since in practice zone I is very narrow relative to zones II–IV, it can be neglected. In this case, zone II may be called mild oxidative stress (MOS), zone III may be called temperate oxidative stress (TOS), and HOS zone IV may be called severe (or strong) oxidative stress (SOS) (Fig. B). Finally, in order to simplify the situation as much as possible and approach more realistic experimental conditions, zone II may be called MOS, while zones III and IV may be combined and jointly called SOS (Fig. C). Certainly, the classifications of oxidative stresses proposed here based on their intensity are rather formal, but in many cases they may be helpful in interpretation of experimental data.
There are several potential limitations for the use of the proposed classifications. Probably the most important one is connected with the parameters selected for characterization of oxidative stress. Usually, in order to describe ROS homeostasis several ROMS and ROSISP parameters are used.Citation6–Citation9 In this case, they would demonstrate different patterns at changing inducer dose/concentration. Unfortunately, at the moment there is no commonly accepted understanding about which of them may be used as primary parameters for characterization of oxidative stresses, and this is critical bottleneck in the proposed systems of oxidative stress classification. Obviously, this issue needs further investigation, but for now it can be suggested that each researcher will have to select the items used primary for this classification. Since each researcher possesses different knowledge, expertise, etc., preferences would likely differ, due to which the same situation could be interpreted in different ways. What can be advised in this case? I suggest that clear and very detailed descriptions of the experimental system and accurate presentation of the results obtained must be followed by comprehensible and closely argued interpretation of the experimental data. This will allow another researcher, being equipped with detailed experimental data, to either accept the author's interpretation, or interpret the same data in a manner more suitable for his/her needs.
Essential biomarkers of oxidative stress to be used for classification
Selection of oxidative stress biomarkers depends on many issues, such as expertise, technical capabilities and financial aspects, that in many cases actually determine the final decision. However, in any case, preference for a certain set of markers must clearly correspond to the goal of the investigation, the model selected and the expected results. Usually, several parameters have to be used simultaneously and in many cases very few of them demonstrate different time-course and dose/concentration patterns. Here I will concentrate only on essential techniques because very expensive and exotic ones are used rarely in practical research in most biochemical and medical laboratories.
The measurement of malonic dialdehyde (MDA) level as a product of ROS-induced peroxidation of lipids was historically the first technique used broadly to characterize ROS-promoted processes. The use of a simple, inexpensive and easily performed method for MDA measurement with thiobarbituric acid (TBA) was critical in its extensive utilization.Citation29 However, low specificity, i.e. interaction of TBA with broad set of compounds like other aldehydes, amino acids, carbohydrates, etc., led to the measurement of products being called TBA-reactive substances (TBARS). The technique can provide more or less accurate data when applied in vitro to evaluate lipid peroxidation either in isolated natural membranes or in artificial ones.Citation29 In spite of the above-mentioned limitations, measurement of TBARS frequently is referred to as MDA measurement. I would recommend being very critical about results of MDA measurement with TBA, especially when dealing with crude extracts.
The TBARS assay and other techniques to measure ROS-induced modification of biomolecules disclosed one very important thing: virtually all compounds formed during these processes may disappear from the system. In other words, in most cases the researcher measures not end products as is frequently claimed but, rather, the steady-state level of compounds of interest. Some researchers even go further, talking about ROS ‘accumulation’.Citation30–Citation32 The dynamic character of ROS levels and levels of products of ROS interaction with cellular components were discussed by us elsewhere.Citation6–Citation9 It is appropriate to mention the measurement of the steady-state level of ROS-modified products by liquid chromatography coupled with mass spectrometry (LC/MS) identification.Citation33–Citation35
The preceding paragraphs demonstrated that serious attention has to be paid to the problem of stability of ROMS in biological systems. Indeed, recognition of ROS homeostasis as a dynamic process may help to understand better the functioning of living organisms. In conclusion, we have to remember that in living organisms there exists not only a steady-state (stationary) ROS level, but also products of their interaction with cellular components that also are not very stable. This statement is perfectly exemplified by the generation and elimination of products of ROS-induced lipid peroxidation such as MDA and 4-hydroxy-2-nonenal (4-HNE or HNE). After formation they may be not only catabolized by certain systems, but also interact with amino groups of proteins leading to formation of Schiff bases and products of their further conversion.Citation36,Citation37 Other products of ROS-induced modification of cellular constituents, like oxidized proteins, are more stable, but they also can be eliminated by proteolytic degradation.Citation37 However, high molecular mass complexes of oxidized proteins, lipids, carbohydrates, and nucleic acids in different combinations may form rather stable intracellular inclusions, such as Alzheimer bodies and lipofuscin, which are very long lived complexes and may accumulate in the cells. However, living organisms possess mechanisms to clean up even these cellular Augean stables, either removing such complexes by autophagy, or eliminating the cells containing accumulated products via apoptosis.Citation38–Citation40
After discussion of these peculiarities of ROS homeostasis, I will list an essential set of parameters used routinely in most laboratories to describe oxidative stress.
