Abstract
Objective: Thiol-disulphide homeostasis (TDH) has a critical role in various clinical disorders. We aimed to assess the association of TDH with acute tonsillopharyngitis (AT) in children.
Methods: This study included 94 (73 viral and 21 bacterial) tonsillopharyngitis patients and 88 control children. Their native thiol, total thiol, and disulphide levels were measured.
Results: Viral and bacterial tonsillopharyngitis patients had lower native thiol levels compared with healthy children (P < 0.001 and P = 0.008, respectively). Both groups had lower total thiol levels compared with control children (P = 0.002 for viral, P = 0.011 for bacterial). The disulphide levels were lower in bacterial than in viral tonsillopharyngitis patients (P = 0.04), and there was a significant difference between viral tonsillopharyngitis patients and the control group (P < 0.001). The native/total thiol ratio in each patient group was lower than in the control group (P < 0.001 for viral, P = 0.017 for bacterial). The disulphide/native thiol and disulphide/total thiol ratios were significantly higher in viral (P < 0.001 for both) and bacterial tonsillopharyngitis patients (P = 0.017 for both) than in healthy children. In all patients, a correlation was found between the levels of C-reactive protein (CRP) and native thiol (r = −0.211, P = 0.04), CRP and total thiol (r = −0.217, P = 0.036), white blood cell (WBC) and native thiol (r = −0.228, P = 0.002), WBC and total thiol (r = −0.191, P = 0.01), and WBC and disulphide (r = 0.160, P = 0.03).
Discussion: TDH is altered in AT in children. The alteration is more prominent in viral than in bacterial tonsillopharyngitis.
Introduction
Acute tonsillopharyngitis (AT) is an acute infection of the tonsils and pharynx, with an annual incidence of 600 000 children below 15 years old in emergency department visits in the United States.Citation1 Most cases in childhood are induced by different viruses, whereas only 15–30% is attributed to group A β-hemolytic streptococcus (GABHS).Citation2 In this disease, the most important concern is to differentiate GABHS from a viral aetiology to prevent suppurative and nonsuppurative complications, inappropriate and unnecessary use of antimicrobials, and hence increased expenses; antimicrobial resistance is therefore important. Although some discriminative signs and symptoms of streptococcal tonsillopharyngitis have been noted, the clinical findings usually overlap; thus, physicians are constrained to laboratory confirmation for accurate diagnosis.
Throat culture and rapid antigen detection test are among the diagnostic methods for GABHS tonsillopharyngitis.Citation3 However, because these two methods have some disadvantages, other diagnostic markers, such as white blood cell (WBC) count, erythrocyte sedimentation rate, and C-reactive protein (CRP) and procalcitonin levels, have been used to increase the diagnostic accuracy in detecting GABHS positivity. Nevertheless, some studies have shown uncertainties in the accuracy of these markers in determining streptococcal tonsillopharyngitis infection.Citation4–Citation7
In infectious diseases, it has been shown that various inflammatory cells are activated, and reactive oxygen species (ROS) are produced to fight against intra- and extracellular microbes.Citation8 This leads to increased potential antioxidant capacity and free radical production.Citation9–Citation11 Thiols are among the mercaptans with sulfhydryl residues, which have a pivotal role in coordinating the antioxidant defense network.Citation12 Oxidants lead thiols to undergo oxidation reaction and form disulphide bonds.Citation13 In case of oxidative stress, cysteine residues are oxidized and reversible mixed disulphides are formed between low-molecular mass thiols and protein thiol groups. Disulphide bonds can again be reduced to thiol groups. In this way, thiol-disulphide homeostasis (TDH) is maintained.Citation14 Intracellular and extracellular thiols form the total thiols, which consist of either free-form or reduced glutathione or are bound to proteins, which is mainly albumin in circulation.Citation15 Total thiol includes both reduced and oxidized thiols, whereas native thiol indicates only the reduced thiol (SH). TDH has been recently shown to play a critical role in various clinical disorders, such as myocardial infarctionCitation16,Citation17 and hyperemesis gravidarumCitation18, but not in infections.
