ABSTRACT
Introduction: The increasing prevalence of hyperglycaemia implicates a state of oxidative stress and inflammation. Traditional and emerging biomarkers associated with increasing hyperglycaemia were assessed to clarify their role they play in hyperglycaemia.
Results: 309 participants attending a rural diabetic screening program were categorised into control and quintile groups based upon glucose levels: 1st quintile - <4.5 mmol/L and 4th, 5th quintile - >6.1 mmol/L. Significant results were obtained for anthropometric data and biochemical markers - glucose, HbA1c and total cholesterol (P < 0.001); oxidative stress: glutathione (P < 0.001), glutathione:glutathione disulfide and 8-hydroxy-2-deoxyguanosine (P < 0.05). Interleukin -1β and inflammatory marker ratios IL-6/IL-10, IL-1β/IL-10, MCP-1/IL-10, IGF-1/IL-10 and IL-6/IL-1β were significant (P < 0.05).
Conclusion: This study provided further evidence that inflammatory and oxidative stress biomarkers may contribute to diagnostic information associated with preclinical increases in BGL. Further we have provided a unique study in the analysis of ratios of inflammatory biomarkers and correlations with increasing BGL.
Introduction
Alterations to homeostatic disturbances in glucose metabolism and resultant hyperglycaemia are causative factors for Type 2 diabetes mellitus (T2DM). Chronic sustained hyperglycaemia also results in micro- and macrovascular complications occurring through a number of mechanisms of which oxidative stress (OS) and inflammatory changes via the innate immune system have increased in interest for medical diagnostics.[Citation1,Citation2] Impaired fasting glucose (IFG) may lead to the development of T2DM and cardiovascular disease (CVD), associated with increased OS [Citation3] along with the ensuing chronic subclinical inflammatory processes apparent with developing insulin resistance.[Citation4] The challenge is to prevent complications associated with IFG of which ∼30% will remain undiagnosed for a significant period of time.[Citation5] The International Diabetes Federation have recognized the importance of the traditional biomarkers (general biochemistry): blood glucose (BGL), haemoglobin A1c (HbA1c), and lipid studies along with life style improvement and drug treatment regimens to combat T2DM and CVD. There is also a recognition that lower blood glucose levels (<7.8 mmol/l) have lower rates of all major end point diabetic complications.[Citation6] However, pathophysiological processes already occur with minor increases in BGL. Traditional biomarkers as predictors for T2DM and CVD remain sub-optimal in indicating disease progression especially when BGL is in the pre-diabetic range. This study further investigated the use of emerging OS and inflammatory markers in conjunction with traditional biomarkers and anthropometric data to establish if there was any association with blood glucose levels (BGLs) of <4.5 mmol/l (1st quintile) and >6.1 mmol/l (4th, 5th quintile) and their utility as potential predictors of T2DM and CVD development. Patients with a BGL indicative of the pre-diabetic state (>6.1 mmol/l) demonstrate an increased propensity to develop T2DM and CVD and emerging biomarkers are of clinical interest in improving risk of diabetes progression. Our hypothesis was that there is a significant difference between inflammatory markers and OS markers at raised BGLs to control BGL levels and indicate the usefulness of these markers as a diagnostic aid in T2DM and CVD prevention.
Chronic hyperglycaemia is considered to be a major causative factor in the establishment of microvascular and macrovascular complications observed in T2DM. Reactive oxygen species (ROS), as a result of hyperglycaemia, are known to damage nucleic acids, lipids, and proteins with the degree or extent of damage related to the duration of hyperglycaemia.[Citation7,Citation8] The associated pathophysiological mechanisms which occur are suggestive of the excess generation of oxygen and nitrogen species and concomitant OS.[Citation9] OS is initiated by hyperglycaemia as a result of increased circulating intracellular and extracellular free radical levels.[Citation10,Citation11] Glutathione (GSH) is a responsive ubiquitous antioxidant and detoxifier of excessive free radicals and ROS [Citation10,Citation12] and readily oxidized to glutathione disulphide (GSSG) making it the major physiological redox reaction.[Citation13] Any oxidation of GSH and a consequential increase in GSSG leads to changes in the GSH:GSSG ratio, which can be utilized as a useful marker in determining an antioxidant status.[Citation14] As decreased levels of GSH have been observed as a consequence of hyperglycaemia, primarily as a result of cysteine depletion and loss of cysteine-assisted membrane transport mechanisms across the red blood cells (RBCs), cellular OS may increase.[Citation15,Citation16] Oxidative damage to DNA and RNA occurs due to nuclear free radical formation as a result of hydrogen peroxide oxidation. The interaction of the hydroxyl radical with the DNA nucleobase guanine forms the product C8-hydroxyguanine (8-OHGua) or the nucleoside 8-hydroxy-2′-deoxyguanosine (8OHDG).[Citation17] Increased levels of 8OHDG have previously been shown to be useful biomarkers in the assessment of atherosclerosis and T2DM.[Citation18] OS and inflammation go hand in hand as a result of hyperglycaemia and increased ROS.[Citation19,Citation20] There is therefore an imperative to observe levels of both oxidative and inflammatory markers in the investigation of the hyperglycaemic state and the progression to T2DM and CVD.