ROS-oxidized lipids
Beside the TBARS method analyzed in detail above, and elsewhere,Citation29 intensity of lipid peroxidation may be evaluated by the measurement of lipid peroxides,Citation41 exhaled alkanes like propane,Citation42,Citation43 and others.Citation44,Citation45 Each of these techniques has positive and negative aspects and interested readers are addressed to the relevant literature because the issue is outside the scope of this paper.
ROS-oxidized proteins
There are many more or less adequate techniques to measure such proteins due to the huge array of products formed by ROS interaction with them,Citation37,Citation46,Citation47 but most techniques are relatively high cost, need high class expensive equipment and qualified personnel. Therefore, one relatively simple technique, i.e. measurement of additional carbonyl groups in proteins formed as a result of their ROS-induced oxidation, is quantified with 2,4-dinitrophenylhydrazine (DNP). The formed hydrazones are easily registered spectrophotometrically.Citation37,Citation48 This technique is frequently used in the version proposed by Levine and colleaguesCitation49 and our long-term experience with it has revealed its reliability and good reproducibility with different models and from experiment to experiment.Citation50–Citation53 A variation of this technique includes immunostaining of DNP-protein adducts with specific antibodies.Citation49
ROS-modified nucleic acids
Interaction of ROS with nucleic acids results in the formation of a big array of diverse compounds. Therefore, there is no widely accepted simple method to evaluate ROS-induced oxidation of nucleic acids. However, in practice, different ROS-modified guanidine derivatives are measured, as well as some other compounds.Citation54,Citation55 But this technique is not broadly used because it needs relatively expensive equipment and well-trained personnel. Unfortunately, to date there is no routine technique that may be used to measure ROS-induced modification of nucleic acids in an ordinary biochemical laboratory.
Low molecular mass markers
Glutathione probably is the most commonly measured and reliable low molecular mass marker of oxidative stress.Citation7,Citation29 Its level is rather dynamic because of glutathione's involvement in many enzymatic and non-enzymatic processes. Ideally, the level of both its reduced and oxidized forms must be measured and their individual concentrations along with their quantitative ratio in different forms are used for characterization of the redox state of the system. Stability at extraction and during glutathione assay is the most important issue here. Routinely, HPLC and enzymatic methods are applied to measuring glutathione levels.Citation29 In some cases, reduced glutathione is included in the so-called group of low molecular mass thiols, which also usually includes cysteine and γ-glutamylcysteine.Citation29,Citation56,Citation57 However, for a long time we have used the enzymatic method to measure reduced and oxidized glutathione and found it to be rather reliable.Citation29,Citation58
In some cases, either alone, or in combination with glutathione, the levels of other low molecular mass antioxidants are measured and also used as markers of oxidative stress. Usually ascorbic acid,Citation59 tocopherol,Citation60,Citation61 uric acid,Citation62,Citation63 carotenoids and anthocyaninsCitation64,Citation65 and some other compounds are measured. However, whether such compounds really play antioxidant roles in vivo has been questioned.Citation9,Citation66 Obviously, despite the existence of many unsolved problems, this approach can be potentially useful for characterization of oxidative stress, particularly for its classification.
Potential applications of the classifications to biomedical, comparative and environmental research
Unquestionably, it would not be easy to apply the classifications of oxidative stress proposed above in sections ‘Time-course based classifications’ and ‘Intensity-based classifications’ to real experimental results. Regarding potential application, we first consider time-course classification as virtually used in some cases, but not systematically applied to understanding the coordinated response to oxidative insults. Acute oxidative stress (short term) is perfectly exemplified by transient oxidative stress induced by a single application of either physical or chemical factors. Such acute stress may be very important for enhancing defense potential to meet subsequent intensive stresses of the same or other nature. In a description of the recently proposed mitohormesis hypothesis, Ristow and SchmeisserCitation13 regarded such transient stress as a hormetic one that may affect the health- and lifespan of organisms. There is substantial body of knowledge supporting this concept.Citation67,Citation68 The only element that must be added here is that not only mitochondria produce ROS in living organisms – NADPH-oxidases,Citation69–Citation71 oxidases of aldehydes, amino acids and carbohydratesCitation72,Citation73 as well as xanthine oxidaseCitation74–Citation76 also may provide substantial amounts of ROS under certain conditions. Moreover, they may do this in a finely controlled spatiotemporal manner. It should be emphasized especially that if an organism can efficiently cope with oxidative stress, and if the stress lasts only minutes or few hours, this may provide a mechanism to improve the physiological state of the organism and even make the system more prepared for the challenges that accompany the next induction of oxidative stress.
