Abstract
Background: We aimed to investigate serum prolidase activity and to investigate its association with oxidative–antioxidative status in patients with developmental dysplasia of the hip (DDH).
Methods: Oxidative status parameters, including lipid hydroperoxide (LOOH), total oxidant status (TOS), and the oxidative stress index (OSI), and antioxidative status parameters, free sulfhydryl groups (Total –SH), and total antioxidative capacity (TAC), as well as serum prolidase activity were assessed in patients with DDH (n = 93), and in healthy controls (n = 82). The severity of dysplasia was evaluated according to the Tonnis grading system.
Results: Serum prolidase activity and the oxidant parameters (LOOH, TOS, and OSI) were significantly higher and the antioxidant parameters (Total –SH and TAC) were significantly lower in patients with DDH compared to the controls (P < 0.005 for all). Serum prolidase activity was positively correlated with the Tonnis grade of DDH and LOOH, TOS, and OSI levels (P < 0.001 for all), but inversely correlated with total –SH and TAC levels (P < 0.001 for all).
Conclusion: Increased levels of serum prolidase activity, LOOH, TOS, and OSI, and decreased levels of total –SH and TAC, may be associated with DDH, and these parameters may be useful adjunctive tools to assess the severity of DDH.
Introduction
Developmental dysplasia of the hip (DDH) is an abnormality in the hip joint of paediatric patients caused by the proximal femur, the shape of the acetabulum, or the soft-tissue structures around them.Citation1 Although the aetiology of DDH is not fully understood, a number of predisposing factors such as ligamentous laxity, breech position, and postnatal predilection are accepted as playing a role. The structures that form the hip develop normally during embryogenesis and gradually become abnormal for a variety of reasons, the main one being the foetal position and presentation at birth (e.g. malposition of the femoral head, abnormal forces acting on the developing hip) and the laxity of ligamentous structures around the hip joint capsule with a failure to maintain the femoral head within the acetabulum.Citation2 New-borns with DDH have also been found to have a higher ratio of collagen III to collagen I when compared with control subjects, which suggests a connective tissue abnormality in those with DDH.Citation2 A previous study performed in our laboratory showed an increased collagen turnover associated with DDH.Citation3 The major component of the abnormal hip joint in patients with DDH is caused by collagen. Therefore, an understanding of the histopathological changes and an investigation into the adjunctive tools for evaluating the severity of DDH are the most important areas of current research.
Prolidase enzyme activity is required for collagen biosynthesis and plays an important role in the breakdown of collagen and the intracellular proteins, especially in the final stage when imidodipeptides containing C-terminal proline or hydroxyproline are cleaved. Collagen is essential for the maintenance of connective tissue, and increased rates of collagen synthesis may lead to a change in the quality of collagen fibres.Citation4 Prolidase enzyme activity and the pathophysiological role of prolidase have been investigated in the context of many different bone- and joint-related diseases by several authors. Studies on osteoarthritis,Citation5 ureamic bone disease,Citation6 and osteogenesis imperfectaCitation7 have shown decreased levels of prolidase activity. However, studies of Legg–Calve–Perthes diseaseCitation8 and idiopathic clubfootCitation4 have found elevated levels of prolidase activity. In these disorders, a relationship has shown between the serum prolidase concentration, disease activity, and/or bone metabolism.
Harmful oxidative reactions have been implicated in over hundred disorders including osteoarthritis.Citation9,Citation10 In pathological conditions, oxygen tension in the synovial fluid is subject to fluctuation as a consequence of ischaemia–reperfusion phenomena, pathological acceleration of tissue metabolism, and sustained abnormal strains on the joint.Citation5 The association of oxidative–antioxidative status parameters and serum prolidase activity has been investigated in several studies.Citation4,Citation8 These studies showed that collagen metabolism may be related to oxidative stress, and this association may have a role in the aetiopathogenesis and/or severity of the disease.