T2DM is characterized by hyperglycaemia primarily associated with insulin resistance but often obesity, dyslipidaemia, hypertension, and accelerated atherosclerosis are clinical comorbidities.[Citation21] T2DM has been further classified as a chronic inflammatory state with evidence of disparate concentrations of cytokines and acute phase reactants (APRs).[Citation22] This inflammatory process and the association with T2DM is also contributory, but not necessarily exclusive to the progression to CVD.[Citation23] More traditional inflammatory markers such as C-reactive protein (CRP) are recognized and utilized as a general high sensitivity systemic marker of inflammatory processes [Citation24] and are of prognostic use in areas such as predicting coronary heart disease.[Citation25] However, CRP lacks specificity for T2DM but in combination with other inflammatory and OS markers may improve risk prediction for T2DM and CVD as utilizing current global risk assessment strategies and scores does still remain sub-optimal.[Citation26] The inflammatory markers interleukins-1β, 6, 10 (IL-1β, IL-6, IL-10), monocyte chemo-attractant protein-1 (MCP-1), and insulin like growth factor-1 (IGF-1) were explored in this study. Chronic low-grade inflammatory responses with activation of the innate immune system have been associated with diabetes, metabolic syndrome (MS), and atherosclerosis.[Citation27–29] Association between inflammation, OS, and BGL has not been comprehensively investigated [Citation30–32] and utilizing these markers to classify participants attending a diabetes health screening clinic into those with possible preclinical CVD is by no means definitive. This study investigated the association of inflammatory and OS markers with normal BGL of <4.5 mmol/l (1st quintile) and those with increased BGL >6.1 mmol/l (4th, 5th quintile).
Methods
Three hundred and nine participants were recruited from the Diabetic Health Screening Clinic (DiabHealth) at Charles Sturt University. The study was approved by the Charles Sturt University Human Ethics Committee (Protocol Number 2006-042) and complies with the standards of the Helsinki agreement for human research. All patients were informed of the aims of the research and any risk prior to consent. As this was a screening clinic, patients were not discriminated or excluded based upon their medical and medication history. The control group (n = 47) were extracted from attendees at the diabetic screening clinic who were non-diabetic, normoglycaemic, normotensive with no evidence of CVD and were non-medicated. Anthropometric data were obtained () in addition to the collection of blood and urine specimens. Specimens collected were analysed for blood glucose and lipids. Biomarkers for OS and inflammation were performed on all patient specimens. Body mass index (BMI) was measured using standardized beam weight scales. BMI is defined as weight in kilograms per height expressed as metres squared and is independent of gender and age. Waist circumference (WC) was measured using a standard measuring tape. Measurements were taken between the top of the hip bone and lowest rib. Blood pressure was measured using a sphygmomanometer with appropriate cuff size after a 5-minute rest. The average of two measurements 1 minute apart was used for systolic and diastolic blood pressure values.
Table 1. Anthropometric and general biomarkers.
Specimen collection and processing
Patients fasted overnight prior to data and blood collection. Whole blood specimens were collected into plain, heparin and EDTA anticoagulated, 7 ml tubes. Serum and plasma was separated after a 10-minute centrifugation at 1000 g. Glucose, total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) cholesterol, HbA1c, and CRP were performed on the day of collection at the local pathology laboratory, in accordance with Australian Laboratory Standards. Plasma/serum/washed RBC lysate samples required for rbcGSH, GSSG, 8OHdG, and interleukins analysis were stored at -80°C prior to batch analysis.
General biochemistry
At the screening, clinic patients had a screening blood glucose performed and bloods collected prior to assessment. Plasma glucose, TC, and TG were determined by standard enzymatic kits and HDL was determined using an immunoinhibition assay. Low-density lipoprotein cholesterol (LDL-C) was calculated according to the Friedewald formula. HbA1c and high sensitivity CRP were analysed by immunoassay. These investigations were carried out at Dorovitch laboratory, a national accredited private pathology laboratory.
