Abstract
Objective: We studied the modulatory effects of homocysteine pre-treatment on the disulfide reduction capacity of tumor and endothelial cells.
Methods: Human MDA-MB-231 breast carcinoma and bovine aorta endothelial cells were pre-treated for 1–24 hours with 0.5–5 mM homocysteine or homocysteine thiolactone. After washing to eliminate any rest of homocysteine or homocysteine thiolactone, cell redox capacity was determined by using a method for measuring disulfide reduction.
Results: Homocysteine pre-treatments for 1–4 hours at a concentration of 0.5–5 mM increase the disulfide reduction capacity of both tumor and endothelial cells. This effect cannot be fully mimicked by either cysteine or homocysteine thiolactone pre-treatments of tumor cells.
Discussion: Taken together, our data suggest that homocysteine can behave as an anti-oxidant agent by increasing the anti-oxidant capacity of tumor and endothelial cells.
Introduction
Homocysteine is a non-proteinogenic amino acid playing key roles in two interconnected metabolic pathways, namely, the activated methyl cycle and the linear trans-sulfuration pathway that allows the conversion of methionine to cysteine.Citation1–Citation6 A dysregulation of intracellular homocysteine metabolism could yield an increased export of this amino acid, leading to hyperhomocysteinemia, which has been associated with an increased risk of cardiovascular diseases.Citation7–Citation10 In spite of decades of experimental efforts, there is no definitive consensus on what could be the molecular mechanisms whereby hyperhomocysteinemia could contribute to cardiovascular disease.Citation11–Citation13 Although it has been shown that high levels of homocysteine can induce endoplasmic reticulum stress,Citation14 protein N-homocysteinylation,Citation15,Citation16 and epigenetic effects through changes in the global methylation status,Citation17–Citation19 the redox active nature of homocysteine has favored the idea of an induction of oxidative stress as the underlying mechanism of homocysteine toxicity.Citation20–Citation23 In contrast, homocysteine can also exert protective effects, including its potent anti-angiogenic and anti-invasive effects previously shown by our group.Citation24–Citation26 Furthermore, homocysteine can also behave as an anti-oxidant compound, at least under certain conditions.Citation27–Citation29 In fact, our group has previously shown that homocysteine treatment is able to induce a potent increase in the activity of ferricyanide reductase, a component of the plasma membrane redox system.Citation30 The present work is aimed to further analyze the capacity of homocysteine to modulate the redox capacity of both endothelial and tumor cells by using a previously described method.Citation31
Materials and methods
Chemicals and reagents
Cell culture media were purchased from Gibco (Grand Island, NY, USA) and Cambrex (Walkersville, MD, USA). Fetal bovine serum (FBS) was provided by Harlan-Seralab (Belton, UK). Homocysteine was supplied by Sigma-Aldrich (St. Louis, MO, USA). Supplements and other chemicals not listed in this section were obtained from Sigma-Aldrich. Plasticware for cell culture was supplied by NUNC (Roskilde, Denmark).
Cell cultures
Human estrogen receptor-negative MDA-MB-231 breast carcinoma cells were supplied by ATCC. Primary bovine aorta endothelial cells (BAEC) were isolated by a collagenase treatment, as previously described.Citation32 Cultures were maintained in RPMI-1640 (MDA-BB.231 cells) or DMEM containing glucose (1 g/l) and 2 mM glutamine (BAEC), both supplemented with the antibiotics penicillin (50 IU/ml), streptomycin (50 µg/ml), and amphoterycin (1.25 µg/ml) and with 10% FBS.
Homocysteine, cysteine, and homocysteine thiolactone treatments
Before measuring cell redox capacity, both MDA-MB231 human breast cancer cells and BAEC were pre-treated with 5 mM homocysteine, cysteine, or homocysteine thiolactone for 4 hours. Experiments were carried out in culture media supplemented or not with 10% FBS. In some experiments, MDA-MB231 tumor cells were pre-treated with 0.05 and 0.5 mM homocysteine for 4 hours. In other experiments, MDA-MB231 tumor cells were pre-treated with 5 mM homocysteine for 1 and 24 hours. Cytotoxicity of pre-treatments was discarded by carrying out MTT assays as previously described.Citation24
Cell redox capacity determination
Cell redox capacity was determined by using the method for measuring disulfide reduction by cultured mammalian cellsCitation31 with modifications. Briefly, control and treated cells were washed three times with cold PBS supplemented with 1 mM CaCl2 and 0.5 mM MgCl2. Afterwards, each plate received 10 ml of reaction buffer (PBS supplemented with 1 mM CaCl2, 0.5 mM MgCl2, 5 mM glucose, 0.1 mM lipoic acid, and 0.1 mM DTNB). At times 0, 15, 30, 45, and 60 minutes, 1 ml aliquots were taken, centrifuged at 300g for 1 minute and absorbance at 412 nm was measured in supernatants.