In this study, we hypothesis that serum prolidase activity and oxidative stress may be associated with severity of DDH. Therefore, we investigated serum prolidase activity, oxidative status (lipid hydroperoxide [LOOH], total oxidant status [TOS], the oxidative stress index [OSI]), antioxidative status (free sulfhydryl groups [total –SH] and total antioxidative capacity [TAC]) in DDH and evaluated their relationship according to radiological severity. We aimed to determine whether an association exists between serum prolidase activity and oxidative status parameters as well as how collagen metabolism is affected in DDH by this degenerative disease.
Patients and methods
This cross-sectional study was conducted in the Department of Orthopaedics and Traumatology, and the Department of Biochemistry, at Harran University Research Hospital between March 2013 and February 2014. In total, 93 unilateral untreated patients with DDH (mean age 17.72 ± 6.54 months) and 82 controls (mean age 19.02 ± 6.69 months) were included after informed consent was obtained from all the participants’ families. The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the local Ethics Committee. The diagnosis of the patients was confirmed using standardized radiographic examination when the patients come first, as previously described.Citation11 Patients with DDH associated with teratological dislocation, bilateral cases, or who had been previously treated or had other neuromuscular causes, clinically unstable medical illness, or the use of any medication within 4 weeks prior to initiation of the study, were excluded. The severity of the dysplasia was evaluated according to the Tonnis grading system.Citation12 Controls were selected when the patients group was completed from among children in the paediatric outpatient clinic that were completely healthy at the time of blood sampling during the routine screening programme. These controls were matched with regard to age and sex.
Collection of blood samples
Venous blood was drawn from antecubital veins after a 2-hour resting period. Samples were collected in empty tubes and stored on ice at 4°C. The serum was separated from the cells by centrifugation at 3000 rpm (2500g) for 10 minutes. Serum samples, for the measurement of LOOH, TOS, OSI, total –SH, and TAC levels, as well as prolidase activity, were stored at −80°C until analysis.
Measurement of serum prolidase activity
Prolidase activity was determined by a photometric method based on measurement of the proline levels produced by prolidase.Citation13 The samples (100 µl) were mixed with 100 µl of physiological serum. A volume of 25 µl of the mixture was preincubated with 75 µl of the preincubation solution (50 mmol/l Tris HCl buffer, pH 7.0, containing 1 mmol/l GSH, and 50 mmol/l MnCl2) at 37°C for 30 minutes. The reaction mixture, which contained 144 mmol/l gly-pro (pH 7.8; 100 µl), was incubated with 100 µl of preincubated sample at 37°C for 5 minutes. To stop the incubation reaction, 1 ml glacial acetic acid was added. After adding 300 µl Tris HCl buffer (pH 7.8) and 1 ml ninhidrin solution (3 g/dl ninhidrin was melted in 0.5 mol/l orthophosphoric acid), the mixture was incubated at 90°C for 20 minutes and cooled with ice. Absorbance was measured at a wavelength of 515 nm to determine the proline levels, as previously described.Citation14 Intra- and interassay coefficients of variation (CV) were lower than 10% for this assay.
Measurement of total free sulfhydryl groups
Serum-free sulfhydryl (−SH; mmol/l) levels were assayed according to the previously described method.Citation15 Briefly, 1 ml of buffer containing 0.1 M Tris; 10 mmol/l EDTA (pH 8.2); and 50 ml of serum, was added to cuvettes, followed by 50 ml of 10 mmol// 5,5′-dithiobis(2-nitrobenzoic acid) in methanol. Blanks were run for each sample as a test. After incubation for 15 minutes at room temperature, the sample absorbance was interpreted at a wavelength of 412 nm on a Jasco V-530 spectrophotometer (Jasco, Easton, MD, USA). Sample and reagent blanks were subtracted. The concentration of the −SH groups was calculated using reduced glutathione as the free −SH group standard, and the results were expressed as millimolars per litre. The CV measurement of the −SH levels in the serum was 3.6%.
Measurement of TAC
Serum TAC was determined using an automated measurement method as previously described.Citation16 This method utilizes hydroxyl radicals, the most potent biological radicals. In the assay, ferrous ion solution, present in reagent 1, is mixed with hydrogen peroxide, present in reagent 2. Other potent radicals are produced, such as brown dianisidinyl radical cation, which is produced by the hydroxyl radicals. This method measures the antioxidant effects of the sample against the potent free radical reactions initiated by the hydroxyl radical. The CV for the measurement of serum TAC levels was <3%. The results are expressed as millimoles of Trolox equivalent per litre (mmol Trolox equiv./l).