OS markers
GSH and GSSG levels were measured from erythrocyte lysate by a glutathione assay kit (Cayman Chemical, MI, USA). As the method incorporates GSH reductase, total GSH is measured. GSH reacts with Ellman’s reagent with the production of 5-thio-2-nitrobenzoic acid (TNB). Absorbance was measured at 405 nm with the absorbance of TNB directly proportional to the GSH concentration in the sample. The GSH:GSSG ratio was determined using the formula (total GSH-2GSSG)/GSSG). Results were calculated using a four parameter logistic fit.
Plasma from 8OHdG was assayed with an enzyme immunosorbent assay kit (Cayman Chemical, MI, USA). The test procedure utilizes an anti-mouse IgG-coated plate and a tracer consisting of an 8OHDG-enzyme conjugate which detects all three oxidised guanine species; 8OHDG from DNA, 8-hydroxyguanosine from RNA, and 8-hydroxyguanine from either DNA or RNA. This kit analysis has the advantage of providing low variability and increased sensitivity compared with assays that utilize an antigen-coated plate which only detect 8OHdG.
Inflammatory markers
IL-1β, IL-6, IL-10, MCP-1, and IGF-1 levels were determined using Enzyme Linked Immunosorbent Assay (ELISA) kits (Elisakit.com, Adelaide Australia). The methods incorporated pre-coated IL, MCP, and IGF capture antibody incubated with their appropriate anti-human (i.e. IL-1β, IL-6, IL-10, MCP-1, IGF-1) biotin-labelled detection antibody. Plates were developed with Streptavidin–horse radish peroxidase conjugate. Colour development occurred utilizing 3,3′,5,5′-tetramethylbenzidine TMB chromogen and was stopped with kit supplied acid solution. The absorbance of the resultant yellow colour was determined at 450 nm. Results were calculated by generating a standard curve using a four parameter logistic fit. All ELISA assays were measured by a Thermo Scientific Multiskan FC™ and data reduction utilized the SkanIt 3.1 software.
Statistical analysis
Data analysis with descriptive data expressed as mean ± standard deviation (x ± SD), for demographic and anthropometric data and median ± IQR (interquartile range) for non-parametric data. A t-test was conducted on descriptive data. The statistical analysis for the 1st and 4th, 5th quintile was the rank-based non-parametric Mann–Whitney test for a two-group comparison. Data analysis was performed with PAWS (Version 22, IBM Co). Data were grouped by fasting blood glucose (BGL) <4.5 mmol/l (1st quintile) vs BGL >6.1 mmol/l (4th, 5th quintile) and compared, with results of P < 0.05 considered significant.
Results
The control group, who had no reported hypertension, CVD or diabetes as well as being medication free, is tabulated shown as a comparison to the clinic groups with results for the control group and 1st, 2nd, 3rd, and 4th, 5th quintiles depicted in . Gender distribution was generally slightly higher for females than males. The study group were divided into quintiles. Statistical analysis of the 1st and 4th, 5th quintiles was based upon a fasting blood glucose (BGL) of Q1 (<4.5 mmol/l) and Q4,5 (>6.1 mmol/l). WC and BMI were significant between groups (P < 0.001) as was the general biomarkers BGL, HbA1c, TG, HDL, TC/HDL ratio, and AIP (P < 0.001).
The comparison of OS and inflammatory markers () compared the 1st and 4th, 5th quintiles. Significant results were observed for GSH (P < 0.001), GSH:GSSG and 8OHDG, and the inflammatory marker IL-1β (P < 0.05). In keeping with our previous publications, GSH/GSSG results were not expressed with reference to Hb concentration. At examination patients, based upon haematocrit observations, were noted not to be anaemic or polycythaemic.
Table 2. Oxidative stress and inflammatory markers.
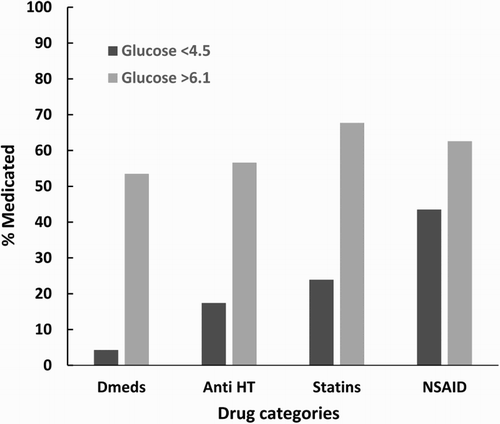
expresses medicated patients with each medication classification portrayed as a percentage of the total for the respective glucose level.
Figure 1. Medication usage chart comparison. Dmeds, diabetic medication; Anti-HT, anti-hypertensives; NSAID, non steroidal anti-inflammatory drugs.
