ABSTRACT
Objectives: The aim of this study was to determine whether non-lethal sepsis induced by cecal ligation and puncture (CLP) modulates oxidative damage and enzymatic antioxidant defenses in diaphragm and hindlimb skeletal muscles (soleus and Extensor Digitorus Longus (EDL)).
Methods: Female Wistar rats were divided into four experimental groups: (1) control animals, (2) animals sacrificed 2 hours or (3) 7 days after CLP, and (4) sham-operated animals. At the end of the experimental procedure, EDL, soleus, and diaphragm muscles were harvested and 4-hydroxynonenal (HNE)-protein adducts and protein carbonyl contents were examined in relation to superoxide dismutase and catalase expression and activities.
Results: We observed that both non-respiratory oxidative (i.e. soleus) and glycolytic skeletal muscles (i.e. EDL) are more susceptible to sepsis-induced oxidative stress than diaphragm, as attested by an increase in 4-HNE protein adducts and carbonylated proteins after 2 hours of CLP only in soleus and EDL.
Discussion: These differences could be explained by higher basal enzymatic antioxidant activities in diaphragm compared to hindlimb skeletal muscles. Together, these results demonstrate that diaphragm is better protected from oxidative stress than hindlimb skeletal muscles during CLP-induced sepsis.
Introduction
Sepsis and related clinical syndromes, such as the multiple organ dysfunction syndrome, are the most frequent causes of morbidity and mortality in intensive care units in the United States and Europe.[Citation1] This syndrome is characterized by systemic inflammation that could be due to a variety of clinical insults including infection, ischemia, pancreatitis, tissue injury or multiple trauma. Skeletal muscle comprises 50–60% of body cell mass and represents one of the organs the most affected by systemic inflammation.[Citation2] Thus, septic patients exhibit weakness and fatigability in both respiratory and limb skeletal muscles.[Citation3–5] Interestingly, meta-analyses and clinical studies showed that deficits in limb muscle strength and mass in critically ill patients may exceed those observed in the diaphragm.[Citation6,Citation7] Moreover, differences between limb muscles are also reported in rodent experimental models of sepsis with a pronounced decrease in muscle strength and release in myofilaments in glycolytic compared to oxidative skeletal muscles.[Citation5,Citation8,Citation9] Altogether, these data highlight that skeletal muscles are differently impacted by systemic inflammation during sepsis depending on function, contractile activity, and metabolic profile. However, the underlying molecular mechanisms remain poorly understood.
Excess production of reactive oxygen species (ROS) occurs in multiple organ failure and septic shock,[Citation10] and oxidative damage has been reported in both respiratory and limb muscles of patients with sepsis and in septic rats.[Citation11–13] In skeletal muscle fibers, ROS are normally synthesized at low levels and are vital for normal force production.[Citation14,Citation15] However, when levels of ROS are excessively produced under inflammatory conditions, muscle force generation may be considerably impaired, leading to further muscle dysfunction and fatigue.[Citation9,Citation14,Citation15] Imbalance between ROS production and its degradation by cellular antioxidants is indeed recognized to stimulate the ubiquitin-proteasome system [Citation16,Citation17] and to impair cytosolic Ca2+ release and myofibrillar Ca2+ sensitivity.[Citation18] During sepsis, neutrophils, macrophages, and endothelial cells release high levels of tumor necrosis factor-α that probably play a major role in sepsis-induced muscle oxidative stress. Indeed, tumor necrosis factor-α acts via the TNF receptor subtype 1, rapidly increasing cytosolic oxidant production in myocytes, whereas antioxidant pretreatment blunts the rise in oxidant production and maintains specific force, arguing for causality.[Citation19,Citation20]
Some studies have explored the effects of sepsis on oxidative stress in respiratory muscles both in humans and rodents and reported contradictory results probably due to differences in experimental protocols (e.g. sample timing or intensity of sepsis).[Citation13,Citation21–24] Even though it is recognized that sepsis also induces weakness in limb skeletal muscle, few studies have explored the relationship between oxidative stress and limb muscle weakness.[Citation13,Citation22] Interestingly, skeletal fiber-type differences in susceptibility to oxidative stress are observed during aging or chronic obstructive pulmonary disease.[Citation25] Such differences could also be observed during sepsis and could potentially contribute to explaining why weakness differently affects skeletal muscles depending on their function (respiratory and limb muscles) and their metabolic profile (glycolytic or oxidative).