Chronic oxidative stress has been regarded as a prolonged increase in ROS levels with certain physiological consequences, including corruption of antioxidant systems and ROS-based regulatory pathways.Citation6,Citation7,Citation9 Such stress frequently parallels diverse pathologies like cardiovascularCitation77 and neurodegenerativeCitation78 diseases, diabetes mellitus,Citation79–Citation80 cancer,Citation81–Citation83 and many others. Here, we may have a vicious circle because in many cases the underlying reasons for many diseases are not known, and moreover it is not clear whether oxidative stress caused the pathology, or vice versa. In any case, thousands of studies have demonstrated that many diseases and environmentally induced stresses are tightly associated with oxidative stress. This knowledge logically predicted the potential application of antioxidants as therapeutic agents, but in many cases these expectations were not confirmed by detailed inspections either in experiments or with meta-analysis.Citation66,Citation84–Citation86 Failure of these approaches was disclosed only recently as it became more or less clear from the perspective of the operation of regulatory pathways regarding ROS defense systems. Certainly, conflicting results on the application of antioxidants, particularly as drugs or food supplements, will not stop their use, which will be continued for a long time because: (i) in some specific cases they really may be helpful, and (ii) pharmacological and food industry companies actively use the antioxidant paradigm as a basement for their billionaire businesses and would not refuse to receive back their investments and gain profit. Serious criticism of the use of antioxidants in the training of athletes was given by Ristow and SchmeisserCitation13: ‘supplementation of antioxidants should not be recommended to healthy athletes due to evidence that antioxidants have counter-productive effects on performance, health and the onset of diseases’. However, that is not the end of the discussion. The intracellular level of antioxidants rarely reaches the concentrations used in vitro to evaluate their antioxidant potential and discussion of this interesting issue may be found in our other publications and in works from other laboratories.Citation4–Citation7,Citation9,Citation66
The idea on classification of oxidative stress based on its intensity was formulated only recentlyCitation5,Citation8 and has been applied in some cases, albeit not systematically. In our laboratory, currently we carry out experiments to demonstrate applicability of the idea to a yeast model system and preliminary data are promising (not shown). However, to date there are no suitable data absolutely supporting the classification over a full range of doses/concentrations of oxidative stress inducer(s). So, we cannot here address interested readers to published work in which all four zones could be definitely described. Considering intensity-based classifications in the literature it is possible to find references to ‘mild’,Citation53,Citation87–Citation89 ‘high’,Citation90 ‘strong’Citation91 and some other kinds of oxidative stress. It has to be emphasized that in the literature we were not able to find clear rationales or explanations as to why the stress was so named. For this reason, the classification proposed in ‘Intensity-based classifications’ of this article, even if will not solve the problem, may provide some clues or stimulate development and application of the classification that is convenient in practical usage and further theoretization. Notably, classifications may be applicable in the fields of biomedical, comparative and especially environmental research where investigations related to oxidative stress are very popular but are not always based on deep theoretical knowledge, leading to problems related to the interpretation of the experimental data.
I would like to underline here the importance of the use of models from different phylogenetic groups to develop a theoretical basis and practical application for further research in the free radical field, particularly for development of classifications. The comparative studies and transfer of knowledge gained in one group (usually from more simple to more complicated) to other ones has provided substantial input in delineation of molecular mechanisms of organisms’ responses to oxidative stress. As in many other cases, bacteria were the first organisms in which the molecular mechanisms of response to oxidative stress were disclosed,Citation92,Citation93 and yeasts were the first eukaryotic organisms where such mechanisms were described in detail.Citation94,Citation95 Generally, plants and animals were the last groups where such molecular mechanisms were described.Citation6 Comparative studies have clearly demonstrated that reversible oxidation of thiol groups is central in coordination of responses to oxidative stress.Citation6 This universal role of thiol groups provides a solid foundation for their use in classification of oxidative stresses and practical applications.
Conclusions and perspectives
Although the definition of oxidative stress was introduced for the first time 30 years ago,Citation1 to date we have no widely accepted classification of this biological phenomenon. This creates certain inconveniences for the description of experimental results. Therefore, in this work, using information from the literature and personal experience, I have proposed some clues to classification of oxidative stresses based on time-course and intensity. Ideally both approaches should be combined, but the current state of our knowledge on disturbances in ROS homeostasis does not let us do that yet.