Measurement of TOS
The TOS of the serum was determined using a previously described automated measurement method.Citation14 Oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a coloured complex with xylenol orange in an acidic medium. The colour intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay was calibrated with hydrogen peroxide, and the results are expressed as micromoles of hydrogen peroxide equivalents per litre (μmol H2O2 equiv./l). The CV for the measurement of the levels of TOS in serum was <3%.
Oxidative stress index
The ratio of TOS to TAC yielded the OSI, an indicator of the degree of oxidative stress.Citation14 For calculations, the resulting unit of TAC was changed to mmol/l, and the OSI value was calculated according to the following formula: OSI (arbitrary unit), TOS (μmol H2O2 equiv./l), TAC (mmol Trolox equiv./l).
Statistical analysis
Statistical analysis was performed using SPSS for Windows software (ver. 16.0; SPSS, Inc., Chicago, IL, USA). Continuous variables are expressed as means ± SD. The normality of the distributions was evaluated with the one-sample Kolmogorov–Smirnov test, revealing a uniform distribution. Comparisons of categorical and continuous variables between the DDH and the control groups were performed using the Chi-squared (χ2) test and the independent samples t-test, respectively. Comparison of laboratory variables between groups categorized according to the Tonnis grading system was performed using one-way analysis of variance (ANOVA) with the least significant difference post hoc LSD test applied. The correlation between serum prolidase activity and oxidative stress markers, including LOOH, TOS, OSI, total –SH, TAC, and Tonnis grade of DDH, was assessed by Pearson's correlation test. Standardised β-regression coefficients and their significance in multiple linear regression analysis were reported. A two-tailed P < 0.05 was considered statistically significant. The power of the study was calculated by post hoc power analysis.
Results
The demographic characteristics of patients with DDH and the control group are as shown in Table . No significant differences were observed in age or female/male ratio between the patients and controls. Serum prolidase activity and oxidant parameters (LOOH, TOS, and OSI) were significantly higher in patients with DDH compared to controls (P < 0.005 for all), whereas antioxidant parameters (total –SH and TAC) were significantly lower in DDH patients (P < 0.005 for all).
Table 1 Demographic characteristics of the DDH and control groups
The comparison included 93 patients with DDH in four subgroups according to the Tonnis grading system: grade 1 (n = 21), grade 2 (n = 24), grade 3 (n = 22), and grade 4 (n = 26). A comparison of demographic characteristics and laboratory parameters according to the Tonnis grading system of the DDH group is as shown in Table . The lowest and highest mean serum prolidase activities were detected in patients with grades 1 and 4, respectively (ANOVA, P < 0.001, Fig. ). In addition, oxidative stress was significantly increased and the antioxidative capacity was significantly decreased when the severity of the disease was higher (ANOVA, P < 0.001).