Inflammatory markers were expressed as ratios () and the 1st and 4th, 5th quintiles were compared. IL-6/IL-10, IL-1β/IL-10, MCP-1/IL-10, IGF-1/IL-10, and IL-6/IL-1β were significant (P < 0.05)
Table 3. Inflammatory marker ratio test.
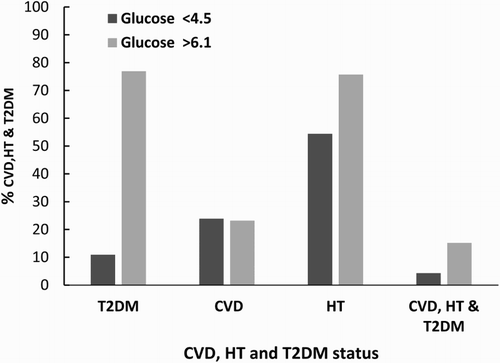
– Patients selected for this study attended the Charles Sturt University rural health diabetic screening programme and were not discriminated on the basis of clinical or medication status. The results express the number of CVD, HT, and T2DM patients as a percentage of the total for each respective group. The CVD, HT, and T2DM group is the combined sum of patients with all three categories present.
Discussion
This study highlights the potential value emerging inflammatory and OS biomarkers may contribute to the screening of patients that exhibit a hyperglycaemic state irrespective of existing treatment regimens targeting T2DM, CVD, and HT. Our results have replicated findings of well-controlled experiments comparing OS and inflammatory markers in diabetes progression, and demonstrated a substantive association with hyperglycaemia, OS, and the inflammatory in a screening population.[Citation10,Citation33–36] The impact of OS and the relationship with the inflammatory response was further supported upon a review of the clinical status indicating a higher prevalence of self-reported CVD and hypertension as shown in most likely due to T2DM, CVD, and HT being associated with inflammatory responses and OS.[Citation18,Citation33,Citation37,Citation38]
Hyperglycaemia is associated with increased risk of T2DM associated with OS and an inflammatory process.[Citation19] Significant differences were observed in WC, BMI, BGL, HbA1c, TG, HDL, and TC/HDL between the normoglycaemic and elevated glycaemia groups (). These findings support their association with T2DM and affirm that traditional markers do assist to some extent in demonstrating that a continuum exists between chronic increasing hyperglycaemia and the progression to and development of T2DM. HbA1c values of 6–6.5% may represent a high risk for the development of diabetes (if used for diagnostic purposes); however, there is still a continuum of risk associated with normal levels of HbA1c below 6%.[Citation39] If a lower cut-off of HbA1c is used, then the likelihood of missing DM is also increased.
The constellation of OS and inflammation factors emerging from and participating with the pro-inflammatory hyperglycaemic state is not fully understood, but of major concern is the progression of patients with elevated glucose to T2DM and the associated morbidity.[Citation27,Citation30,Citation40] The results of this study indicate that both OS and inflammation provided significant results when groups were categorized with a BGL of <4.5 mmol/l and > 6.1 mmol/l (), irrespective of any medication. The OS markers GSH, GSH:GSSG, and 8OHdG showed significant results. GSH has previously been shown to demonstrate an association with IFG and OS and the link to diabetes.[Citation7,Citation41,Citation42] It has also been demonstrated that GSH:GSSG showed a significant difference between a control group and IFG, supporting the notion that early thiol-related OS occurs.[Citation14] If this is the case, and as demonstrated in this study, the significant GSH, GSH:GSSG results when glucose levels were >6.1 mmol/l support the concept of monitoring OS during sustained hyperglycaemia. A consequence of this is an increased risk of micro- and macrovascular complications [Citation43] due to the increased ROS contributing to the structural and functional damage of endothelial cells and smooth muscle proliferation, a factor in the development of atherogenesis.[Citation44,Citation45] 8OHdG is also considered an important biomarker for OS in diabetes progression and indicator of the pre-diabetic state.[Citation7] There is still some controversy over whether OS is triggered by sudden onset hyperglycaemia or at least pronounced glucose variability [Citation46]; however, there is merit in the continuous monitoring of BGL along with OS biomarkers and antioxidant status for diabetes progression and complications screening. This would further aid in establishing whether a patient is at risk of developing T2DM or its complications based upon the additional information obtained for OS and inflammation.
It has also been demonstrated that there are positive and negative OS associations, which have been linked to lipid levels.[Citation47–49] In this study, lipid studies did show a significant difference between glucose levels <4.5 mmol/l and levels >6.1 mmol/l. As we did not discriminate between disease and the medication state, the OS markers provide an important additional diagnostic tool, along with inflammatory markers in their utility in screening and interpreting possible diabetes risk in mild-to-moderate hyperglycaemic patients.