After each assay, cell number in each dish was determined by using a Coulter counter. The absorbance values obtained in the integrated measure of cell redox capacity were normalized to equal number of cells.
All quantitative data are expressed as means ± standard deviation (S.D.). Two-tailed Student's t-test was used for evaluations of pairs of means, to establish which groups differed from the control group.
Results and discussion
Homocysteine pre-treatment increases the redox capacity of both MDA-MB231 human breast cancer and bovine aortic endothelial cells (BAEC)
Regarding the dual pro-/anti-oxidant role of homocysteine, our group had previously demonstrated that 5 mM homocysteine pre-treatment can increase plasma membrane redox activities in both tumor and endothelial cells, thus increasing their capacity to reduce extracellular oxidant agents.Citation30 The first goal of the present study was to test whether homocysteine pre-treatment could induce an increased cell redox activity as determined by the method for the integrated measure of cell redox capacity.Citation31 This method is based on the redox reactivity of α-lipoic acid whereby it is reduced within cells to dihydrolipoic acid, which effluxes and quantitatively reduces extracellular DTNB. Hence, this method is useful to assess the disulfide reduction capacity of intact cells.
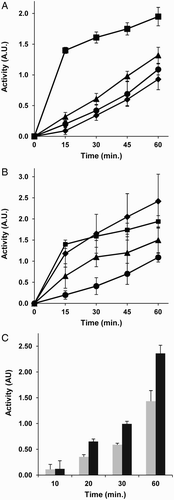
Results of the present study clearly show that, indeed, 5 mM homocysteine pre-treatment for 1–4 hours increases the disulfide-reducing capacity of both MDA-MB231 human breast carcinoma cancer (Fig. A and B) and BAEC (Fig. C). It is important to note that homocysteine interference with the redox assay should be discarded, since it is only present during the pre-treatment and is not present during the redox assay. The need to use this non-physiological concentration of homocysteine to assess regulatory effects in short-term cell culture incubations was previously discussed elsewhere.Citation30 Nonetheless, we could find out statistically significant increases in this total disulfide reduction capacity even at the pathophysiologically relevant concentration of 0.5 mM homocysteine (corresponding to a very severe hyperhomocysteinemia) for both MDA-MB231 tumor cells (Fig. A) and BAEC (results not shown). Since homocysteine was removed after pre-treatment and cells were washed before the experimental determination of cell redox capacity, the above mentioned effects of homocysteine cannot be attributed to a direct interference of its reactive sulfhydryl group on the assay.
Figure 1 Homocysteine pre-treatment increases the disulfide reduction capacity of MDA-MB231 human breast cancer cells and BAEC. (A) Time-course curves of the disulfide reduction capacity of intact cells after pre-treatments with different concentrations of homocysteine, as described in Material and Methods. Estrogen receptor-negative MDA-MB-231 human breast cancer cells were pre-treated for 4 hours with 0 mM (rhombus), 0.05 mM (circles), 0.5 mM (triangles), or 5 mM (squares) homocysteine. Values for 0.5 and 5 mM homocysteine pre-treatments were statistically significant as compared with those of controls (P < 0.05 and P < 0.01, respectively). (B) Time-course curves of the disulfide reduction capacity of intact cells after different pre-treatment times with 5 mM homocysteine: controls (circles) and 1 hour (rhombus), 4 hours (squares), and 24 hours (triangles). Values for 1 and 4 hours of pre-treatment with 5 mM homocysteine were statistically significant as compared with those of controls (P < 0.01). (C) The total disulfide reduction capacity of intact BAEC after 4 hours of pre-treatments with or without 5 mM homocysteine was measured, as described in Material and Methods. Activity values after different times of incubation in arbitrary units are represented for control, untreated cells (gray bars) and 5 mM homocysteine-treated cells (black bars). Depicted data are means ± S.D. of values of three independent experiments. From 20 minutes on, values for 4 hours of pre-treatment with 5 mM homocysteine were statistically significant as compared with those of controls (P < 0.01).