The present study was therefore designed to determine whether non-lethal sepsis induced by cecal ligation and puncture (CLP) in rats modulates oxidative damage and enzymatic antioxidant defenses in both respiratory (diaphragm) and hindlimb skeletal muscles (soleus and Extensor Digitorus Longus (EDL)). We hypothesize that (1) diaphragm muscles are better protected from oxidative stress than hindlimb muscles; (2) glycolytic skeletal muscle (EDL) is more susceptible to sepsis-induced oxidative stress than oxidative skeletal muscle (soleus).
Materials and methods
In vivo studies were performed in accordance with the recommendations of European Community directive 2010/63/EU and were authorized by agreement n° 02076.01, with the approval of the local ethics committee.
CLP model
Two-month-old female Wistar rats (≈340 g) were obtained from Janvier Labs (Le Genest Saint Isle, France) and housed in the Animal Care Facility of the Faculty of Medicine in Brest, accredited for live animal experimentation (N° B29–019–04) by the Department of Veterinary Services, French Ministry of Agriculture. Rats were maintained on a 12:12-hour dark–light cycle. Water was available ad libitum, and standard laboratory diet was provided, changed, and monitored daily. To explore short-term and long-term effects of sepsis, the animals were divided into three experimental groups: control animals, animals sacrificed 2 hours after CLP, and animals sacrificed 7 days after CLP. The CLP procedure was realized as previously described.[Citation26] Briefly, under aseptic conditions, an incision in the midabdominal wall was performed to allow exposure of the cecum. The cecum was ligated at its base, below the ileocecal valve, and was perforated 10 times with a 21-gauge needle allowing the passage of some fecal material into the abdominal cavity when gentle pressure was applied. In these conditions, most of the animals could survive for > 2 weeks (mortality rate: 33% by day 10).[Citation5] A sham group was added in which animals underwent a similar abdominal incision and cecum manipulation without ligation or puncture. Then, the abdominal cavity was sutured the same way in the sham and septic groups. At the end of the experimental procedure, animals were anesthetized with intraperitoneally injected sodium pentobarbital (120 mg/kg). EDL, soleus, and diaphragm muscles were removed and immediately frozen in liquid nitrogen.
Antioxidant enzyme activities
Muscles were homogenized in cold buffer containing 20 mM Tris–HCl, 250 mM sucrose, 40 mM KCl, and 2 mM EDTA, pH 7.2 (1:10 w/v), and centrifuged at 700g for 10 minutes at 4°C. Supernatants were collected and used for measurement of catalase and CuZn-superoxide dismutase (SOD) activities or centrifuged again at 15,000g for manganese-dependent SOD (MnSOD) assays. Total protein was quantified by Bradford’s method using BSA as standard.
The principle of the SOD activity assay is based on the inhibition of nitroblue tetrazolium (NBT) reduction. Illumination of riboflavin in the presence of O2 and electron-donor-like methionine generates superoxide anions and was used as the basis of a SOD assay. The reduction of NBT by superoxide radicals to blue-colored formazan was measured at 580 nm. One unit of SOD activity is defined as the amount of enzyme required to inhibit the reduction of NBT by 50% under the specified conditions. The reaction mixture contained 13 mM methionine, 2.64 mM NBT, and 0.26 mM riboflavin with hemolysate in a total volume of 200 µl. The solution was illuminated for 20 minutes with a lamp of 15 W. A control without an enzyme source was also included. The absorbance was measured at 580 nm. The values were expressed in percentage of inhibition and results were expressed as U/mg protein. Inhibition of CuZnSOD by addition of 1 mM sodium cyanide to the reaction buffer allowed measurement of mitochondrial MnSOD activity.
Catalase activity was measured by the hydrogen peroxide (H2O2) degradation assay. Briefly, 100 μl tissue homogenate was added to a quartz cuvette containing 0.8 ml of 0.05 M sodium phosphate buffer (pH 7.0) and 100 μl of 100 mM H2O2 was added to start the reaction. Catalase activity was determined by measuring the decrease in absorbance (H2O2 degradation) at 240 nm for 5 minutes and expressed as U/mg protein. One unit (U) of catalase activity was defined as 1 μmol of H2O2 consumed/minute.