Despite huge progress in the field of ROS research, involvement of more and more people in this area and the existence of many good theoretical and experimental works, the absence of acceptable classifications has substantially limited our capabilities to systematize gained knowledge. Although this and our previous papersCitation8,Citation9 and recent work from SiesCitation5 have highlighted this problem, the problem is not solved. Rather, this is the first step in the field and I hope it may attract the attention of interested persons and will shed light on the outstanding questions. This would be done by the collective application of efforts that will stimulate ROS homeostasis studies in biomedical, comparative and environmental fields. Which prospective issues may provide progress in classifications and their practical use? The first, a ranging of parameters used as biomarkers of oxidative stress to identify the leading (dominant) and secondary ones, if possible. The second, identification and evaluation of potential applications for oxidative stress classifications, with especial attention to regulatory mechanisms involved in response to oxidative stress. The third, the failure of antioxidant application in treatment of certain diseases, environmental hazards and other fields requires revision of our knowledge in the area of ROS homeostasis and development of multifaceted balanced systems, particularly regarding the application of antioxidants for investigation of oxidative stress. The fourth, since the postgenomic era provides us with very powerful tools it would be beneficial to use diverse manipulations of genomes or pathways involved in the realization of genetic information (such as different stages of gene expression) to apply to and develop the classification. Finally, I suggest that some mathematical and statistical tools must be applied to formalize the classification of oxidative stresses in order to obtain the quantitative characteristics of the systems under oxidative insults. Concerning the last issue, some previous ideas were proposed for quantitative analysis of hormetic relationshipsCitation68 that virtually corresponds to curve 1 in Fig. A and I hope that cooperation with mathematicians may be efficient in providing certain formal tools for quantitative description of oxidative stresses.
Disclaimer statements
Contributors None.
Funding None.
Conflict of interest The author declares that there is no conflict of interests regarding the publication of this paper.
Ethical approval None.
Acknowledgements
The author is grateful to Drs H. Sies, K. Storey, J. Storey, R. Levine, M. Nikinmaa, A. Boldyrev, V. Skulachev, H. Semchyshyn, M. Hermes-Lima, O. Lushchak, and M. Bayliak for long-term personal communications which stimulated the author's interest to the field of oxidative stress. The idea of this work was formulated and discussed with many colleagues partially owing to financial support and great facilities provided by the Institute for Advanced Study, Delmenhorst, Germany, for the author.
References
- Sies H. Oxidative stress: introductory remarks. In: Sies H, (ed.) Oxidative stress. London: Academic Press; 1985. p. 1–8.
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997;82(2):291–5. doi: 10.1113/expphysiol.1997.sp004024
- Sies H, Jones DP. Oxidative stress. In: Fink G, (ed.) Encyclopaedia of stress. San Diego: Elsevier; 2007. p. 45–8.
- Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem 2014;289(13):8735–41. doi: 10.1074/jbc.R113.544635
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol 2015;4:180–3. doi: 10.1016/j.redox.2015.01.002
- Lushchak VI. Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp Biochem Physiol C Toxicol Pharmacol 2011;153(2):175–90. doi: 10.1016/j.cbpc.2010.10.004
- Lushchak VI. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 2011;101(1):13–30. doi: 10.1016/j.aquatox.2010.10.006
- Lushchak VI. Classification of oxidative stress based on its intensity. Exp Clinic Sci J 2014;13:922–37.
- Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 2014;224:164–75. doi: 10.1016/j.cbi.2014.10.016
- Aksentijević D, Zervou S, Faller KM, McAndrew DJ, Schneider JE, Neubauer S, et al. Myocardial creatine levels do not influence response to acute oxidative stress in isolated perfused heart. PLoS ONE 2014;9(10):e109021. doi: 10.1371/journal.pone.0109021
- Hosamani R, Krishna G, Muralidhara. Standardized Bacopa monnieri extract ameliorates acute paraquat-induced oxidative stress, and neurotoxicity in prepubertal mice brain. Nutr Neurosci 2014. doi:10.1179/1476830514Y.0000000149. Epub ahead of print.