Cysteine does not mimic the inducing effect of homocysteine on MDA-MB231 tumor cell total redox capacity
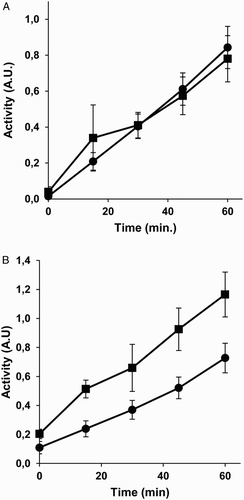
To test whether the inducing effects of homocysteine pre-treatment on cell total redox capacity could be due exclusively to the reactive sulfhydryl group of this amino acid, we carried out pre-treatments with 5 mM cysteine for 4 hours prior to determination of redox activity. Figure A shows that 5 mM cysteine pre-treatment for 4 hours had no inducing effect on MDA-MB231 tumor cell total redox capacity. In contrast, this pre-treatment elicited a slight but significant increase in BAEC total redox capacity (Fig. B).
Figure 2 Cysteine cannot fully mimic the effects of homocysteine on the disulfide reduction capacity of intact cells. The disulfide reduction capacity of intact MDA-MB-231 human breast cancer cells (A) and BAEC (B) after 4 hours of pre-treatments with (squares) or without (circles) 5 mM cysteine was measured, as described in Material and Methods. Depicted data are means ± S.D. of values of three independent experiments. Values for 4 hours of pre-treatment with 5 mM cysteine were statistically significant in BAEC as compared with those of controls (P < 0.05).
Homocysteine thiolactone does not mimic the inducing effect of homocysteine on MDA-MB231 tumor cell total redox capacity
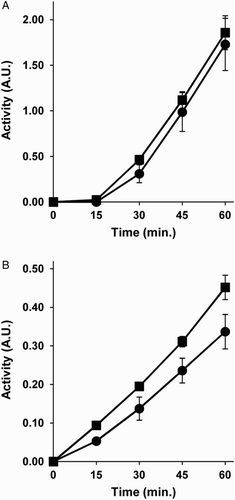
In the presence of high concentrations of homocysteine, the synthesis of homocysteine thiolactone is enhanced because of the reduction of both transmethylation and trans-sulfuration. Since protein homocysteinylation by homocysteine thiolactone has been suggested to underlie the physiopathological effects of hyperhomocysteinemia,Citation15,Citation16 we next wanted to test whether homocysteine thiolactone could mimic the inducing effect of homocysteine on MDA-MB231 tumor cells and BAEC redox capacity. Results depicted in Fig. clearly show that this does not seem the case, since 5 mM homocysteine thiolactone treatment had no significant effect on MDA-MB231 tumor cell redox capacity (Fig. A) and only a weak, less than a 50% increase on BAEC redox capacity (Fig. B). These results are consistent with the remarkable differences we found previously in the effects of homocysteine and its thiolactone derivative on angiogenesis.Citation24,Citation33
Figure 3 Homocysteine thiolactone cannot mimic the effects of homocysteine on the disulfide reduction capacity of intact cells. The disulfide reduction capacity of intact MDA-MB-231 human breast cancer cells (A) and BAEC (B) after 4 hours of pre-treatments with (squares) or without (circles) 5 mM homocysteine thiolactone was measured, as described in Material and Methods. Depicted data are means ± S.D. of values of three independent experiments. Values for 4 hours of pre-treatment with 5 mM homocysteine thiolactone were statistically significant in BAEC as compared with those of controls (P < 0.05).