This study aimed to assess the association of TDH with AT in children.
Materials and methods
Study design
This study was approved by the local ethics committee (Erzurum Regional Training and Research Hospital ethical committee), and informed consent forms were obtained from the legal guardians of the participants. A total of 94 tonsillopharyngitis patients with compatible symptoms, physical examination, and laboratory findings, and 88 age- and gender-matched control children, without any apparent infection, cardiovascular disease, cancer, kidney disease, liver disorder, diabetes, or rheumatologic disorder, were included in the study.
The tonsillopharyngitis diagnosis was determined by paediatric infectious diseases fellows. The discrimination between bacterial and viral aetiology was made by the help of patients’ clinical properties and bacteriological and laboratory methods, based on the guideline of the Infectious Diseases Society of America.Citation3 Hemogram, CRP tests, and throat culture were carried out for the patients and control children.
Sample collection
The blood samples were collected in plain tubes, and serum was separated after centrifugation at 1500 × g for 10 minutes and stored at −80°C until analysis. The total thiol content of the sample was measured with the help of a modified Ellman reagent. Half of the difference between the total and native thiols provided the dynamic disulphide amount. After the determination of the native thiol and disulphide levels, the disulphide/thiol ratio was calculated as previously described by Erel and Neselioglu.Citation19
Statistical analyses
Statistical analyses were carried out with the SPSS Statistics for Windows, version 17.0 (SPSS Inc., Chicago, USA). The normality of the distribution of variables was determined by the Kolmogorov–Smirnov test. The results are presented as means ± SD or medians (min–max) according to the normal distribution. Differences among groups were analysed by using the ANOVA post hoc LSD or Kruskal–Wallis test and Mann–Whitney U tests. A P value lower than 0.05 was regarded as statistically significant. Spearman's rank correlation was applied to test the correlation among the results in the study groups.
Results
Of the 94 patients studied, 73 (77.7%) had viral and 21 (22.3%) had bacterial (GABHS) tonsillopharyngitis. No other bacterium was found. The ages of the participants ranged from 36 to 204 months for viral tonsillopharyngitis patients, 55 to 203 months for bacterial tonsillopharyngitis patients, and 36 to 192 months for healthy children. Males accounted for 36 (49.3%) of the viral tonsillopharyngitis patients, 11 (52.4%) of the bacterial tonsillopharyngitis patients, and 44 (50%) of the control children. Table shows the CRP and WBC levels obtained. The levels of the inflammatory markers WBC (P = 0.014) and CRP (P < 0.001) were higher in bacterial than in viral tonsillopharyngitis patients. All patients had higher WBC counts and CRP levels compared with the control children (Table ).
Table 1 The laboratory values of the patients and control children
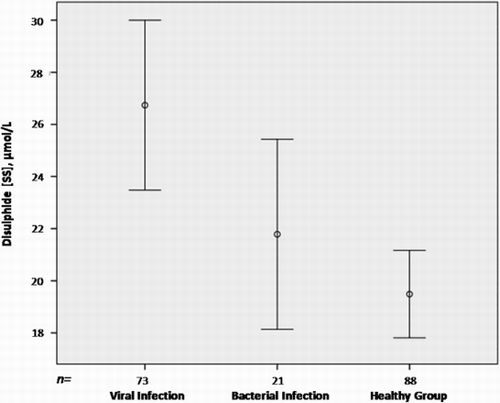
Both viral and bacterial tonsillopharyngitis patients had lower native thiol values compared with the control children (P < 0.001 and P = 0.008, respectively) (Fig. ). Also, both patient groups had lower total thiol values compared with the control children (P = 0.002 for viral tonsillopharyngitis, P = 0.011 for bacterial tonsillopharyngitis). No significant difference in native and total thiol levels was found between viral and bacterial tonsillopharyngitis patients. The disulphide levels were significantly different among the three groups, with bacterial tonsillopharyngitis patients having lower serum disulphide levels compared with viral tonsillopharyngitis patients (P = 0.04) (Fig. ). The difference between the viral infection patients and the control children was also significant (P < 0.001).