Elevated levels of the pro-inflammatory IL-1β have been associated with hyperglycaemia, insulin resistance, obesity, all factors which contribute to the development of T2DM.[Citation50] The current study confirmed that a significant increase in IL-1β is associated with hyperglycaemia and supports previous findings of elevated IL-1β in T2DM, possibly as a result of chronic inflammation and insulin resistance.[Citation51] Studies have shown that the neutralization of IL-1β assists in decreasing the inflammatory response in autoimmune, malignancy, and other common disease states such as T2DM.[Citation52,Citation53] If so, knowledge of IL-1β levels may provide an indication of earlier treatment intervention to allay the development of progression to T2DM and CVD in hyperglycaemia. Elevated IL-6, another pro-inflammatory marker, has also been demonstrated in hyperglycaemia/hyperinsulinaemia [Citation54,Citation55] and in a previous study of ours.[Citation33] The anti-inflammatory marker IL-10 in this study was not significantly different between the two glucose groups. IL-10 is considered a potent anti-inflammatory; however, mixed results have been observed.[Citation56] Esposito has pointed out that a low innate production capacity of IL-10 may help identify subjects at more risk for inflammation in MetS.[Citation27,Citation57] Drug therapy to either control T2DM or prevent the development of comorbidities is required if diet and lifestyle improvements are unable to modify and improve the risk factors resulting from hyperglycaemia. While the anti-DM, anti-HT. and statin use is much higher in the elevated glucose group (>6.1 mmol/l), the use of NSAIDS was also heavily utilized in the low glucose (<4.5 mmol/l) group (). This may exert an effect on the anti-inflammatory effects of IL-10; however, it has been shown that statins not only reduce cholesterol and TG but also can independently decrease CRP levels.[Citation58]
Recognizing in this study that IL-1β was the only individual inflammatory marker demonstrating a significant difference, a ratio test of the emerging biomarkers, with CRP included, was investigated for any degree of significance between the Q1 and Q4,5 groups (). Previous studies into prognostic inflammatory outcomes have revealed an association of IL6/IL10 in systemic inflammatory responses and post-operative surgical outcomes to infections in chronic alcoholics.[Citation59,Citation60] Studies on inflammatory markers have noted that participants with a combined elevation of both IL-6 and IL-1β had about a three-fold increase in risk of developing diabetes, whereas low levels of IL-1β alone demonstrated no substantial increase in risk.[Citation32] However to the best of our knowledge, inflammatory marker ratios and any association with hyperglycaemia have not been fully investigated, albeit the recognition that there are numerous possible interactions between inflammatory markers and the presence of T2DM.[Citation61,Citation62] A greater understanding of the interactions via ratio testing may therefore provide valuable information in T2DM and CVD progression. Significant results were obtained with IL-6/IL-10, IL-1β/IL-10, MCP-1/IL-10, IGF-1/IL-10, and IL-6/IL-1β (). Studies reviewing IL-6 and tumour necrosis factor–α (TNF-α) have established roles in the regulation of APRs with other investigations providing a possible link with these two biomarkers and cardiovascular events.[Citation63] Additionally, an association with TNF-α receptor 2, IL-6, and CRP has been demonstrated, with elevated levels of CRP noted as a strong independent predictor of T2DM. CRP as a possible mediator between TNF-α receptor 2 and IL-6 IL-6/IL-10 has been shown to decrease significantly in post-operative alcoholic patients with infections.[Citation60] It has been previously established that the anti-inflammatory IL-10 is induced by pro-inflammatory cytokines such as IL-6, thereby affording some protection in states of inflammation.[Citation64] There is also growing evidence linking lower levels of IL-10, independently of increased levels of IL-1β, with obesity, MetS, and CVD.[Citation65] Our results show that IL-1β/IL-10 was significantly different between the low BGL versus high BGL group, whereas IL-10 did not increase significantly compared to the control group suggesting that IL-10 is not responding to small increases in BGL. This could also reflect possible interactions between IL-6, MCP-1, or IGF-1, whether acting dependently or independently of IL-10, with the ratio providing a more sensitive indicator in the development and pathophysiology of T2DM and CVD. The pro-inflammatory IL-6/IL-1β ratio was significantly higher where the individual analysis of the inflammatory markers other than IL-1β was not; possibly indicating the requirement for caution in the use of individual biomarkers as a screening mechanism when investigating T2DM and CVD. IL-1β has been shown to be increased in T2DM [Citation35]; however, its interaction with functional β-cell mass leading to overt T2DM may not be reversible by glucose lowering therapy,[Citation66] thus further highlighting that inflammatory cytokine and OS ratios may provide additional information on progressive hyperglycaemia in a screening cohort and their potential use as clinical indicators of T2DM and CVD disease progression and targets for therapy.