Western immunoblot analyses
Cytosolic protein extraction was performed from skeletal muscles in cold lysis buffer containing 10 mM Tris–HCl (pH 7.4), 0.5 M sucrose, 50 mM NaCl, 5 mM EDTA, 30 mM Na4P2O7, 1% NP-40, 0.25% sodium deoxycholate, 50 mM NaF, 100 µM sodium orthovanadate, and protease inhibitor cocktail (Sigma P8340, 5 µl/ml). The samples were homogenized using a Polytron homogenizer at 4°C. Each sample was then incubated on ice for 30 minutes followed by 3 × 10 s of sonication. The homogenates were transferred to microcentrifuge tubes and centrifuged at 12,000g for 12 minutes at 4°C. The protein concentration of the supernatant was determined by a Lowry assay using bovine serum albumin as standard. Samples were then diluted in SDS–PAGE sample buffer [50 mM Tris–HCl (pH 6.8), 2% SDS, 10% glycerol, 5% β-mercaptoethanol, and 0.1% bromophenol blue] and heated 5 minutes at 95°C until analyses. Samples containing 50 µg of proteins were resolved on 10 or 15% SDS–PAGE. The proteins were transferred at 240 mA for 90 minutes onto a 0.2-µm nitrocellulose membrane. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline/0.05% Tween-20 for 1 hour at room temperature. Primary antibodies were incubated overnight at 4°C with appropriated primary antibodies: anti-CuZnSOD (1:1000, Enzo Life Sciences), anti-MnSOD (1:5000, Stressgen), anti-catalase (1:700, Sigma Aldrich), and α-actin (1:2000, Sigma Aldrich). Thereafter, membranes were washed with Tris-buffered saline/0.05% Tween-20 and incubated for 1 hour at room temperature with infrared dye-conjugated secondary antibodies (LI-COR, Lincoln, NE, U.S.A). After washing, membrane images were digitized using the Odyssey Imaging System (LI-COR, Lincoln, NE, U.S.A). All blots were scanned, and densitometric analysis of the bands was conducted using a GS-800 Imaging densitometer and QuantityOne software.
Protein carbonylation
Analysis of protein carbonyl levels was performed using an OxyBlot kit (Millipore). Muscle tissues were lysed in 0.05 M Kpi–0.1 mM EDTA buffer. Protein concentration was defined by the Lowry protein assay. Protein extracts (10 µg) were derivatized with 1% DNPH. After 15-minute incubation, the reaction was stopped by the addition of the supplied neutralization solution and β-mercaptoethanol. Molecular weight protein standards containing attached DNP residues and muscle protein extracts were separated on SDS–PAGE and transferred onto nitrocellulose membranes (BioRad). Membranes were blocked and then incubated overnight at 4°C with anti-DNP primary antibody (1:100) supplied with the OxyBlot kit. After washes, secondary IgG anti-rabbit goat IRDye 680 antibody (Sciencetec) was added for 1 hour in the dark at room temperature. The membranes were then washed and the blots scanned using an Odyssey Infrared Imaging System (LI-COR, Biosciences). The membranes were finally stripped and reprobed with antibodies to α-actin to verify equal protein loading, because α-actin is not altered in skeletal muscle in response to sepsis.
4-HNE analysis
Detection of 4-hydroxy-2-nonenal (4-HNE) adducts, a marker for lipid peroxidation was performed by Western blotting. For each sample, 30 μg of proteins was loaded into a well for analysis and separated on SDS–PAGE. After transferring protein onto nitrocellulose membranes (BioRad), the membranes were incubated in a solution of 250 mM sodium borohydride in 100 mM MOPS, pH 8.0, for 15 minutes. Sodium borohydride chemically reduced the aldehyde moiety on HNE to an alcohol, since the primary antibody would recognize only 4-hydroxy-2-nonenal-bound lipoic acid. Membranes were blocked for 30 minutes in 5% milk in 1× PBS, then incubated overnight at 4°C with primary antibody 4-HNE, which was a gift from Pr. Luke Szweda (OMRF, Oklahoma City, OK, U.S.A). Thereafter, the membranes were washed with PBST and incubated for 1 hour at room temperature with infrared dye-conjugated secondary antibodies (LI-COR, Lincoln, NE, U.S.A). After washing, blot images were digitized using the Odyssey Imaging System (LI-COR, Lincoln, NE, U.S.A). All blots were scanned, and densitometric analysis of the bands was conducted using a GS-800 Imaging densitometer and QuantityOne software. Membranes were stripped and reprobed with antibodies to α-actin to verify equal protein loading.
