Abstract
Objectives: The etiology of multiple myeloma (MM) is not exactly known. This study investigated the role of thiol/disulfide homeostasis in the etiopathogenesis of MM.
Methods: Some 50 patients with MM (aged 39–84 years) and 50 sex-matched healthy volunteer controls (aged 50–91 years) participated in this study. Venous blood samples were collected, and levels of native thiols, total thiols, and disulfide were measured.
Results: Native and total thiol levels in the control group were determined to be higher than in the study and patient groups (P<0.001). Disulfide levels were found to be higher in the control group than in the study group and higher in newly diagnosed patients than in outpatients who were undergoing treatment (P=0.002). The ratios of thiol levels were found to be similar in both the study and control groups (P>0.05).
Discussion: The results of the study show that although there was a decrease in the levels of disulfide, native thiol, and total thiol, the balance of thiol/disulfide was maintained. This is the first study to research the homeostasis of dynamic thiol/disulfide from the perspective of the new method that was used. We hope that this study will encourage and facilitate further studies in this area.
Introduction
Multiple myeloma (MM) is a malignancy of bone marrow that is characterized by the clonal proliferation of plasma cells. MM accounts for 1% of all malignancies and 10% of all hematological malignancies.Citation1 Abnormal proliferating plasma cells produce monoclonal immunoglobulins (M-protein, M-component, paraprotein) and cause symptoms and findings such as renal impairment, hypercalcemia, pathological bone fracture, bone marrow deficiency, and hyperviscosity by suppressing the normal immunoglobulins. The etiology of this disease is not exactly known. Weakened immunity, radiation, certain viruses, chemicals, and chromosome abnormalities caused by these are responsible for the etiology.Citation2 Recent research into MM etiopathogenesis has looked into mutation risk and parameters of oxidative stress that induces the neoplastic transformation period.Citation3–Citation5 The oxidant and antioxidant systems are stabilized in the human body, thereby continuing homeostasis.Citation3 The oxidant–antioxidant balance is important for cell function and normal metabolism. Imbalance between pro-oxidants and antioxidants causes oxidative stress. Proteins, lipids, and DNA are target molecules for oxidative damage. Reactive oxygen species that occur due to changes in these molecules and other free radicals increase mutation risk and hasten the neoplastic transformation period.Citation3–Citation5
Thiols are organic compounds known as mercaptans. They possess the sulfydryl group (-SH) which occurs when sulfur and oxygen are added to carbon atoms.Citation6 A major part of the plasma thiol pool contains albumin and protein thiols. A minor part contains low-molecular-weight thiols such as cysteine, cysteinyl glycine, glutathion, homocystein, and gamma glutamylcysteine.Citation7 Thiols can undergo oxidation reaction via oxidants and form disulfide bonds.Citation8 When oxidative stress increases, oxidation of cysteine residues can lead to the reversible formation of mixed disulfides of molecular weights between thiols and protein thiol groups. Disulfide bonds can also be reduced to thiol groups, thereby maintaining thiol/disulfide homeostasis.Citation9
Homeostasis of dynamic thiol/disulfide plays an important part in cell signal mechanisms, transcription factors, regulation of enzymatic activations, apoptosis and signal transduction, antioxidant protection, and detoxification.Citation10,Citation11 Abnormalities of dynamic thiol/disulfide homeostasis have been shown to be evident in diseases such as malignanciesCitation12, hyperemesis gravidarumCitation13, cardiovasculary diseasesCitation14, and diabetes mellitus.Citation15
This study addressed the role of thiol/disulfide homeostasis in the etiopathogenesis of MM. The role of chromosomal mutations and the effects of dynamic thiol/disulfide homeostasis on important events in the cell that are based on acquired factors of MM etiology were taken into consideration. An impaired dynamic thiol/disulfide homeostasis was hypothesized in this study. According to our knowledge, there has been no other study into the relationship between MM and homeostasis of dynamic thiol/disulfide.
Material and methods
Sample data collection
The study was conducted in the hematology clinic of Ankara Ataturk Training and Research Hospital between 2013 and 2015. We enrolled 50 patients with MM in the study group; 35 of them (19 male, 16 female) had been newly diagnosed and 15 of them (9 male, 6 female) were outpatients who were already being treated. There were 50 healthy people (19 male, 31 female) in the control group.
The patients had been diagnosed with MM by using anamnesis physical examination, laboratory evaluations (serum protein and immunofixation electrophoresis in 24-hour urine), bone marrow aspiration, and biopsy. Exclusion criteria in both the study and control groups were antioxidant drug use or having systemic diseases (diabetes mellitus, liver failure, coronary artery diseases, or active infections).