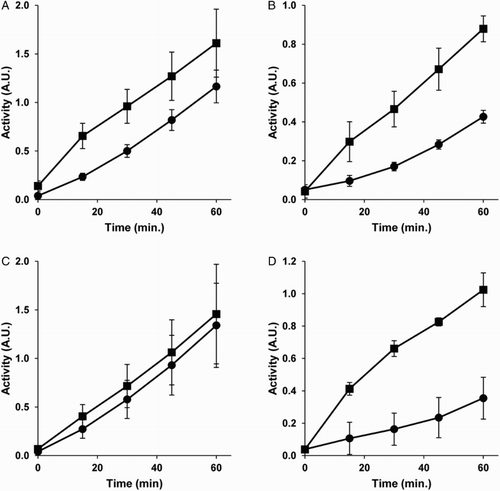
All the experiments described so far were carried out with pre-incubations in complete culture media in the presence of 10% FBS. Under these conditions, it could be likely that most of the homocysteine thiolactone reacted with serum proteins, thus yielding a much lower effective concentration of this compound. This could be a reason why we did not observe any relevant effect of homocysteine thiolactone pre-treatment on tumor cell total redox capacity. Therefore, we decided to test the effects of pre-treatments carried out with cells maintained in culture media devoid of FBS. Figure shows that 5 mM homocysteine pre-treatment for 4 hours in the absence of FBS still induced significant increases in both MDA-MB231 tumor cell and BAEC total redox capacity (Fig. A and B, respectively). In contrast, the results obtained after 5 mM homocysteine thiolactone pre-treatment for 4 hours in the absence of FBS were very different for tumor and endothelial cells. In the case of MDA-MB231 tumor cells, the pre-treatment had no effect on its total redox capacity (Fig. C), whereas the same pre-treatment had a clear and significant inducing effect on BAEC total redox capacity (Fig. D).
Figure 4 Pre-treatments in the absence of serum reveal different effects of homocysteine thiolactone on MDA-MB231 human breast cancer cells and BAEC disulfide reduction capacity. The disulfide reduction capacity of intact MDA-MB-231 human breast cancer cells (A and C) and BAEC (B and D) after 4 hours of pre-treatments with (squares) or without (circles) 5 mM homocysteine (A, B) or 5 mM homocysteine thiolactone (C and D) was measured, as described in Material and Methods. Depicted data are means ± S.D. of values of three independent experiments. In (A), (B), and (D) (but not in C) values for pre-treated samples were statistically significant as compared with those of controls (P < 0.05).
Effects of homocysteine and homocysteine thiolactone treatments on MB231 tumor cells and BAEC morphology
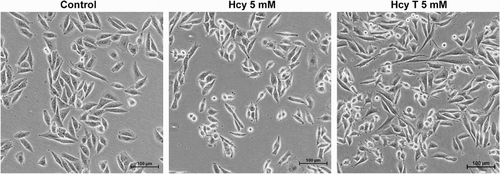
Since we had previously shown that homocysteine treatment could induce morphological differentiation of BAEC cultured in the absence of serum,Citation24 we wanted to test whether the treatment conditions used in the present study could induce any alteration in the viability and morphology of treated cells. MTT assays detected no relevant effects of 5 mM treatment for 4 hours on BAEC and MDA-MB231 cell viability (results not shown). On the other hand, Fig. shows that 5 mM homocysteine or homocysteine thiolactone treatments induced similar slight effects on MDA-MB231 tumor cell morphology, with an increased subpopulation of smaller, more ‘rounded’ and refringent cells.
Figure 5 Effects of the pre-treatments on the morphology of MDA-MB231 human breast cancer cells. Photographs were taken of MDA-MB231 human breast cancer cells after 4 hours of treatment in the absence (control) or presence of 5 mM homocysteine (Hcy) or homocysteine thiolactone (Hcy T). Photographs were taken at a 200× magnification under a Nikon Eclipse TI microscope. Representative images are shown. Three independent experiments were carried out with similar results.
Concluding remarks
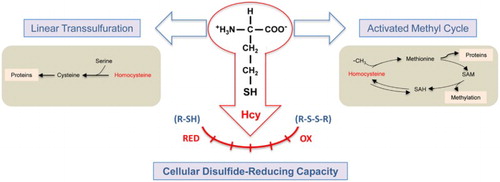
Taken altogether, data from this study show that homocysteine has modulatory effects on disulfide reduction capacity of intact cells (Fig. ). These effects cannot be fully mimicked by either cysteine or homocysteine thiolactone in the case of tumor cells. In conclusion, the present study shows new modulatory effects of homocysteine on cellular redox status to be added to its previously shown effects on plasma membrane electron transport systems.Citation30 Further experimental efforts will be needed in the future to reveal the underlying mechanisms for the differential effects of homocysteine versus cysteine or homocysteine thiolactone pre-treatments of tumor versus endothelial cells. It is tempting to speculate on potential effects of the imbalance of both epigenetic and redox regulation caused by high concentrations of homocysteine. It is known that high concentrations of homocysteine cause accumulation of S-adenosylhomocysteine (Fig. ), which is known to be a potent inhibitor of methylases. Therefore, this could create a strong imbalance in epigenetic regulations. On the other hand, it has been shown that S-adenosylhomocysteine accumulation suppresses the expression of glutathione peroxidase 1 to promote oxidative stress.Citation34
Figure 6 Some major metabolic and modulatory functions of homocysteine. To the well-known metabolic roles of homocysteine as an intermediate metabolite in both the lineal trans-sulfuration pathway and the activated methyl cycle, our results add a relevant modulatory effect of this amino acid on cellular disulfide-reducing capacity.