Figure 1 Graph demonstrating that the lowest and highest mean serum paraoxonase activities were detected in patients with grade 1 and grade 4 DDH, respectively (ANOVA, P < 0.001)
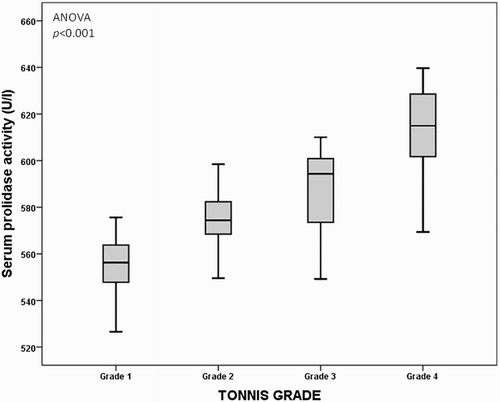
Table 2 Demographic characteristics of the DDH according to Tonnis grade
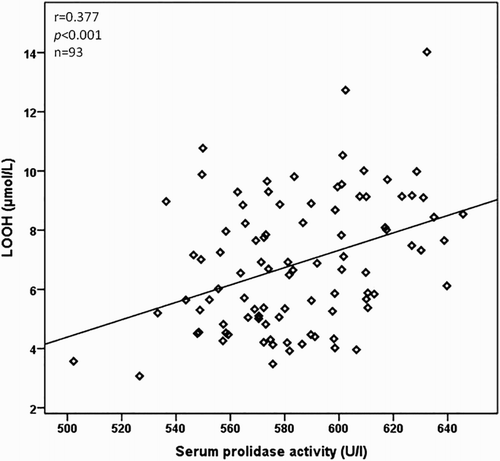
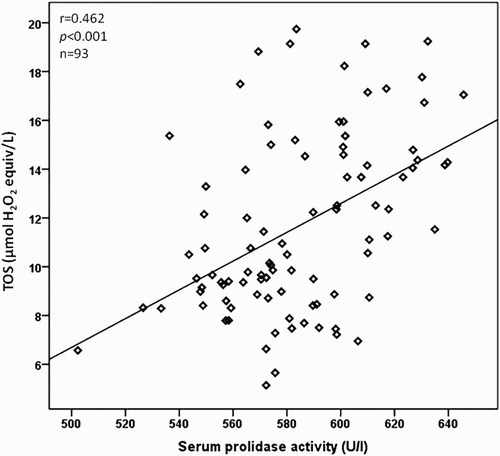
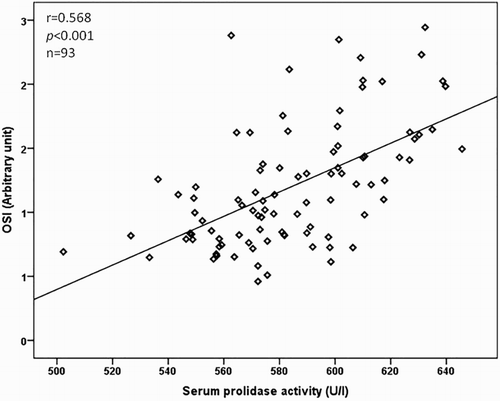
The relationship between the serum prolidase activity, clinical characteristics, and laboratory parameters is as presented in Table . The serum prolidase activity was positively correlated with the Tonnis grade of DDH and LOOH, TOS, and OSI levels (P < 0.001 for all), but inversely correlated with total –SH and TAC levels (P < 0.001 for all) in a bivariate analysis. The correlations between serum prolidase activity and LOOH, TOS, and OSI levels are as shown in Figs. –, respectively. In a multiple regression analysis, we found that the Tonnis grade of DDH (β = 0.711, P < 0.001), TOS (β = 0.675, P = 0.040), and OSI (β = 0.789, P = 0.042) levels were independently associated with serum prolidase activity (Table ).
Table 3 Relationship between serum prolidase activity and clinical characteristics and laboratory parameters
We also investigated the correlation between LOOH, TOS, and OSI parameters. The LOOH level was positively correlated with TOS (r = 0.717, P < 0.001) and OSI (r = 0.643, P < 0.001) in patients with DDH. The power of the study was calculated as 96.2%.