Fasting blood samples were obtained from volunteers to plain tubes. Sera were separated after centrifugation at 1600 g for 10 minutes and stored at −80°C until analysis.
This study was approved by the Local Ethical Committee of the Yildirim Beyazit University Medical Faculty and an informed consent form was completed by all participants.
Biochemical assays
Thiol/disulfide homeostasis parameter measurement
Thiol/dysulfide homeostasis tests were measured by the automated spectrophotometric method as described by Erel and Neselioglu.Citation16 Short disulfide bonds were first reduced with sodium borohydride to form free functional thiol groups. Unused reductant sodium borohydride was consumed and removed with formaldehyde to prevent reduction of DTNB (5,5’-dithiobis-(2-nitrobenzoic) acid). All of the thiol groups, including reduced and native, were determined after their reaction with DTNB. The dynamic disulfide amount represented half of the difference between the total and native thiols.
After determining the concentrations of native thiols, total thiols, and disulfide and the ratios of disulfide to total thiol (SS/(SH + SS)) and disulfide to native thiol (SS/SH), the ratios of native thiol to total thiol (SH/(SH + SS)) were calculated. Measurements were made by an Autocobas 501 autoanalyser (Roche-Hitachi, Mannheim, Germany).
The levels of total protein and albumin were measured with commercially available assay kits (Roche, Germany). Electrophoresis of serum immune fixation, serum protein, and urine protein was performed on patient samples using agarose gel and an Interlab Microgel instrument (Interlab Rome, Italy).
Statistics
The distribution of continuous variables such as age, native thiol, and disulfide were examined visually and by the Shapiro–Wilk test. All continuous variables were stated as mean plus/minus standard deviation (mean ± SD) and median (minimum–maximum); categorical variables were shown as count (%).
A relationship was found concerning the ages of the study and control groups. ANCOVA was therefore used to eradicate the age effect during the comparison of thiol/disulfide levels between the two groups. Univariate analyses were used because the age effect was found to be insignificant. The Mann–Whitney U test and Kruskal–Wallis test were performed to compare controls with MM patients in general. These tests were based on the diagnostic time with respect to the biochemical measurements. Chi-square tests were used to evaluate the distributions of gender, type of myeloma, etc. in the groups. For myeloma type and phase, a statistical inference could not be made because the cell counts were insufficient.
The Sperman and polyserial correlation coefficients were calculated to evaluate the relationship between the measurements. A P<0.05 was accepted as statistically significant.
All analyses and statistical calculations were performed using IBM SPSS Statistics 21.0 (IBM Corp., NY Released 2012. IBM SPSS Statistics for Windows, Version 21.0).
Results
The median age of the study group was 66.5 years (min–max: 39–84) and the median age of the control group was 68 years (min–max: 50–91) (Table ). The median age of the control group was higher than of the study group (P=0.043). The median age of the 35 newly diagnosed patients was 64 years (min–max: 39–84) and the median age of 15 patients who were undergoing treatment was 72 years (min–max: 41–83). The median age of the newly diagnosed patients was lower than that of the control group and of the outpatients who were already being treated. The distribution of gender was found to be similar in the control and study groups (P=0.109 and 0.184, respectively).
Table 1 Comparisons of demographic features and native thiol, total thiol, disulfide, total protein, and albumin measurements across groups
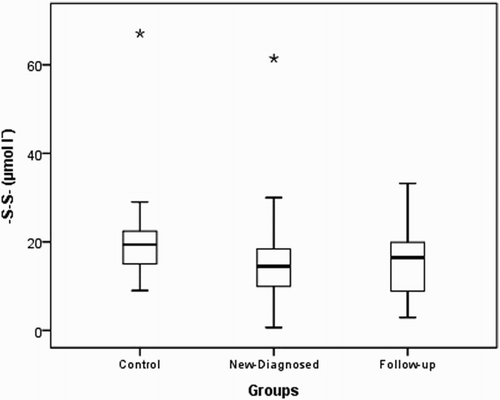
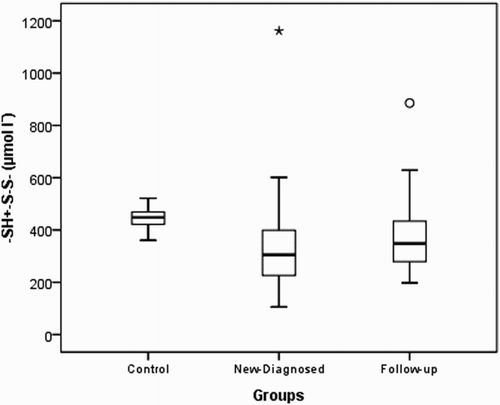
Native thiol levels in the control group were determined to be higher than those in the study and patient groups (P<0.001). Disulfide levels were found to be higher in the control group than in the study group and higher in newly diagnosed patients than in outpatients who were undergoing treatment. Total thiol levels were higher in the control group than in the study and patient groups. Thiol levels were found to be similar in the study and control groups (P=0.659 and 0.540, respectively). The distributions of the native thiol, disulfide, and total thiol levels in the control group are shown in Figs. –.