Statistical analyses
Normality and equality of variance were checked before the statistical analysis was conducted. If the conditions were met, a one-way ANOVA was used to compare variables across groups of animals. Significant main effects or interactions were further analyzed by the Fischer LSD post hoc test. If data were not normally distributed, they were first analyzed using an ANOVA on ranks, and then further examined with the Dunn’s test. For all statistical analyses, the level of significance was set at P < 0.05.
Results
Effects of sepsis on skeletal muscle oxidative damage
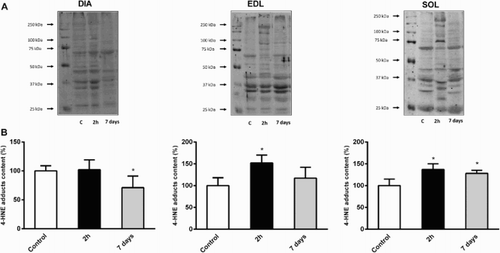
To determine how CLP can modulate oxidative damage in respiratory and hindlimb skeletal muscles, we quantified 4-HNE and carbonylated proteins in cytosolic extracts. 4-HNE is the most intensively studied lipid peroxidation end product, whereas the appearance of carbonyl groups (such as aldehyde or ketone groups) in proteins is the result of several oxidative modification reactions. We detected a significant increase of carbonylated protein content in soleus and EDL 2 hours after CLP (+23 and +29% vs. control animals, respectively, P < 0.05), whereas no modification was observed in diaphragm muscle (). shows that 4-HNE protein adducts exhibit the same pattern of expression in soleus (+37%, P < 0.05), EDL (+52%, P < 0.01) and diaphragm (+2%, NS). Carbonylated proteins reached control values in soleus and EDL 7 days after sepsis induction, but remained unchanged in diaphragm muscle (). Conversely, 4-HNE protein adducts remained significantly higher 7 days after CLP in soleus (+28% vs. control animals, P < 0.05, ). Oxidative damage markers returned to baseline 7 days after sepsis induction in EDL, and even decreased in diaphragm (−29% vs. control animals, P < 0.05, ).
Figure 1. Evolution of protein carbonylation in DIA, EDL, and SOL muscles 2 hours and 7 days after CLP. (a) Representative immunoblots of protein carbonyl adducts in skeletal muscles. (b) Means ± SD of protein carbonyl adducts. n = 5–6 per group. Values are expressed as fold change relative to control group, P < 0.05 as compared to control group.
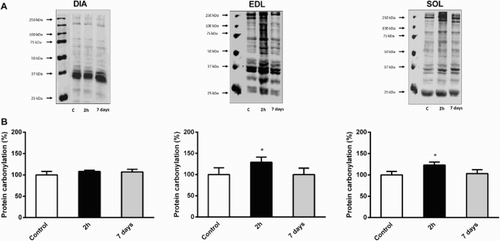
Figure 2. Evolution of 4-hydroxynonenal (4-HNE) in DIA, EDL, and SOL muscles 2 hours and 7 days after CLP. (a) Representative immunoblots of 4-HNE in skeletal muscles. (b) Means ± SD of 4-HNE adducts. n = 5–6 per group. Values expressed as fold change relative to control group, P < 0.05 as compared to control group.