Discussion
In this study, we investigated serum prolidase activity (to evaluate collagen metabolism) and oxidative stress markers. We also investigated the relationships among these markers. According to the obtained results from this study, we assume that there is an association between the oxidative–antioxidative status and prolidase activity in DDH. Notably, the prolidase enzyme contains –SH groups, which possess potent antioxidative power.Citation4 Hence, increased prolidase activity is expected in the presence of high total –SH and TAC levels. We observed increased prolidase activity and expected to find elevated total –SH and TAC levels, but this was not the case. Therefore, we conclude that the increased oxidative stress observed in DDH highlighted that the condition is a degenerative disease, which causes degradation in ligamentous, osteoarticular, musculotendinous, and neurovascular structures, including collagen. As a result, metabolism increases and causes the elevation of prolidase activity.Citation3,Citation17 Furthermore, severity of DDH may create oxidative stress in the body because LOOH, TOS, and OSI levels are very high.Citation3 The levels of antioxidants (Total –SH and TAC) increased in response to oxidative stress. Ultimately, the elevated concentrations of oxidants consume the antioxidative components, and TAC and total –SH levels decrease depending on the severity of the increased oxidative stress.Citation10,Citation18
DDH is one of the most common congenital disorders of the lower limbs that evolves over time.Citation19 At birth, the neonatal acetabulum is completely composed of cartilage, with a thin rim of fibrocartilage called the labrum. The ligamentum teres also elongates and thickens, and it may take up valuable space within the acetabulum. The transverse acetabular ligament is often hypertrophic as well, which may impede reduction.Citation20 More importantly, the inferior capsule of the hip assumes an hourglass shape, eventually presenting an opening that is smaller in diameter than the femoral head. A multifactorial pathogenesis of DDH is commonly accepted.Citation21 There are several studies on the pathological differences in collagen content, and distribution and metabolism of the joint capsule and ligaments in patients with DDH.Citation22–Citation25 Pathological changes were documented in an anatomical study of six infants with DDH that included a loss of proteoglycans and an increase in collagen deposition in the lateral acetabular roof, manifesting as a ridge of degenerating acetabular cartilage.Citation2 Significant changes in the type of collagen, the fibril diameter, and the nature of the crosslinks in the joint capsules of subjects with DDH were reported, but these changes were probably tissue specific since no detectable change could be observed in the skin of these subjects compared to the controls.Citation26,Citation27 This change in structure of collagen in DDH leads to increased collagen breakdown and turnover. It is known that increased rates of collagen synthesis may lead to a change in the quality of collagen fibres.Citation4
Serum prolidase enzyme activity is affected by oxidative stress and plays an important role in the breakdown of collagen in the final step of degradation. Since the identification of increased collagen fibres in the joint capsule of patients with DDH, investigation of the histopathological changes in DDH has become more important. Although prolidase activity is found in a wide variety of diseases, reports have been contradictory. Several studies have reported that prolidase activity decreases in diseases such as osteoarthritis,Citation5 uraemic bone disease,Citation6 and osteogenesis imperfecta,Citation7 while increased prolidase activity has been described in Legg–Calve–Perthes diseaseCitation8 and idiopathic clubfoot.Citation4 In the current study, the increased serum prolidase levels in patients with DDH compared to controls may be a marker of increased collagen turnover and may be responsible for the altered histological structure of the joint capsule and other soft tissues. Furthermore, in this study the highest prolidase levels were seen in patients with grade 4, and the lowest prolidase levels were in patients with grade 1; this may be explained by the severity of the disease. However, the above-mentioned changes in biochemical parameters may indicate that DDH is a systematic disorder; it is not known why other joints are spared in unilateral patients. More detailed clinical studies are needed to clarify this point.
In several previous studies performed in our laboratory, reactive oxygen species levels have also been shown to be elevated in numerous diseases such as osteoarthritis, rheumatoid arthritis, idiopathic clubfoot, and Legg–Calve–Perthes disease.Citation4,Citation8,Citation9,Citation28 In the current study, we evaluated oxidative/antioxidative status. With respect to an increase in oxidative stress, our findings concurred with those of previous studies.Citation4,Citation8,Citation9,Citation28 Increased LOOH, TOS, and OSI and decreased total –SH and TAC levels may play a role in the aetiopathogenesis of DDH.
In addition, in the present study, serum prolidase activity positively correlated with LOOH, TOS, and OSI levels and inversely correlated with total –SH and TAC levels. These results may suggest an association between collagen turnover and oxidative stress in patients with DDH. We would like to highlight the importance of evaluating prolidase activity and oxidative status in subjects according to the severity of DDH. It is not clear whether oxidative stress is a cause and/or a result of this disease.Citation4 However, our findings provide evidence that the presence of severe oxidative stress causes collagen turnover alterations, and implicated not only oxidative stress, but also increases in prolidase enzyme activity. Additionally, in the current study a positive correlation between serum prolidase activity and Tonnis grade of DDH showed that there was a significant relationship between the severity of DDH and prolidase activity.