Figure 1 Box-plot of native thiol (-SH) in experimental groups (asterisk and circle indicate outliers).
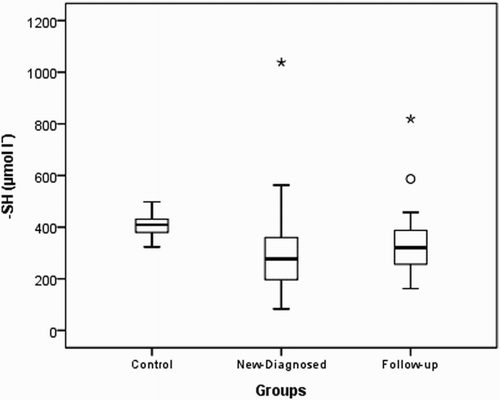
Figure 3 Box-plot of total thiol (-SH+-S-S-) in experimental groups (asterisk and circle indicate outliers).
Median protein levels in the control and study groups were 7.50 g/dl (min–max: 6.80–8.00) and 8.05 g/dl (min–max: 4.68–16.50), respectively; no relationship was found between the groups (Table , P=0.081). Total protein levels were found to be similar in the control and study groups (P=0.103).
IGG kappa type MM was found in 34.3% (n=12) of newly diagnosed patients and 33.3% of the outpatients who were undergoing treatment. Median M-protein levels of the newly diagnosed patients and outpatients were 5220.0 (min–max: 200.0–13 600.0) and 2060.0 (min–max: 72.0–5270.0), respectively. The serum M-protein levels were lower in outpatients (P=0.002) (Table ).
Table 2 Comparisons of myeloma types and M protein levels across patient groups
No relationships were observed between thiol levels and thiol ratios to serum/urine M-protein, stage and total protein (P>0.05) (Table ).
Table 3 Relationships between thiols, disulfide, ratios of thiols-disulfide and M proteins, total protein, albumin, and stage
There was a positive but mild relationship between albumin and thiol (native and total); there was a positive but weak relationship between albumin and disulfide.
Discussion
Proteins, lipids, and DNA are target molecules for oxidative damage. The presence of excessive amounts of free radicals or a disability of the antioxidant system causes detrimental effects such as membrane damage, changes in protein function, lipid denaturation, and structural damage to DNA.Citation17 These changes increase mutation risk and the duration of neoplastic transformation.Citation3–Citation5 Based on these hypotheses, in recent years an increasing number of studies have been carried out to evaluate oxidant and antioxidant status in MM etiopathogenesis.
Several studies have shown that the levels of malondialdehyde (MDA), a product of lipid peroxidation, increase, the levels of antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx) decrease,Citation5,Citation18 and the levels of catalase and non-enzymatic antioxidants (vitamin C and E) decrease in patients with MM.Citation5 In addition, Gangemi et al. (2012) reported significantly increased levels of serum advanced oxidation protein products (AOPPs) in untreated MM patients and S-nitrosylated proteins in MM patients.Citation19 These studies support the relationship between MM and oxidative stress.
There are several studies that concern changes in oxidative parameters after treatment. Kuku et al. (2005) researched the changes of SOD, catalase, GPx, and nitric oxide (NO) levels after vincristine-adriamycin-dexametasone treatment in 14 newly diagnosed MM patients. They found that all enzyme activities and oxidative stress parameters decreased after treatment.Citation20 Mehdi et al. (2013) found that total antioxidant capacity (TAC), glutathione, ascorbic acid (vitamin C), vitamin E, and antioxidant enzyme levels increased and AOPPs, MDA, and adenosine deaminase (ADA) levels decreased after the induction therapy in 30 MM patients.Citation21 These studies have shown that oxidative stress decreased after therapy.