- Ngo JK, Pomatto LC, Davies KJ. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol 2013;1(1):258–64. doi: 10.1016/j.redox.2013.01.015
- Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 2014;12(2):288–341. doi: 10.2203/dose-response.13-035.Ristow
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59
- Walsh ME, Shi Y, Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic Biol Med 2014;66:88–99. doi: 10.1016/j.freeradbiomed.2013.05.037
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 2002;418(6895):344–8. doi: 10.1038/nature00829
- Hulbert J, Clancy DJ, Mair W, Braeckman BP, Gems D, Partridge L. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Exp Gerontol 2004;39(8):1137–43. doi: 10.1016/j.exger.2004.04.006
- Masoro EJ. Hormesis and the antiaging action of dietary restriction. Exp Gerontol 1998;33(1–2):61–6. doi: 10.1016/S0531-5565(97)00071-5
- Walker G, Houthoofd K, Vanfleteren JR, Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech Ageing Dev 2005;126(9):929–37. doi: 10.1016/j.mad.2005.03.014
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 2007;6(4):280–93. doi: 10.1016/j.cmet.2007.08.011
- Piper MD, Skorupa D, Partridge L. Diet, metabolism and lifespan in Drosophila. Exp Gerontol 2005;40(11):857–62. doi: 10.1016/j.exger.2005.06.013
- Magwere T, Goodall S, Skepper J, Mair W, Brand MD, Partridge L. The effect of dietary restriction on mitochondrial protein density and flight muscle mitochondrial morphology in Drosophila. J Gerontol A Biol Sci Med Sci 2006;61(1):36–47. doi: 10.1093/gerona/61.1.36
- Schoffen JP, Santi Rampazzo AP, Cirilo CP, Zapater MC, Vicentini FA, Comar JF, et al. Food restriction enhances oxidative status in aging rats with neuroprotective effects on myenteric neuron populations in the proximal colon. Exp Gerontol 2014;51:54–64. doi: 10.1016/j.exger.2014.01.001
- Schroeder EA, Shadel GS. Crosstalk between mitochondrial stress signals regulates yeast chronological lifespan. Mech Ageing Dev 2014;135:41–9. doi: 10.1016/j.mad.2013.12.002
- Szafranski K, Mekhail K. The fine line between lifespan extension and shortening in response to caloric restriction. Nucleus 2014;5(1):56–65. doi: 10.4161/nucl.27929
- Valero T. Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des 2014;20(35):5507–9. doi: 10.2174/138161282035140911142118
- Goutelle S, Maurin M, Rougier F, Barbaut X, Bourguignon L, Ducher M, et al. The Hill equation: a review of its capabilities in pharmacological modelling. Fundam Clin Pharmacol 2008;22(6):633–48. doi: 10.1111/j.1472-8206.2008.00633.x
- Frank SA. Input–output relations in biological systems: measurement, information and the Hill equation. Biol Direct 2013;8(1):31. doi: 10.1186/1745-6150-8-31
- Lushchak VI, Semchyshyn HM, Lushchak OV. Classic methods for measuring of oxidative damage: TBARS, xylenol orange, and protein carbonyls. In: Abele D, Vazquez-Medina J, Zenteno-Savin T, (eds.) Oxidative stress in aquatic ecosystems. Blackwell: Publishing Ltd; 2012. p. 420–31.
- Nakahata S, Morishita K. PP2A inactivation by ROS accumulation. Blood 2014;124(14):2163–5. doi: 10.1182/blood-2014-08-594093
- Peng J, Li Z, Wen X, Li W, Shi H, Yang L, et al. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet 2014;10(10):e1004664. doi: 10.1371/journal.pgen.1004664
- Zhong L, Wang L, Xu L, Liu Q, Jiang L, Zhi, Y, et al. The role of NOS-mediated ROS accumulation in an early phase Cu-induced acute cytotoxicity in MCF-7 cells. Biometals 2015;28(1):113–22. doi: 10.1007/s10534-014-9807-7
- Barth J, Bergner SV, Jaeger D, Niehues A, Schulze S, Scholz M, et al. The interplay of light and oxygen in the reactive oxygen stress response of Chlamydomonas reinhardtii dissected by quantitative mass spectrometry. Mol Cell Proteomics 2014;13(4):969–89. doi: 10.1074/mcp.M113.032771
- Li DQ, Zhao J, Li SP. High-performance liquid chromatography coupled with post-column dual-bioactivity assay for simultaneous screening of xanthine oxidase inhibitors and free radical scavengers from complex mixture. J Chromatogr A 2014;1345:50–6. doi: 10.1016/j.chroma.2014.03.065
- Wang H, Sun Y, Guo W, Fang C, Fawcett JP, Li W, et al. Determination of a deuterohemin-peptide conjugate in rat plasma by liquid chromatography–tandem mass spectrometry and application to a preclinical pharmacokinetic study. J Pharm Biomed Anal 2014;98:401–6. doi: 10.1016/j.jpba.2014.06.026
- Chancerelle Y, Mathieu J, Kergonou JF. Recognition and elimination of senescent erythrocytes: implication of antibodies specific for malonic dialdehyde-protein adducts, as demonstrated by flow cytometry. Biochem Mol Biol Int 1994;34(6):1259–70.