Some limitations of this study should be considered. A limited number of subjects were evaluated and we did not measure the levels of tissue prolidase activity.
In conclusion, it is not possible to conclude from this study whether oxidative stress is a cause of DDH or a result of the disease. It is certainly possible to conclude that increased LOOH, TOS, and OSI levels result in oxidative injury in the context of this disease, and that increased prolidase activity results in increased collagen metabolism, as observed in degenerative diseases. Therefore, we may conclude that serum prolidase activity and oxidative–antioxidative status parameters may be adjunctive tools to assess the severity of DDH. The findings of this study demonstrate for the first time the relationship between serum prolidase activity and the severity of DDH. Further prospective randomised clinical studies with larger sample sizes are needed to elucidate whether these alterations are consistent and clinically relevant.
Disclaimer statements
Contributors All authors contributed equally.
Funding This project was funded by the Harran University Scientific Research Coordination Committee (2011/3/963).
Conflicts of interest The authors declare that they have no relevant financial involvement with any commercial organization with direct financial interest in the subject or materials discussed in this manuscript. The authors have no conflicts of interest to declare.
Ethics approval Approval of Local Ethics Committee reference number: B.30.2.HRÜ.0.20.05.00.101.5/142.
References
- Ertürk C, Altay MA, Işikan UE. A radiological comparison of Salter and Pemberton osteotomies to improve acetabular deformations in developmental dysplasia of the hip. J Pediatr Orthop B 2013;22:527–32. doi: 10.1097/BPB.0b013e32836337cd
- Herring JA. Tachdjian's pediatric orthopaedics. In: Herring JA (ed.), Developmental dysplasia of the hip. Vol. 1. 5th ed. Philadelphia: Elsevier Saunders Company. Chapter 16, 2014. pp. 483–579.
- Soran N, Altindag O, Aksoy N, Cakır H, Taşkın A, Soran M, et al. The association of serum prolidase activity with developmental dysplasia of the hip. Rheumatol Int 2013;33(8):1939–42. doi: 10.1007/s00296-013-2672-9
- Altay MA, Erturk C, Aksoy N, Taskın A, Isıkan UE. A preliminary study pointing out the role of serum prolidase activity and oxidative-antioxidative status parameters during the treatment process of patients with idiopathic clubfoot. Scand J Clin Lab Invest 2011;71(7):576–82. doi: 10.3109/00365513.2011.596661
- Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int 2007;27:339–44. doi: 10.1007/s00296-006-0247-8
- Evrenkaya TR, Atasoyu EM, Kara M, Unver S, Gultepe M. The role of prolidase activity in the diagnosis of uremic bone disease. Ren Fail 2006;28:271–4. doi: 10.1080/08860220600577726
- Galicka A, Nazaruk J. Stimulation of collagen biosynthesis by flavonoid glycosides in skin fibroblasts of osteogenesis imperfecta type I and the potential mechanism of their action. Int J Mol Med 2007;20(6):889–95.
- Altay MA, Erturk C, Aksoy N, Taskin A, Bilge A, Celik H, et al. Serum prolidase activity and oxidative-antioxidative status in Legg-Calve-Perthes disease. J Pediatr Orthop B 2011;20(4):222–6. doi: 10.1097/BPB.0b013e32834493df
- Ertürk C, Altay MA, Selek S, Koçyiğit A. Paraoxonase-1 activity and oxidative status in patients with knee osteoarthritis and their relationship with radiological and clinical parameters. Scand J Clin Lab Invest 2012;72(5):433–9. doi: 10.3109/00365513.2012.687116
- Rahman T, Hosen I, Islam MMT, Shekhar HU. Oxidative stress and human health. Adv Biosci Biotechnol 2012;3:997–1019. doi: 10.4236/abb.2012.327123
- Ertürk C, Altay MA, Yarimpapuç R, Koruk I, Işikan UE. One-stage treatment of developmental dysplasia of the hip in untreated children from two to five years old. A comparative study. Acta Orthop Belg 2011;77(4):464–71.