MM studies over the past 2 years have also investigated levels of total thiol, lipophilic antioxidant enzymes (paraoxonase and arylesterase), total oxidant, and antioxidant status by the Erel method. These also reported changes in the oxidant–antioxidant balance and that those parameters affected the prognosis.Citation22–Citation24 Based on these findings, it could be accepted that oxidative stress affects the pathogenesis of MM, even if it has different mechanisms.Citation25
Concerning thiol/disulfide homeostasis, Ellidag et al. (2014) investigated only a single side of this balance in 40 patients with MM. They found that the levels of total thiol in the patients were significantly lower than those in the control group. As a result, it appeared that the sulfydryl groups of thiol compounds had an important role in the defense against free radicals. Decreased thiol levels and increased intracellular oxidants impairment the activation of several enzymes, so these factors may be involved in MM etiopathogenesis.Citation26 In a preliminary study, Erel and Neselioglu (2014) showed that plasma disulfide levels were higher in patients with heterogeneous diseases such as MM. They reported that aggressively growing tumors, especially MM, showed the lowest disulfide levels.Citation16
According to the authors’ knowledge, this is the first study to investigate the relationship between MM and thiol/disulfide homeostasis. Thiol/disulfide homeostasis plays an important role in antioxidant protection, detoxification, apoptosis, and cellular signaling mechanisms. We found that the native thiol, total thiol, and disulfide levels of the study group were lower than those of the control group. Similarly, native thiol, total thiol, and disulfide levels were lower in outpatients and newly diagnosed patients than in the control group. The ratios of thiol levels were determined to be similar in all groups. These results show that, although there was a decrease in disulfide, native thiol, and total thiol levels, the balance of thiol/disulfide was maintained. Oxidative and antioxidative parameters encompass a wide and varying range, so that their cumulative effects and balance levels determine the systemic effects, rather than their individual changes. Although the parameters of our study group were lower than those of the control group, these combined parameters did not change the systemic oxidative effect because there was no change in total homeostasis.
There was a mild positive relationship between albumin and both native and total thiol. It was not surprising to find such a positive correlation between these parameters because decreasing in albumin correlates with the stage of MM and the antioxidant effects of thiol are reduced because of decreased thiol levels. Similarly, there was a weak positive relationship between albumin and disulfide levels. Again, no significant correlation was found between total protein and disulfide levels. The possible reasons for this finding were that the basal total protein levels of both patient groups were found to be at normal levels and there was no significant difference between them and the control group. There was no significant correlation between M proteins, which show disease burden, and measured parameters. The relatively limited numbers of patients were thought to be a reason for evaluating correlational status objectively.
Mechanisms that affect general cellular functions – such as thiol/disulfide homeostasis, which has effects on antioxidant protection, detoxification, apoptosis, transcription, and cellular signal transduction mechanisms – may play a role in the etiology of diseases such as MM that have as-yet unknown etiologies. When one considers that factors such as weakened immunity, radiation, certain viruses, and chemicals, which are all accepted as possible reasons in MM etiology, have indirect rather than direct effects, oxidative metabolism and thiol/disulfide homeostasis, which affect cellular function and cause chromosomal abnormalities in ways similar to others, may also be included in the etiology with similar mechanisms. Certainly, a direct etiological cause–effect relationship cannot be determined in cross-sectional case-control studies. Such studies rather shed a light on the specific etiologies of MM.
This is the first study to research oxidative homeostasis from the perspective of the new method that was used. The major limitation of our study was its small sample size. The sample size was not sufficient to allow generalization to all patients with MM. An evaluation of oxidant and antioxidant parameters was not included in this study because several studies have already evaluated oxidant and antioxidant parameters in patients with MM. In addition, thiol/disulfide homeostasis has other cellular functions such as cell signal mechanisms, apoptosis, and signal transduction. Further studies are needed with larger sample groups.
Conclusions
MM is a hematologic malignancy that does not have a curing therapy. Studies into curing therapies are interesting. Deeper understanding of the etiopathogenesis of this disease is thought to help in treatment. To that purpose, we set up a study using a method that had not been used previously in MM patients. This study researched dynamic thiol/disulfide homeostasis in MM patients. There was no difference in the thiol/disulfide balance. We hope that this study will encourage and facilitate further studies in this area.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflicts of interest The authors report no conflicts of interest.
Ethics approval This study was approved by the ethics committee of Yildirim Beyazit University Medical Faculty.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2015;65:5–29. doi: 10.3322/caac.21254
- Bergsagel PL. Epidemiology, etiology, and molecular pathogenesis. In: Richardson PG, Anderson KC, (eds.) Multiple Miyeloma. London & Chicago: Remedica Publishing; 2004. p. 1–24.