Disclaimer statements
Contributors E.D.S. and L.R.C. carried out most of the experiments. C.C. and J.J.S. carried out additional and confirmative experiments. A.R.Q. and M.A.M. revised results, discussed them and revised the preliminary draft of the manuscript. M.A.M. conceived the experimental work and wrote the preliminary draft of the manuscript. All the authors approved the final version of the manuscript.
Funding This work was supported by grants BIO2014-56092-R (MINECO and FEDER) and P12-CTS-1507 (Andalusian Government and FEDER). The ‘CIBER de Enfermedades Raras’ is an initiative from the ISCIII (Spain). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. Thanks are due to Mrs. Norma McVeigh McIvor for the professional English proof-reading and editing of the manuscript.
Conflicts of interest The authors declare that they have no financial or other conflict of interest.
Ethics approval None.
ORCID
Miguel Ángel Medina http://orcid.org/0000-0001-7275-6462
References
- Selhub J. Homocysteine metabolism. Annu Rev Nutr 1999;19:217–46. doi: 10.1146/annurev.nutr.19.1.217
- Finkelstein JD. Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost 2000;26:219–25. doi: 10.1055/s-2000-8466
- Medina M, Urdiales JL, Amores-Sánchez MI. Roles of homocysteine in cell metabolism: old and new functions. Eur J Biochem 2001;268:3871–82. doi: 10.1046/j.1432-1327.2001.02278.x
- Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 2004;24:539–77. doi: 10.1146/annurev.nutr.24.012003.132418
- Williams KT, Schalinske KL. Homocysteine metabolism and its relation to health and disease. Biofactors 2010;36:19–24.
- Perla-Kajan J, Jakubowski H. Paraoxonase 1 and homocysteine metabolism. Amino Acids 2012;43:1405–17. doi: 10.1007/s00726-012-1321-z
- Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31
- El-Khairy L, Ueland PM, Nygard O, Refsum H, Vollset SE. Lifestyle and cardiovascular disease risk factors as determinants of total cysteine in plasma: the Hordaland Homocysteine Study. Am J Clin Nutr 1999;70:1016–24.
- Tehlivets O. Homocysteine as a risk factor for atherosclerosis: is its conversion to s-adenosyl-L-homocysteine the key to deregulated lipid metabolism?. J Lipids 2011;2011:702853. doi: 10.1155/2011/702853
- Ueland PM, Clarke R. Homocysteine and cardiovascular risk: considering the evidence in the context of study design, folate fortification, and statistical power. Clin Chem 2007;53:807–9. doi: 10.1373/clinchem.2007.085480
- Medina MA, Amores-Sánchez MI. Homocysteine: an emergent cardiovascular risk factor? Eur J Clin Invest 2000;30:754–62. doi: 10.1046/j.1365-2362.2000.00691.x
- Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am J Clin Nutr 2000;72:324–32.
- Ueland PM. Importance of chemical reduction in plasma and serum homocysteine analysis. Clin Chem 2008;54:1085–6. doi: 10.1373/clinchem.2007.100438
- Outinen PA, Sood SK, Pfeifer SI, Pamidi S, Podor TJ, Li J, et al. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood 1999;94:959–67.
- Jakubowski H. Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J 1999;13:2277–83.
- Jakubowski H. Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J Nutr 2000;130:377S–81S.