Figure 1 Native thiol levels by infection type. Control children had higher native thiol values than both viral and bacterial tonsillopharyngitis patients.
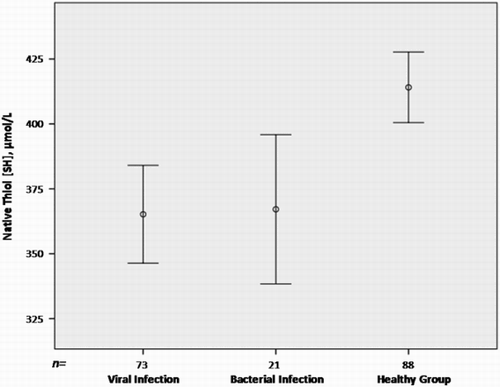
Figure 2 Disulphide levels by infection type. Viral tonsillopharyngitis patients had higher serum disulphide levels than bacterial tonsillopharyngitis patients.
The native/total thiol ratio was significantly lower in each patient group than in the control children (viral tonsillopharyngitis, P < 0.001; bacterial tonsillopharyngitis, P = 0.017). This ratio was not statistically significantly different between viral and bacterial tonsillopharyngitis patients. The disulphide/native thiol and disulphide/total thiol ratios were significantly higher in the viral (P < 0.001 for both) and bacterial tonsillopharyngitis groups (P = 0.017 for both) than in the control children (Fig. ). These ratios were not statistically significantly different between viral and bacterial tonsillopharyngitis patients.
Figure 3 Disulphide/native thiol ratios by infection type. The disulphide/native thiol ratio was higher in the viral and bacterial tonsillopharyngitis patients than in the control children.
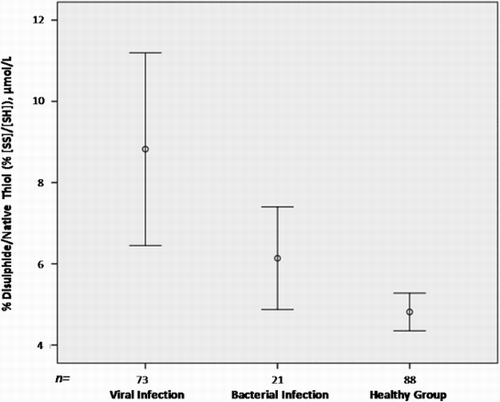
A negative correlation was found between the levels of CRP and native thiol (r = −0.211, P = 0.04) and those of CRP and total thiol (r = −0.217, P = 0.036) in all tonsillopharyngitis patients. Also, there was a correlation between the levels of WBC and native thiol (r = −0.228, P = 0.002), WBC and total thiol (r = −0.191, P = 0.01), and WBC and disulphide (r = 0.160, P = 0.03) (Table ).
Table 2 Spearman's correlation analysis of native thiol, total thiol, and disulphide values
Discussion
In the present study, the disulphide/native thiol and disulphide/total thiol ratios were found to be significantly higher in children with tonsillopharyngitis. To the best of our knowledge, this is the first investigation on the correlation between TDH and AT in children. Thiols are found in all body cells. In an oxidizing environment, these endogenous molecules contribute aerobic cells to maintain their reducing state.Citation20 Some molecules, such as free cysteine, glutathione, and cysteine residues in proteins, contribute to blood total thiols.Citation21 Thiols are protective against damage induced by free radicals. Both the intracellular and extracellular redox states of thiols play a critical role in the determination of protein structure and function, the regulation of enzymatic activity of transcription factors, and antioxidant protection.Citation20 In the current study, the native and total thiol levels were found to be lower in both patient groups than in healthy children. This may indicate that the levels of thiol-containing proteins or other molecules decreased. Furthermore, the native/total thiol ratio was found to be lower in each patient group than in the controls. The decreased thiol levels may be indicator of oxidative stress in these patients.