Conclusion
Our current results support previous findings that inflammation and OS are associated with increased BGLs and suggest that GSH, GSH:GSSG, 8OHdG, and IL-1β may play a role in aiding clinicians in identifying hyperglycaemic patients with inflammatory and OS pathology and are at risk of developing T2DM and CVD. Our novel findings observed with the inflammatory marker ratios have not been previously reported. Using anthropomorphic, traditional markers, and emerging markers and/or ratios in hyperglycaemic management may provide valuable information leading to more preventative measures at a clinical level. As these novel ratio findings are largely untested in the hyperglycaemic state, further investigations are required to interrogate our findings. Future research will expand this analysis on inflammatory and OS markers and whether they can assist in charecterizing diabetes and its progression from a pre-diabetic (IFG) level.
Disclaimer statements
Contributors All authors contributed equally.
Conflicts of interest The authors declare that there is no conflict of interest regarding the publication of this article.
Ethics approval The study was approved by the Charles Sturt University Human Ethics Committee (Protocol Number 2006-042) and complies with the standards of the Helsinki agreement for human research.
Acknowledgements
Roche Australia provided the glucose measuring sticks and glucometers. Bev de Jong provided technical assistance for Simon McDonald from the CSU Spatial Data Analysis Network assisted in the statistical analysis.
ORCiD
Eugene G. Butkowski http://orcid.org/0000-0002-4308-0843
References
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a
- Collier B, Dossett LA, May AK, Diaz JJ. Glucose control and the inflammatory response. Nutr Clin Pract. 2008;23(1):3–15. doi: 10.1177/011542650802300103
- Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93(12):1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D
- Haffner SM. Pre-diabetes, insulin resistance, inflammation and CVD risk. Diabetes Res Clin Pr. 2003;61(Suppl 1):S9–S18. doi: 10.1016/S0168-8227(03)00122-0
- Gholap NN, Davies MJ, Mostafa SA, Khunti K. Diagnosing type 2 diabetes and identifying high-risk individuals using the new glycated haemoglobin (HbA1c) criteria. Br J Gen Pract. 2013;63(607):165–167. doi: 10.3399/bjgp13X663244
- Federation ID. Global guidelines for type 2 diabetes. Brussels: International Diabetes Federation; 2012.
- Al-Aubaidy HA, Jelinek HF. 8-Hydroxy-2-deoxy-guanosine identifies oxidative DNA damage in a rural prediabetes cohort. Redox Rep. 2010;15(4):155–160. doi: 10.1179/174329210X12650506623681
- Tatsch E, Bochi GV, Piva SJ, et al. Association between DNA strand breakage and oxidative, inflammatory and endothelial biomarkers in type 2 diabetes. Mutat Res. 2012;732(1–2):16–20. doi: 10.1016/j.mrfmmm.2012.01.004
- Bandeira SDM, da Fonseca LJS, Guedes GdS, Rabelo LA, Goulart MOF, Vasconcelos SML. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci. 2013;14(2):3265–3284. doi: 10.3390/ijms14023265
- Al-Aubaidy HA, Jelinek HF. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur J Endocrinol. 2011;164(6):899–904. doi: 10.1530/EJE-11-0053
- Whiting PH, Kalansooriya A, Holbrook I, Haddad F, Jennings PE. The relationship between chronic glycaemic control and oxidative stress in type 2 diabetes mellitus. Br J Biomed Sci. 2008;65(2):71–74. doi: 10.1080/09674845.2008.11732800
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390(3):191–214. doi: 10.1515/BC.2009.033
- Wu G, Fang Y-Z, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–492.
- Jelinek HF, Al-Aubaidy HA, Maschirow L, Meidinger S, Jamil DA, Butkowski E. Glutathione: Glutathione sulfide redox imbalance in early impaired fasting glucose. Cardiol Angiol. 2014;2(4):223–229. doi: 10.9734/CA/2014/11389
- Bannai S, Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membr Biol. 1986;89(1):1–8. doi: 10.1007/BF01870891
- Toroser D, Sohal RS. Age-associated perturbations in glutathione synthesis in mouse liver. Biochem J. 2007;405(Pt 3):583–589. doi: 10.1042/BJ20061868
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C. 2009;27(2):120–139. doi: 10.1080/10590500902885684
- Maschirow L, Khalaf K, Al-Aubaidy HA, Jelinek HF. Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes – Biomarkers as a possible tool for early disease detection for rural screening. Clin Biochem. 2015;48(9):581–585. doi: 10.1016/j.clinbiochem.2015.02.015
- de Carvalho VF, Guedes CP, Goncalves PL, de Cassia Goncalves AR. The role of hyperglycemia in the induction of oxidative stress and inflammatory process. Nutricion hospitalaria. 2012;27(5):1391–1398.