- Lushchak VI. Free radical oxidation of proteins and its relationship with functional state of organisms. Biochem (Moscow) 2007;72(8):809–27. doi: 10.1134/S0006297907080020
- Klionsky DJ, Codogno P. The mechanism and physiological function of macroautophagy. J Innate Immun 2013;5(5):427–33. doi: 10.1159/000351979
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res 2014;24(1):24–41. doi: 10.1038/cr.2013.168
- Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 2014;20(3):460–73. doi: 10.1089/ars.2013.5371
- Hermes-Lima M, Willmore WG, Storey KB. Quantification of lipid peroxidation in tissue extracts based on Fe(III) xylenol orange complex formation. Free Radic Biol Med 1995;19(3):271–80. doi: 10.1016/0891-5849(95)00020-X
- Kivits GA, Ganguli-Swarttouw MA, Christ EJ. The composition of alkanes in exhaled air of rats as a result of lipid peroxidation in vivo. Effects of dietary fatty acids, vitamin E and selenium. Biochim Biophys Acta 1981;665(3):559–70. doi: 10.1016/0005-2760(81)90271-X
- Li P, Xu G, Wang C, Gong Y, He Y. Breath pentane: an indicator for early and continuous monitoring of lipid peroxidation in hepatic ischaemia–reperfusion injury. Eur J Anaesthesiol 2009;26(6):513–9. doi: 10.1097/EJA.0b013e328326f7b7
- Leung KS, Galano JM, Durand T, Lee JC. Current development in non-enzymatic lipid peroxidation products, isoprostanoids and isofuranoids, in novel biological samples. Free Radic Res 2014;1:1–11.
- Niki E. Biomarkers of lipid peroxidation in clinical material. Biochim Biophys Acta 2014;1840(2):809–17. doi: 10.1016/j.bbagen.2013.03.020
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003;25(3–4):207–18. doi: 10.1007/s00726-003-0011-2
- Luo S, Levine RL. Methionine in proteins defends against oxidative stress. FASEB J 2009;23(2):464–72. doi: 10.1096/fj.08-118414
- Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012, Article ID 736837.
- Lenz AG, Costabel U, Shaltiel S, Levine RL. Determination of carbonyl groups in oxidatively modified of proteins by reduction with tritiated sodium borohydride. Anal Biochem 1989;177(2):419–25. doi: 10.1016/0003-2697(89)90077-8
- Kubrak OI, Lushchak OV, Lushchak JV, Torous IM, Storey JM, Storey KB, et al. Chromium effects on free radical processes in goldfish tissues: comparison of Cr(III) and Cr(VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp Biochem Physiol C Toxicol Pharmacol 2010;152(3):360–70. doi: 10.1016/j.cbpc.2010.06.003
- Vasylkiv OYu, Kubrak OI, Storey KB, Lushchak VI. Cytotoxicity of chromium ions may be connected with induction of oxidative stress. Chemosphere 2010;80(9):1044–9. doi: 10.1016/j.chemosphere.2010.05.023
- Husak VV, Mosiichuk NM, Maksymiv IV, Sluchyk IY, Storey JM, Storey KB, et al. Histopathological and biochemical changes in goldfish kidney due to exposure to the herbicide Sencor may be related to induction of oxidative stress. Aquat Toxicol 2014;155:181–9. doi: 10.1016/j.aquatox.2014.06.020
- Matviishyn TM, Kubrak OI, Husak VV, Storey KB, Lushchak VI. Tissue-specific induction of oxidative stress in goldfish by 2,4-dichlorophenoxyacetic acid: mild in brain and moderate in liver and kidney. Environ Toxicol Pharmacol 2014;37(2):861–69. doi: 10.1016/j.etap.2014.02.007
- Jena NR. DNA damage by reactive species: mechanisms, mutation and repair. J Biosci 2012;37(3):503–17. doi: 10.1007/s12038-012-9218-2
- Cui L, Ye W, Prestwich EG, Wishnok JS, Taghizadeh K, Dedon PC, et al. Comparative analysis of four oxidized guanine lesions from reactions of DNA with peroxynitrite, singlet oxygen, and γ-radiation. Chem Res Toxicol 2013;26(2):195–202. doi: 10.1021/tx300294d
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr 2004;134(3):489–92.