Previous studies have shown that WBC and CRP may not be sensitive enough to distinguish bacterial from viral infection in febrile childrenCitation22; however, our results revealed higher CRP levels in bacterial than in viral tonsillopharyngitis patients. Further, each patient group had higher CRP levels compared with healthy children. Additionally, this study showed that the native and total thiol levels were negatively correlated with the CRP levels in tonsillopharyngitis patients. This result may indicate that thiol levels decrease due to inflammation. Infection markers are used widely, and CRP has been an important marker in AT as in most of the infections.Citation6 In the diagnosis of GABHS pharyngitis, RADT and/or throat culture remains the gold standard diagnostic method.Citation3 However, culture is a time-consuming procedure, and the cost-effectiveness of RADT is still uncertain, especially in low-resource settings.Citation2 Measurement of the CRP level has been suggested to increase the diagnostic accuracy in the detection of GAS-positive patients when RADT is not available.Citation5 Conversely, Alper et al.Citation2 showed that leukocytosis (≥10 000/ml) and CRP positivity were more prevalent in throat culture-negative than in GABHS-positive children. In our study, the WBC counts were higher in bacterial than in viral tonsillopharyngitis patients. Further, all tonsillopharyngitis patients had higher WBC counts compared with the control subjects. Our analysis also showed a correlation in that both the native and total thiol levels decreased and the disulphide levels increased in tonsillopharyngitis patients when the WBC counts increased. This finding supports the antioxidant function of TDH in infectious oxidative stress conditions.
Humans have various antioxidant defense systems to overwhelm the uncontrolled production of ROS, including both enzymatic (catalase, superoxide dismutase, and glutathione peroxidase) and non-enzymatic [vitamin A (carotenoids), vitamin E (α-tocopherol), vitamin C (ascorbic acid), reduced glutathione (GSH), selenium, and uric acid) components against the effects of ROS.Citation23,Citation24 Increased ROS has been shown to be associated with infectious diseases, such as hepatitis, influenza, HIV, and septic shock, as well as some noninfectious diseases.Citation23 Numerous studies have previously shown that the plasma levels or the activities of total peroxide, malondialdehyde, serum paraoxonase, and arylesterase were significantly lower in patients with infections, such as osteomyelitis, sepsis, tuberculosis, brucellosis, and hepatitis B, compared with healthy controls.Citation9,Citation25–Citation28 Also, in AT patients, the levels of malondialdehyde, reduced glutathione, β-carotene, retinol, α-tocopherol, and ascorbic acid were found to be lower compared with control subjects.Citation24 Further, Cemek et al. reported the antioxidant activities shown by erythrocyte superoxide dismutase and glutathione peroxidase, serum catalase, ceruloplasmin, albumin, and total bilirubin in children with acute tonsillitis.Citation29
Similarly to our study, previous works have shown that native thiol levels decrease in oxidative stress due to infections, such as brucellosis and Crimean-Congo haemorrhagic fever.Citation8,Citation30 Native thiol groups are among the main antioxidant components of serum and prevent tissue damage by reacting with free oxygen radicals and lipid peroxides and by neutralizing these molecules. Our study is the first investigation to show a correlation between TDH and an infection in children.
Erel et al. found that plasma disulphide levels were higher in patients with degenerative diseases, such as smoking, diabetes, obesity, and pneumonia, were lower in patients with proliferative diseases, such as multiple myeloma, urinary bladder cancer, colon cancer, and renal cancer. Also, aggressive-growing tumours have been shown to have the lowest disulphide levels, whereas slow-growing ones had subnormal values.Citation19 In the present study, viral tonsillopharyngitis patients had lower disulphide levels compared with both bacterial tonsillopharyngitis patients and healthy children. This is a defense mechanism. Oxidative stress increases in viral infections, and thiols augment the severity of viral infections. Thiols prevent viruses from attaching to cellular receptors. Whenever the level of thiols decreases, the level of disulphides increases and viruses are prevented from attaching to the receptors. Increases in plasma and lymphocyte disulphide levels have been previously shown in AIDS patients, similarly to our study.Citation31 Further, bacterial infections, with the help of oxidative enzymes, such as myeloperoxidase and NADPH oxidase in granulocytes, produce oxidizing molecules. In this way, as the thiol levels decrease, the disulphide levels increase.