- Lin Y, Berg AH, Iyengar P, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem. 2005;280(6):4617–4626. doi: 10.1074/jbc.M411863200
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17(5):350–364. doi: 10.1016/S0889-1591(03)00048-5
- Dallmeier D, Larson MG, Vasan RS, et al. Metabolic syndrome and inflammatory biomarkers: a community-based cross-sectional study at the Framingham Heart Study. Diabetol Met Syn. 2012;4:28. doi: 10.1186/1758-5996-4-28
- Schöttker B, Herder C, Rothenbacher D, et al. Proinflammatory cytokines, adiponectin, and increased risk of primary cardiovascular events in diabetic patients with or without renal dysfunction: results from the ESTHER study. Diabetes Care. 2013;36(6):1703–1711. doi: 10.2337/dc12-1416
- Salazar J, Martinez MS, Chavez M, et al. C-reactive protein: clinical and epidemiological perspectives. Cardiol Res Prac. 2014;2014:10.
- Koenig W, Sund M, Fröhlich M, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring trends and determinants in cardiovascular disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99(2):237–242. doi: 10.1161/01.CIR.99.2.237
- Herder C, Karakas M, Koenig W. Biomarkers for the prediction of type 2 diabetes and cardiovascular disease. Clin Pharmacol Ther. 2011;90(1):52–66. doi: 10.1038/clpt.2011.93
- Esposito K, Giugliano D. The metabolic syndrome and inflammation: association or causation? Nutr Metab Cardiovasc Dis. 2004;14(5):228–232. doi: 10.1016/S0939-4753(04)80048-6
- Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.S114
- Plutzky J. Inflammatory pathways in atherosclerosis and acute coronary syndromes. Am J Cardiol. 2001;88(8A):10–15. doi: 10.1016/S0002-9149(01)01924-5
- de Rekeneire N, Peila R, Ding J, et al. Diabetes, hyperglycemia, and inflammation in older individuals: the health, aging and body composition study. Diabetes Care. 2006;29(8):1902–1908. doi: 10.2337/dc05-2327
- Schmidt FM, Weschenfelder J, Sander C, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PloS one. 2015;10(3):e0121971. doi: 10.1371/journal.pone.0121971
- Spranger J, Kroke A, Möhlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetes. 2003;52(3):812–817. doi: 10.2337/diabetes.52.3.812
- Butkowski EG, Brix LM, Al-Aubaidy HA, Kiat H, Jelinek HJ. Diabetes, oxidative stress and cardiovascular risk. J Med Clin Sci. 2016;5(1):17–23.
- Calabrese V, Cornelius C, Leso V, et al. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim Biophys Acta. 2012;1822(5):729–736. doi: 10.1016/j.bbadis.2011.12.003
- Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrin Diab Obes. 2010;17(4):314–321.
- van Exel E, Gussekloo J, de Craen AJM, Frölich M, Bootsma-van der Wiel A, Westendorp RGJ. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: the Leiden 85-plus study. Diabetes. 2002;51(4):1088–1092. doi: 10.2337/diabetes.51.4.1088
- Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed Res Int. 2014;2014:11. doi: 10.1155/2014/406960
- Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30(1):49–59. doi: 10.1111/j.1755-5922.2010.00218.x
- Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33(3):562–568. doi: 10.2337/dc09-1524
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscl Throm Vasc. 2004;24(5):816–823. doi: 10.1161/01.ATV.0000122852.22604.78
- Lowe GD. Can haematological tests predict cardiovascular risk? The 2005 Kettle Lecture. Br J Haematol. 2006;133(3):232–250. doi: 10.1111/j.1365-2141.2006.06021.x
- Nwose EU, Richards RS, Kerr PG, Tinley R, Jelinek HF. Oxidative damage indices for the assessment of subclinical diabetic macrovascular complications. Br J Biomed Sci. 2008;65(3):136–141. doi: 10.1080/09674845.2008.11732817
- Sekhar RV, McKay SV, Patel SG, et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34(1):162–167. doi: 10.2337/dc10-1006
- Al-Aubaidy HA, Jelinek HF. Oxidative DNA damage: antioxidant response in postprandial hyperglycaemia in type 2 diabetes mellitus. Br J Diab Vasc Dis. 2011;11(2):87–91. doi: 10.1177/1474651411405259
- Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339(1–2):1–9. doi: 10.1016/j.cccn.2003.09.010
- Choi S-W, Benzie IFF, Ma S-W, Strain JJ, Hannigan BM. Acute hyperglycemia and oxidative stress: direct cause and effect? Free Radical Biol Med. 2008;44(7):1217–1231. doi: 10.1016/j.freeradbiomed.2007.12.005
- Kikuchi H, Nanri A, Hori A, et al. Lower serum levels of total cholesterol are associated with higher urinary levels of 8-hydroxydeoxyguanosine. Nutr Metab. 2013;10(1):1–5. doi: 10.1186/1743-7075-10-59
- Miyamoto M, Kotani K, Ishibashi S, Taniguchi N. The relationship between urinary 8-hydroxydeoxyguanosine and metabolic risk factors in asymptomatic subjects. Med Princ Pract. 2011;20(2):187–190. doi: 10.1159/000319774
- Subash P, Gurumurthy P, Sarasabharathi A, Cherian KM. Urinary 8-OHdG: a marker of oxidative stress to DNA and total antioxidant status in essential hypertension with South Indian population. Ind J Clin Biochem. 2010;25(2):127–132. doi: 10.1007/s12291-010-0024-z
- Koenen TB, Stienstra R, van Tits LJ, et al. Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1β transcription in human adipose tissue. Diabetes. 2011;60(2):517–524. doi: 10.2337/db10-0266
- Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079–2086. doi: 10.2337/diabetes.53.8.2079
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–3732. doi: 10.1182/blood-2010-07-273417
- Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. Treatment with an interleukin 1 beta antibody improves glycemic control in diet induced obesity. Cytokine. 2008;44(1):141–148. doi: 10.1016/j.cyto.2008.07.004
- Fishel MA, Watson G, Montine TJ, et al. Hyperinsulinemia provokes synchronous increases in central inflammation and β-amyloid in normal adults. Arch Neurol. 2005;62(10):1539–1544. doi: 10.1001/archneur.62.10.noc50112
- Morohoshi M, Fujisawa K, Uchimuraa I, Numano F. Glucose-dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes. 1996;45(7):954–959. doi: 10.2337/diab.45.7.954
- Straczkowski M, Kowalska I, Nikolajuk A, Krukowska A, Gorska M. Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care. 2005;28(8):2036–2037. doi: 10.2337/diacare.28.8.2036
- Esposito K, Pontillo A, Giugliano F, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endo Metab. 2003;88(3):1055–1058. doi: 10.1210/jc.2002-021437
- Averna M, Lo Verde A. Statins and metabolic syndrome. ICS. 2003;1253:243–246.
- Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27(7):1262–1264. doi: 10.1097/00003246-199907000-00005
- Sander M, Irwin M, Sinha P, Naumann E, Kox W, Spies C. Suppression of interleukin-6 to interleukin-10 ratio in chronic alcoholics: association with postoperative infections. Inten Care Med. 2002;28(3):285–292. doi: 10.1007/s00134-001-1199-9
- Donath Marc Y, Dalmas É, Sauter Nadine S, Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 2013;17(6):860–872. doi: 10.1016/j.cmet.2013.05.001
- Xia W, Wei BAO, Jun LIU, et al. Inflammatory markers and risk of type 2 diabetes. Diabetes Care. 2013;36(1):166–175. doi: 10.2337/dc12-0702
- Biswas S, Ghoshal PK, Mandal SC, Mandal N. Relation of anti- to pro-inflammatory cytokine ratios with acute myocardial infarction. Korean J Intern Med. 2010;25(1):44–50. doi: 10.3904/kjim.2010.25.1.44
- Weis F, Beiras-Fernandez A, Schelling G, et al. Stress doses of hydrocortisone in high-risk patients undergoing cardiac surgery: effects on interleukin-6 to interleukin-10 ratio and early outcome. Crit Care Med. 2009;37(5):1685–1690. doi: 10.1097/CCM.0b013e31819fca77
- Chang JS, Chang CC, Chien E, et al. Association between interleukin 1beta and interleukin 10 concentrations: a cross-sectional study in young adolescents in Taiwan. BMC Pediatr. 2013;13:313. doi: 10.1186/1471-2431-13-123
- Zhao G, Dharmadhikari G, Maedler K, Meyer-Hermann M. Possible role of interleukin-1beta in type 2 diabetes onset and implications for anti-inflammatory therapy strategies. PLoS Comput Biol. 2014;10(8):e1003798. doi: 10.1371/journal.pcbi.1003798