Effects of sepsis on CAT activation in skeletal muscle
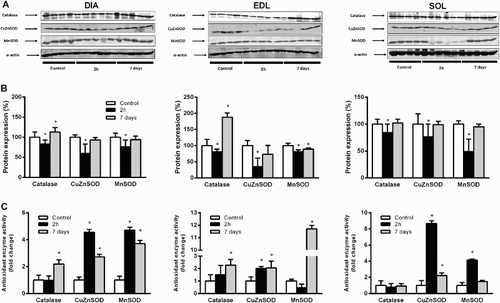
shows the effects of sepsis on antioxidant protein content and enzymatic activities in skeletal muscles. We reported significant decreases of CAT protein content in soleus (−16%, P < 0.05), EDL (−17%, P < 0.05) and diaphragm (−20%, P < 0.05) after 2 hours of CLP ((a,b)). No simultaneous modification in CAT enzymatic activities was reported in these skeletal muscles ((c)). CAT protein content became significantly higher than control animals 7 days after CLP in diaphragm (+13%, P < 0.05) and EDL (+88%, P < 0.01). Such increases were associated with increases in enzymatic activities (+219% vs. control animals for diaphragm and +228% for EDL, P < 0.01). However, these parameters remained unchanged 7 days after sepsis induction in soleus muscle. No significant difference was observed between the sham group and control group.
Figure 3. Evolution of antioxidant enzyme protein content and activities in DIA, EDL, and SOL muscles 2 hours and 7 days after CLP. (a) Representative immunoblots of antioxidant enzymes in skeletal muscles. Means ± SD of catalase, CuZnSOD, and MnSOD protein content (b) and enzymatic activities (c). n = 5–6 per group. Values expressed as fold change relative to control group, P < 0.05 as compared to control group.
Effects of sepsis on CuZnSOD activation in skeletal muscle
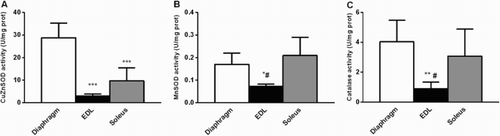
As shown in , the CuZnSOD activity level was 3- and 10-fold higher in the diaphragm than in the soleus and EDL, respectively (P < 0.001). CuZnSOD protein content was significantly lower 2 hours after CLP in soleus (−24%, P < 0.05), EDL (−65%, P < 0.01) and diaphragm (−40%, P < 0.05). Surprisingly, the study of enzymatic activities did not follow the same pattern. Indeed, such decreases were associated with significant increases in enzymatic activities in soleus, EDL, and diaphragm muscle ((c)). After 7 days of sepsis induction, CuZnSOD enzymatic activities decreased compared with animals killed after 2 hours of sepsis, but remained significantly higher than control animals in soleus, EDL, and diaphragm ((c)). However, CuZnSOD protein content reached normal values in hindlimb and respiratory skeletal muscles 7 days after sepsis induction. No significant difference was observed between the sham group and control group.
Effects of sepsis on MnSOD activation in skeletal muscle
MnSOD protein content decreased 2 hours after CLP in soleus (−51%, P < 0.01), EDL (−19%, P < 0.05) and diaphragm (−19%, P < 0.05). On the contrary, we observed that MnSOD enzymatic activities significantly increased at the same time in soleus and diaphragm, whereas such activity remained unchanged in EDL ((c)). MnSOD activity remained significantly higher in diaphragm 7 days after sepsis induction, whereas it reached control values in soleus ((c)). On the contrary, MnSOD activity increased twofold in EDL compared to values of control animals after 7 days of sepsis induction (P < 0.001). Protein content reached control values after 7 days in diaphragm and soleus, whereas it remained lower in EDL. No significant difference was observed between the sham group and control group.
Discussion
Among potential triggers underlying muscle weakness during sepsis, ROS might contribute to atrophy and contractile dysfunction in both respiratory and non-respiratory skeletal muscle. Our data show that CLP, a widely used experimental model of sepsis in rats,[Citation27] induces early transient oxidative damage in hindlimb skeletal muscle, whereas the antioxidant enzymatic system appears sufficiently potent to preserve the diaphragm from oxidative damage. These results highlight that the diaphragm is better protected from oxidative stress than hindlimb skeletal muscle during CLP-induced sepsis.