One limitation of this study is that, despite the selectively performed throat cultures and high levels of acute phase reactants in the bacterial group, whether or not the results of the throat cultures are real infections or carrier states may never be known. These results were regarded as bacterial infection due to clinical conviction. Also, the disulphide/thiol ratios were not compared with other oxidative stress markers, such as total peroxide, malondialdehyde, serum paraoxonase, and arylesterase, among others.
In conclusion, TDH is both altered and shifts to the left, oxidized side in patients with AT. The alteration is more prominent in viral than in bacterial tonsillopharyngitis, possibly due to the presence of a host defense mechanism or as a secondary occurrence to pathogenetic mechanisms exerted by the infection.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflicts of interest There is no conflicts of interest.
Ethics approval The study has been approved by the local ethical committee.
Acknowledgement
English polishing of this manuscript was performed by SPİ Global.
References
- National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. [Cited: 2015 Jun 1]. Available from: <http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf>
- Alper Z, Uncu Y, Akalin H, Ercan I, Sinirtas M, Bilgel NG. Diagnosis of acute tonsillopharyngitis in primary care: a new approach for low-resource settings. J Chemotherapy 2013;25(3):148–55. doi: 10.1179/1973947813Y.0000000071
- Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012;55(10):e86–102. doi: 10.1093/cid/cis629
- Putto A, Meurman O, Ruuskanen O. C-reactive protein in the differentiation of adenoviral, Epstein–Barr viral and streptococcal tonsillitis in children. Eur J Pediatr 1986;145(3):204–6. doi: 10.1007/BF00446066
- Christensen AM, Thomsen MK, Ovesen T, Klug TE. Are procalcitonin or other infection markers useful in the detection of group a streptococcal acute tonsillitis? Scand J Infect Dis 2014;46(5):376–83. doi: 10.3109/00365548.2014.885656
- Kaplan EL, Wannamaker LW. C-reactive protein in streptococcal pharyngitis. Pediatrics 1977;60(1):28–32.
- Koo CY, Eisenhut M. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. Can inflammatory markers distinguish streptococcal from viral tonsillitis? Emerg Med J 2011;28(8):715–7.
- Esen R, Aslan M, Kucukoglu ME, Cıkman A, Yakan U, Sunnetcioglu M, et al. Serum paraoxonase activity, total thiols levels, and oxidative status in patients with acute brucellosis. Wien Klin Wochenschr 2015;127(11–12):427–33. doi: 10.1007/s00508-015-0720-z
- Serefhanoglu K, Taskin A, Turan H, Timurkaynak FE, Arslan H, Erel O. Evaluation of oxidative status in patients with brucellosis. Braz J Infect Dis 2009;13(4):249–51. doi: 10.1590/S1413-86702009000400001
- Dundaroz R, Erenberk U, Turel O, Demir AD, Ozkaya E, Erel O. Oxidative and antioxidative status of children with acute bronchiolitis. J Pediatr (Rio J) 2013;89(4):407–11. doi: 10.1016/j.jped.2012.12.001
- Karsen H, Sunnetcioglu M, Ceylan RM, Bayraktar M, Taskin A, Aksoy N, et al. Evaluation of oxidative status in patients with Fasciola hepatica infection. Afr Health Sci 2011;(11 Suppl. 1):S14–8.
- Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr 2000;72(2 Suppl.):653–69.
- Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem 2013;288(37):26489–96. doi: 10.1074/jbc.R113.462929
- Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med 2009;47(10):1329–38. doi: 10.1016/j.freeradbiomed.2009.08.021
- Chianeh YR, Prabhu K. Protein thiols as an indicator of oxidative stress. Arch Med Rev J 2014;23(3):443–56.