- Donnelly KJ, Chan KW, Cosgrove AP. Delayed diagnosis of developmental dysplasia of the hip in Northern Ireland: can we do better? Bone Joint J 2015;97-B(11):1572–6. doi: 10.1302/0301-620X.97B11.35286
- Ozcan O, Gultepe M, Ipcioglu O, Bolat B, Kayadibi H. Optimization of the photometric enzyme activity assay for evaluating real activity of prolidase. Turk J Biochem 2007;32:12–16.
- Ekin S, Arısoy A, Gunbatar H, Sertogullarindan B, Sunnetcioglu A, Sezen H, et al. The relationships among the levels of oxidative and antioxidative parameters, FEV1 and prolidase activity in COPD. Redox Rep 2016 15:1–4. [Epub ahead of print].
- Yildiz A, Demirbag R, Yilmaz R, Gur M, Altiparmak IH, Akyol S, et al. The association of serum prolidase activity with the presence and severity of coronary artery disease. Coron Artery Dis 2008;19:319–25. doi: 10.1097/MCA.0b013e32830042ba
- Demirkol A, Uludag M, Soran N, Aksoy N, Gun K, Incebıyık S, et al. Total oxidative stress and antioxidant status in patients with carpal tunnel syndrome. Redox Rep 2012;17:234–8. doi: 10.1179/1351000212Y.0000000027
- Hilali N, Aksoy N, Vural M, Camuzcuoglu H, Taskin A. Oxidative status and serum prolidase activity in tubal ectopic pregnancy. J Pak Med Assoc 2013;63(2):169–72.
- Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013;2013:956792. doi: 10.1155/2013/956792
- Li Y, Xu H, Li J, Yu L, Liu Y, Southern E, et al. Early predictors of acetabular growth after closed reduction in late detected developmental dysplasia of the hip. J Pediatr Orthop B 2015;24(1):35–39. doi: 10.1097/BPB.0000000000000111
- Eberhardt O, Wirth T, Fernandez FF. Arthroscopic anatomy of the dislocated hip in infants and obstacles preventing reduction. Arthroscopy 2015;31(6):1052–9. doi: 10.1016/j.arthro.2014.12.019
- Rhodes AM, Clarke NM. A review of environmental factors implicated in human developmental dysplasia of the hip. J Child Orthop 2014;8(5):375–9. doi: 10.1007/s11832-014-0615-y
- Staheli LT. Fundamentals of pediatric orthopedics. In: Staheli LT (ed.), Hip and femur. 4th ed. Philadelphia: Wolters Kluwer Health. Chapter 11, 2008. pp. 201–30.
- Shi D, Dai J, Zhu P, Qin J, Zhu L, Zhu H, et al. Association of the D repeat polymorphism in the ASPN gene with developmental dysplasia of the hip: a case-control study in Han Chinese. Arthritis Res Ther 2011;13:R27. doi: 10.1186/ar3252
- Gold SL, Burge AJ, Potter HG. MRI of hip cartilage: joint morphology, structure, and composition. Clin Orthop Relat Res 2012;470:3321–31. doi: 10.1007/s11999-012-2403-7
- Loder RT, Skopelja EN. The epidemiology and demographics of hip dysplasia. ISRN Orthop 2011; 2011:238607.
- Zhao L, Tian W, Pan H, Zhu X, Wang J, Cheng Z, et al. Variations of the COL1A1 gene promoter and the relation to developmental dysplasia of the hip. Genet Test Mol Biomarkers 2013;17(11):840–3. doi: 10.1089/gtmb.2013.0179
- Blatt SH. To swaddle, or not to swaddle? Paleoepidemiology of developmental dysplasia of the hip and the swaddling dilemma among the indigenous populations of North America. Am J Hum Biol 2015;27(1):116–28. doi: 10.1002/ajhb.22622
- Karakoc M, Altindag O, Keles H, Soran N, Selek S. Serum oxidative-antioxidative status in patients with ankylosing spondylitis. Rheumatol Int 2007;27:1131–4. doi: 10.1007/s00296-007-0352-3