Excessive generation of ROS greatly contributes to the development of sepsis-induced multiple organ dysfunctions.[Citation28–33] Among these, increasing evidence supports that such ROS overproduction plays a pivotal role in the induction of sepsis-induced skeletal muscle atrophy and contractile dysfunctions.[Citation9,Citation34,Citation35] During sepsis, several sources of ROS production have been identified, including NADPH oxidase, xanthine oxidase, NOS, and impairment in the mitochondrial electron transport chain.[Citation36–39] As a defensive strategy, muscle fibers are capable of stimulating expression and activities of antioxidant enzymes to remove harmful ROS. Here, we analyzed SOD activity, one of the major enzymatic systems responsible for protecting cells against ROS-induced damage. Our results revealed an increase of SOD activity 2 hours after sepsis induction in both respiratory and non-respiratory skeletal muscles. Surprisingly, our results showed that these increases of enzymatic activities were associated with a paradoxical decrease in protein levels. We suppose that post-translational modifications could explain whether reduced protein levels are associated with enhanced activity. Interestingly, recent studies observed a strong relationship between redox signaling and steady-state levels of protein phosphorylation, thereby affecting the activities and expression of antioxidant enzymes.[Citation40–42] Furthermore, the presence and activities of antioxidant enzymes in cells are strongly regulated by transcriptional, translational, and post-translational mechanisms as a consequence of changes in the cellular redox potential.[Citation43] Thus, we hypothesized that oxidative stress in sepsis could be correlated, at least in part, with the protein expression and phosphorylation of antioxidant enzymes. In this context, it would be interesting to examine the expression and activity levels of MnSOD and CuZnSOD in combination with their phosphorylation states.
An increase in SOD activity results in an overproduction of H2O2 which can induce membrane damage through lipid peroxidation or react with iron via Fenton chemistry to generate hydroxyl radicals. Excessive cellular H2O2 is normally removed by CAT and glutathione peroxidase (GPx), leading to detoxification of superoxide radicals to water and oxygen. Here, increases in total SOD activity in both respiratory and non-respiratory skeletal muscles are not associated with a concomitant increase in CAT activity after 2 hours of sepsis induction. This imbalance between catalase and SOD activities is followed by oxidative damage in both glycolytic and oxidative hindlimb muscles (i.e. EDL and soleus), as attested by elevations in carbonylated proteins and 4-HNE adduct levels. These results are in accordance with oxidative damage previously observed in human vastus lateralis 24 hours after sepsis induction.[Citation13] The excessive H2O2 production related to this imbalance between SOD and CAT activities probably contributes to sepsis-induced skeletal muscle atrophy through the activation of calpains/caspase 3 and ubiquitin-proteasome systems.[Citation44,Citation45] Seven days after sepsis induction, we observed that oxidative damage returned to baseline values in EDL, whereas such damage appeared to be persistent in soleus, as indicated by high levels of 4-HNE adducts. Such discrepancies between these skeletal muscles are associated with a different regulation in antioxidant enzyme activities, since catalase, CuZnSOD, and MnSOD activities remained higher in EDL, whereas they dropped to baseline in soleus.
It is well known that respiratory muscle failure is one of the main organ dysfunctions occurring during severe sepsis, contributing to the elevated mortality seen in this syndrome. Rodent endotoxemia and septic shock models are also recognized to induce diaphragmatic contractile dysfunction.[Citation12,Citation46–48] In contrast to hindlimb skeletal muscles, we did not report increases in protein carbonylation and lipid peroxidation in the diaphragm of septic rats after 2 hours and 7 days of sepsis induction. These results are in agreement with previous studies showing that oxidative damage did not increase in respiratory skeletal muscles in human with severe sepsis, or in the rat CLP model.[Citation13,Citation22,Citation39] The lack of oxidative damage in diaphragm compared to hindlimb skeletal muscle could be first explained by a better antioxidant defense system. Indeed, baseline CuZnSOD activity is reported in our study to be 3- and 10-fold higher in diaphragm compared to soleus and EDL, respectively. Curiously, the lack of oxidative damage is not associated with a concomitant upregulation of catalase activity in our study, suggesting that other antioxidant defense systems may react to protect the diaphragm cellular environment from H2O2. GPx could play a key role since it is established that diaphragm exhibits higher GPx activity than soleus or gastrocnemius muscles.[Citation49,Citation50] Also, we cannot exclude that non-enzymatic antioxidant defenses are higher in diaphragm. Indeed, it has been previously reported that reduced glutathione concentrations are higher in rat diaphragm compared to soleus.[Citation51] Second, rat diaphragm is an oxidative skeletal muscle that exhibits higher oxidative enzyme activities (e.g. citrate synthase, malate dehydrogenase, and SDH) compared with soleus, and most importantly EDL.[Citation52,Citation53] Interestingly, it has been demonstrated that oxidative, but not glycolytic, myofibers increase NO production and iNOS expression in response to cachectic stimulus (i.e. LPS), thus promoting enhanced antioxidant gene expression.[Citation54] Moreover, previous studies have highlighted that glycolytic muscle fibers possess cellular properties that potentiate mitochondrial ROS production compared with oxidative myofibers.[Citation55,Citation56] All these findings ultimately suggest that (1) higher antioxidant defense and (2) lower ROS production in response to sepsis provide a better protection of diaphragm against sepsis-related oxidative damage.