- Hultberg B, Andersson A, Isaksson A. Hypomethylation as a cause of homocysteine-induced cell damage in human cell lines. Toxicology 2000;147:69–75. doi: 10.1016/S0300-483X(00)00189-X
- Fuso A, Seminara L, Cavallaro RA, D'Anselmi F, Scarpa S. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci 2005;28:195–204. doi: 10.1016/j.mcn.2004.09.007
- Kalani A, Kamat PK, Tyagi SC, Tyagi N. Synergy of homocysteine, microRNA, and epigenetics: a novel therapeutic approach for stroke. Mol Neurobiol 2013;48:157–68. doi: 10.1007/s12035-013-8421-y
- Perna AF, Ingrosso D, De Santo NG. Homocysteine and oxidative stress. Amino Acids 2003;25:409–17. doi: 10.1007/s00726-003-0026-8
- Topal G, Brunet A, Millanvoye E, Boucher JL, Rendu F, Devynck MA, et al. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radic Biol Med 2004;36:1532–41. doi: 10.1016/j.freeradbiomed.2004.03.019
- Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol 2005;289:H2649–56. doi: 10.1152/ajpheart.00548.2005
- Ventura E, Durant R, Jaussent A, Picot MC, Morena M, Badiou S, et al. Homocysteine and inflammation as main determinants of oxidative stress in the elderly. Free Radic Biol Med 2009;46:737–44. doi: 10.1016/j.freeradbiomed.2008.11.002
- Rodríguez-Nieto S, Chavarría T, Martínez-Poveda B, Sánchez-Jiménez F, Quesada AR, Medina MA. Anti-angiogenic effects of homocysteine on cultured endothelial cells. Biochem Biophys Res Commun 2002;293:497–500. doi: 10.1016/S0006-291X(02)00232-2
- Chavarría T, Rodríguez-Nieto S, Sánchez-Jiménez F, Quesada AR, Medina MA. Homocysteine is a potent inhibitor of human tumor cell gelatinases. Biochem Biophys Res Commun 2003;303:572–5. doi: 10.1016/S0006-291X(03)00382-6
- Chavarría T, Sánchez-Jiménez F, Quesada AR, Medina MA. Homocysteine inhibits the proliferation and invasive potential of HT-1080 human fibrosarcoma cells. Biochem Biophys Res Commun 2003;301:540–4. doi: 10.1016/S0006-291X(02)03081-4
- Wilcken DE, Wang XL, Adachi T, Hara H, Duarte N, Green K, et al. Relationship between homocysteine and superoxide dismutase in homocystinuria: possible relevance to cardiovascular risk. Arterioscler Thromb Vasc Biol 2000;20:1199–202. doi: 10.1161/01.ATV.20.5.1199
- Drunat S, Moatti N, Paul JL, Cogny A, Benoit MO, Demuth K. Homocysteine-induced decrease in endothelin-1 production is initiated at the extracellular level and involves oxidative products. Eur J Biochem 2001;268:5287–94. doi: 10.1046/j.0014-2956.2001.02460.x
- Zappacosta B, Mordente A, Persichilli S, Minucci A, Carlino P, Martorana GE, et al. Is homocysteine a pro-oxidant?. Free Radic Res 2001;35:499–505. doi: 10.1080/10715760100301511
- Rodríguez-Alonso J, Montañez R, Rodríguez-Caso L, Medina MA. Homocysteine is a potent modulator of plasma membrane electron transport systems. J Bioenerg Biomembr 2008;40:45–51. doi: 10.1007/s10863-008-9127-0
- Biaglow JE, Donahue J, Tuttle S, Held K, Chrestensen C, Mieyal J. A method for measuring disulfide reduction by cultured mammalian cells: relative contributions of glutathione-dependent and glutathione-independent mechanisms. Anal Biochem 2000;281:77–86. doi: 10.1006/abio.2000.4533
- Gospodarowicz D, Moran J, Braun D, Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A 1976;73:4120–4. doi: 10.1073/pnas.73.11.4120
- Martínez-Poveda B, Chavarría T, Sánchez-Jiménez F, Quesada AR, Medina MA. An in vitro evaluation of the effects of homocysteine thiolactone on key steps of angiogenesis and tumor invasion. Biochem Biophys Res Commun 2003;311:649–53. doi: 10.1016/j.bbrc.2003.10.044
- Barroso M, Florindo C, Kalwa H, Silva Z, Turanov AA, Carlson BA, et al. Inhibition of cellular methyltransferases promotes endothelial cell activation by suppressing glutathione peroxidase 1 protein expression. J Biol Chem 2014;289:15350–62. doi: 10.1074/jbc.M114.549782