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009
- Gupte A, Rusell JM. Elevated copper and oxidative stres in cancer cells as a target for cancer treatment. Cancer Treat Rev 2009;35:32–46. doi: 10.1016/j.ctrv.2008.07.004
- Sharma A, Tripathi M, Satyam A, Kumar L. Study of antioxidant levels in patients with multiple myeloma. Leukemia Lymphoma 2009;50:809–15. doi: 10.1080/10428190902802323
- Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr 2000;72 (2 Suppl): 653S–69S.
- Turell L, Radi R, Alvarez B. The thiol pool in plasma: the central contribution of albümin to redox processes. Free Radic Biol Med 2013;65:244–53. doi: 10.1016/j.freeradbiomed.2013.05.050
- Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem 2013;288:26489–96. doi: 10.1074/jbc.R113.462929
- Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med 2009;47:1329–38. doi: 10.1016/j.freeradbiomed.2009.08.021
- Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol 2006;28:551–64. doi: 10.1016/j.bcp.2005.10.044
- Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 2010;48:749–62. doi: 10.1016/j.freeradbiomed.2009.12.022
- Prabhu A, Sarcar B, Kahali S, Yuan Z, Johnson JJ, Adam KP et al. Cysteine catabolism: a novel metabolic pathway contributing to glioblastoma growth. Cancer Res 2014;74:787–96. doi: 10.1158/0008-5472.CAN-13-1423
- Ergin M, Cendek BD, Neselioglu S, Avsar AF, Erel O. Dynamic thiol-disulfide homeostasis in hyperemesis gravidarum. J Perinatol 2015;35:788–92. doi: 10.1038/jp.2015.81
- Kundi H, Ates I, Kiziltunc E, Cetin M, Cicekcioglu H, Neselioglu S, et al. A novel oxidative stress marker in acute myocardial infarction; thiol/disulfide homeostasis. Am J Emerg Med 2015;33:1567–71. doi: 10.1016/j.ajem.2015.06.016
- Ates I, Kaplan M, Yuksel M, Mese D, Alisik M, Erel O, et al. Determination of thiol/disulfide homeostasis in type 1 diabetes mellitus and the factors associated with thiol oxidation. Endocrine 2016;51(1):47–51. doi: 10.1007/s12020-015-0784-6
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulfide homeostasis. Clin Biochem 2014;47:326–32. doi: 10.1016/j.clinbiochem.2014.09.026
- Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev 2013;2013:963520. doi: 10.1155/2013/963520
- Zima T, Štipek S, Crkovská J, Plátenik J, Merta M, Tesaŕ V. Antioxidant enzymes and lipid peroxidation in patients with multiple myeloma. Neoplasma 1996;43:69–73.
- Gangemi S, Allegra A, Alonci A, Cristani M, Russo S, Speciale A, et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res 2012;61:1063–7. doi: 10.1007/s00011-012-0498-7
- Kuku I, Aydogdu I, Bayraktar N, Kaya E, Akyok O, Erkurt MA. Oxidant/antioxidant parameters and their relationship with medical treatment in multiple myeloma. Cell Biochem Funct 2005;23:47–50. doi: 10.1002/cbf.1127
- Mehdi WA, Zainulabdeen JA, Mehde AA. Investigation of the antioxidant status in multiple myeloma patients: effects of therapy. Asian Pac J Cancer Prev 2013;14:3663–7. doi: 10.7314/APJCP.2013.14.6.3663
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008
- Ellidag HY, Aydin O, Eren E, Yilmaz N, Ergin M. Decreased hdl-dependent paraoxonase and arylesterase enzyme activity may indicate a worse prognosis in multiple myeloma. Asian Pac J Cancer Prev 2014;15:9847–51. doi: 10.7314/APJCP.2014.15.22.9847
- Faridvant Y, Oskuyi AE, Khadem-Ansari MH. Serum 8-isoprostane levels and paraoxonase 1 activity in patients with stage I multiple myeloma. Redox Rep 2016;26:1–5.
- Imbesi S, Musolino C, Allegra A, Saija A, Morabito F, Calapai G, et al. Oxidative stress in oncohematologic diseases: an update. Expert Rev Hematol 2013;6:317–25. doi: 10.1586/ehm.13.21
- Ellidah HY, Eren E, Aydin O, Yildirim M, Sezer C, Yilmaz N. Multiple myeloma: relationship to antioxidant esterases. Med Princ Prac 2014;23:18–23. doi: 10.1159/000355826