- Kim EK, Cha CJ, Cho YJ, Cho YB, Roe JH. Synthesis of gamma-glutamylcysteine as a major low-molecular-weight thiol in lactic acid bacteria Leuconostoc spp. Biochem Biophys Res Commun 2008;369(4):1047–51. doi: 10.1016/j.bbrc.2008.02.139
- Lushchak OV, Kubrak OI, Lozinsky OV, Storey JM, Storey KB, Lushchak VI. Chromium(III) induces oxidative stress in goldfish liver and kidney. Aquat Toxicol 2009;93(1):45–52. doi: 10.1016/j.aquatox.2009.03.007
- Sinhorin VD, Sinhorin AP, Teixeira JM, Miléski KM, Hansen PC, Moreira PS, et al. Effects of the acute exposition to glyphosate-based herbicide on oxidative stress parameters and antioxidant responses in a hybrid Amazon fish surubim (Pseudoplatystoma sp). Ecotoxicol Environ Saf 2014;106:181–7. doi: 10.1016/j.ecoenv.2014.04.040
- Bruynsteen L, Janssens GP, Harris PA, Duchateau L, Valle E, Odetti P, et al. Changes in oxidative stress in response to different levels of energy restriction in obese ponies. Br J Nutr 2014;112(8):1402–11. doi: 10.1017/S0007114514001974
- Hódi A, Földesi I, Ducza E, Hajagos-Tóth J, Seres AB, Klukovits A et al. Tocopherol inhibits the relaxing effect of terbutaline in the respiratory and reproductive tracts of the rat: the role of the oxidative stress index. Life Sci 2014;105(1–2):48–55. doi: 10.1016/j.lfs.2014.04.023
- Ahn SH, Lee SH, Kim BJ, Lim KH, Bae SJ, Kim EH, et al. Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos Int 2013;24(12):2961–70. doi: 10.1007/s00198-013-2377-7
- Rovenko BM, Perkhulyn NV, Gospodaryov DV, Sanz A, Lushchak OV, Lushchak VI. High consumption of fructose rather than glucose promotes a diet-induced obese phenotype in Drosophila melanogaster. Comp Biochem Physiol A Mol Integr Physiol 2015;180:75–85. doi: 10.1016/j.cbpa.2014.11.008
- Semchuk NM, Lushchak OV, Falk J, Krupinska K, Lushchak VI. Inactivation of genes, encoding tocopherol biosynthetic pathway enzymes, results in oxidative stress in outdoor grown Arabidopsis thaliana. Plant Physiol Biochem 2009;47(5):384–90. doi: 10.1016/j.plaphy.2009.01.009
- Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014;6(2):466–88. doi: 10.3390/nu6020466
- Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045
- Falone S, D'Alessandro A, Mirabilio A, Petruccelli G, Cacchio M, Di Ilio C, et al. Long term running biphasically improves methylglyoxal-related metabolism, redox homeostasis and neurotrophic support within adult mouse brain cortex. PLoS ONE 2012;7(2):e31401. doi: 10.1371/journal.pone.0031401
- Lushchak VI. Dissection of the hormetic curve: analysis of components and mechanisms. Dose Response 2014;12(3):466–479. doi: 10.2203/dose-response.13-051.Lushchak
- Hayes P, Knaus UG. Balancing reactive oxygen species in the epigenome: NADPH oxidases as target and perpetrator. Antioxid Redox Signal 2013;18(15):1937–45. doi: 10.1089/ars.2012.4895
- Paik YH, Kim J, Aoyama T, De Minicis S, Bataller R, Brenner DA. Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal 2014;20(17):2854–72. doi: 10.1089/ars.2013.5619
- Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci 2012;69(14):2345–63. doi: 10.1007/s00018-012-1011-8
- Moriwaki Y, Yamamoto T, Higashino K. Distribution and pathophysiologic role of molybdenum-containing enzymes. Histol Histopathol 1997;12(2):513–24.
- Rashidi MR, Beedham C, Smith JS, Davaran S. In vitro study of 6-mercaptopurine oxidation catalysed by aldehyde oxidase and xanthine oxidase. Drug Metab Pharmacokinet 2007;22(4):299–306. doi: 10.2133/dmpk.22.299
- Huang CC, Chen KL, Cheung CH, Chang JY. Autophagy induced by cathepsin S inhibition induces early ROS production, oxidative DNA damage, and cell death via xanthine oxidase. Free Radic Biol Med 2013;65:1473–86. doi: 10.1016/j.freeradbiomed.2013.07.020
- Kane D, Hansell JA, Herrera EA, Allison BJ, Niu Y, Brain KL, et al. Xanthine oxidase and the fetal cardiovascular defence to hypoxia in late gestation ovine pregnancy. J Physiol 2014;592(3):475–89. doi: 10.1113/jphysiol.2013.264275
- Kovac S, Domijan AM, Walker MC, Abramov AY. Seizure activity results in calcium- and mitochondria-independent ROS production via NADPH and xanthine oxidase activation. Cell Death Dis 2014, Article ID:e1442.