- Kundi H, Erel O, Balun A, Çiçekçioğlu H, Cetin M, Kiziltunç E, et al. Association of thiol/disulphide ratio with syntax score in patients with NSTEMI. Scand Cardiovasc J 2015;49(2):95–100. doi: 10.3109/14017431.2015.1013153
- Kundi H, Ates I, Kiziltunc E, Çiçekçioğlu H, Cetin M, Kiziltunç E, et al. A novel oxidative stress marker in acute myocardial infarction; thiol/disulphide homeostasis. Am J Emerg Med 2015;49(2):95–100.
- Ergin M, Cendek BD, Neselioglu S, Avsar AF, Erel O. Dynamic thiol-disulfide homeostasis in hyperemesis gravidarum. J Perinatol 2015;35(10):788–92. doi: 10.1038/jp.2015.81
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem 2014;47(18):326–32. doi: 10.1016/j.clinbiochem.2014.09.026
- da Costa CM, dos Santos RCC, Lima ES. A simple automated procedure for thiol measurement in human serum samples. J Bras Patol Med Lab 2006;42(5):345–50. doi: 10.1590/S1676-24442006000500006
- Turell L, Radi R, Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med 2013;65:244–53. doi: 10.1016/j.freeradbiomed.2013.05.050
- Peltola V, Toikka P, Irjala K, Mertsola J, Ruuskanen O. Discrepancy between total white blood cell counts and serum C reactive protein levels in febrile children. Scand J Infect Dis 2007;39(6–7):560–5. doi: 10.1080/00365540601158722
- Ginter E, Simko V, Panakova V. Antioxidants in health and disease. Bratisl LekListy 2014;115(10):603–6.
- Cemek M, Dede S, Bayiroğlu F, Caksen H, Cemek F, Yuca K. Oxidant and antioxidant levels in children with acute otitis media and tonsillitis: A comparative study. Int J Pediatr Otorhinolaryngo 2005;69(6):823–7. doi: 10.1016/j.ijporl.2005.01.016
- TekinKoruk S, Aksoy N, Hamidanoglu M, Karsen H, Unlu S, Bilinc H. The activity of paraoxonase and arylesterase in patients with osteomyelitis. Scand J Clin Lab Invest 2012;72(7):513–7. doi: 10.3109/00365513.2012.700058
- Li Y, Zhai R, Li H, Mei X, Qiu G. Prognostic value of serum paraoxonase and arylesterase activity in patients with sepsis. J Int Med Res 2013;41(3):681–7. doi: 10.1177/0300060513483412
- Torun E, Gedik AH, Cakir E, Umutoglu T, Gok O, Kilic U. Serum paraoxonase 1 activity and oxidative stress in pediatric patients with pulmonary tuberculosis. Med Princ Pract 2014;23(5):426–31. doi: 10.1159/000363700
- Duygu F, Tekin Koruk S, Aksoy N. Serum paraoxonase and arylesterase activities in various forms of Hepatitis B virus infection. J Clin Lab Anal 2011;25(5):311–6. doi: 10.1002/jcla.20473
- Cemek M, Caksen H, Cemek F, Bayiroğlu F, Dede S, Dülger H, Ustün R. Investigation of antioxidant status in children with acute otitis media and tonsillitis. Int J Pediatr Otorhinolaryngol 2004;68(11):1381–5. doi: 10.1016/j.ijporl.2004.05.003
- Karadag-Oncel E, Erel O, Ozsurekci Y, Caglayik DY, Kaya A, Gozel MG, et al. Plasma Oxidative Stress and Total Thiol Levels in Crimean-Congo Hemorrhagic Fever. Jpn J Infect Dis 2014;67(1):22–6. doi: 10.7883/yoken.67.22
- Walmsley SL, Winn LM, Harrison ML, Uetrecht JP, Wells PG. Oxidative stress and thiol depletion in plasma and peripheral blood lymphocytes from HIV-infected patients: toxicological and pathological implications. AIDS 1997;11(14):1689–97. doi: 10.1097/00002030-199714000-00005