- Lee A, Jeong D, Mitsuyama S, Oh JG, Liang L, Ikeda Y, et al. The Role of SUMO-1 in cardiac oxidative stress and hypertrophy. Antioxid Redox Signal 2014;21(14):1986–2001. doi: 10.1089/ars.2014.5983
- Kamat PK, Kalani A, Kyles P, Tyagi SC, Tyagi N. Autophagy of mitochondria: a promising therapeutic target for neurodegenerative disease. Cell Biochem Biophys 2014;70(2):707–19. doi: 10.1007/s12013-014-0006-5
- Bollineni RC, Fedorova M, Blüher M, Hoffmann R. Carbonylated plasma proteins as potential biomarkers of obesity induced type 2 diabetes mellitus. J Proteome Res 2014;13(11):5081–93. doi: 10.1021/pr500324y
- Pimson C, Chatuphonprasert W, Jarukamjorn K. Improvement of antioxidant balance in diabetes mellitus type 1 mice by glutathione supplement. Pak J Pharm Sci 2014;27:1731–37.
- Townsend DM, Lushchak VI, Cooper AJ. A comparison of reversible versus irreversible protein glutathionylation. Adv Cancer Res 2014;122:177–98. doi: 10.1016/B978-0-12-420117-0.00005-0
- El Gaafary M, Büchele B, Syrovets T, Agnolet S, Schneider B, Schmidt CQ, et al. An α-acetoxy-tirucallic acid isomer inhibits Akt/mTOR signaling and induces oxidative stress in prostate cancer cells. J Pharmacol Exp Ther 2015;352(1):33–42. doi: 10.1124/jpet.114.217323
- Marina R, González P, Ferreras MC, Costilla S, Barrio JP. Hepatic Nrf2 expression is altered by quercetin supplementation in X-irradiated rats. Mol Med Rep 2015;11(1):539–46.
- Day BJ. Antioxidant therapeutics: Pandora's box. Free Radic Biol Med 2014;66:58–64. doi: 10.1016/j.freeradbiomed.2013.05.047
- Murphy MP. Antioxidants as therapies: can we improve on nature? Free Radic Biol Med 2014;66:20–3. doi: 10.1016/j.freeradbiomed.2013.04.010
- Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2 – an update. Free Radic Biol Med 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008
- Lushchak OV, Lushchak VI. Sodium nitroprusside induces mild oxidative stress in Saccharomyces cerevisiae. Redox Rep 2008;13(4):144–52. doi: 10.1179/135100008X308885
- Giavarotti L, Simon KA, Azzalis LA, Fonseca FL, Lima AF, Freitas MC et al. Mild systemic oxidative stress in the subclinical stage of Alzheimer's disease. Oxid Med Cell Longev 2013;2013(11):1–8. doi: 10.1155/2013/609019
- Atamaniuk TM, Kubrak OI, Husak VV, Storey KB, Lushchak VI. The mancozeb-containing carbamate fungicide tattoo induces mild oxidative stress in goldfish brain, liver, and kidney. Environ Toxicol 2014;29(11):1227–35.
- Wong VW, Rustad KC, Glotzbach JP, Sorkin M, Inayathullah M, Major MR, et al. Pullulan hydrogels improve mesenchymal stem cell delivery into high-oxidative-stress wounds. Macromol Biosci 2011;11(11):1458–66.
- Huerta-García E, Pérez-Arizti JA, Márquez-Ramírez SG, Delgado-Buenrostro NL, Chirino YI, Iglesias GG, et al. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic Biol Med 2014;73:84–94. doi: 10.1016/j.freeradbiomed.2014.04.026
- Demple B. Regulation of bacterial oxidative stress genes. Annu Rev Genet 1991;25(1):315–37. doi: 10.1146/annurev.ge.25.120191.001531
- Lushchak VI. Oxidative stress and mechanisms of protection against it in bacteria. Biokhimiya (Moscow) 2001;66(5):592–609.
- Lushchak VI. Oxidative stress in yeast. Biochemistry (Moscow) 2010;75(3):281–96. doi: 10.1134/S0006297910030041
- Jamieson DJ. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast 1998;14(16):1511–27. doi: 10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S