In summary, this study demonstrated that CLP in rats rapidly increases enzymatic antioxidant defense in both diaphragm and hindlimb skeletal muscles. However, these responses seem more efficient in diaphragm, thus protecting it against sepsis-related oxidative damage. These findings provide new insights for understanding clinical data observing that diaphragm is better protected from muscle weakness than limb muscles during sepsis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002
- Lara TM, Wong MS, Rounds J, Robinson MK, Wilmore DW, Jacobs DO. Skeletal muscle phosphocreatine depletion depresses myocellular energy status during sepsis. Arch Surg. 1998;133(12):1316–1321. doi: 10.1001/archsurg.133.12.1316
- Bolton CF. Sepsis and the systemic inflammatory response syndrome: neuromuscular manifestations. Crit Care Med. 1996;24(8):1408–1416. doi: 10.1097/00003246-199608000-00022
- Larsson L, Li X, Edstrom L, Zackrisson H, Argentini C, Schiaffino S. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: mechanisms at the cellular and molecular levels. Crit Care Med. 2000;28(1):34–45. doi: 10.1097/00003246-200001000-00006
- Rossignol B, Gueret G, Pennec JP, et al. Effects of chronic sepsis on contractile properties of fast twitch muscle in an experimental model of critical illness neuromyopathy in the rat. Crit Care Med. 2008;36(6):1855–1863. doi: 10.1097/CCM.0b013e318176106b
- Baldwin CE, Bersten AD. Alterations in respiratory and limb muscle strength and size in patients with sepsis who are mechanically ventilated. Phys Ther. 2014;94(1):68–82. doi: 10.2522/ptj.20130048
- Prentice CE, Paratz JD, Bersten AD. Differences in the degree of respiratory and peripheral muscle impairment are evident on clinical, electrophysiological and biopsy testing in critically ill adults: a qualitative systematic review. Crit Care Resusc. 2010;12(2):111–120.
- Alamdari N, Toraldo G, Aversa Z, et al. Loss of muscle strength during sepsis is in part regulated by glucocorticoids and is associated with reduced muscle fiber stiffness. Am J Physiol Regul Integr Comp Physiol. 2012;303(10):R1090–R1099. doi: 10.1152/ajpregu.00636.2011
- Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37(10 Suppl):S354–S367. doi: 10.1097/CCM.0b013e3181b6e439
- Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–223. doi: 10.1016/S0140-6736(02)09459-X
- Hammarqvist F, Luo JL, Cotgreave IA, Andersson K, Wernerman J. Skeletal muscle glutathione is depleted in critically ill patients. Crit Care Med. 1997;25(1):78–84. doi: 10.1097/00003246-199701000-00016
- Barreiro E, Comtois AS, Mohammed S, Lands LC, Hussain SN. Role of heme oxygenases in sepsis-induced diaphragmatic contractile dysfunction and oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L476–L484. doi: 10.1152/ajplung.00495.2001
- Pascual-Guardia S, Arbol F, Sanchez E, et al. Inflammation and oxidative stress in respiratory and limb muscles of patients with severe sepsis. Med Clin (Barc). 2013;141(5):194–200. doi: 10.1016/j.medcli.2012.05.026
- Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol. (1985) 2007;102(4):1664–1670. doi: 10.1152/japplphysiol.01102.2006
- Reid MB. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc. 2001;33(3):371–376. doi: 10.1097/00005768-200103000-00006
- Derbre F, Ferrando B, Gomez-Cabrera MC, et al. Inhibition of xanthine oxidase by allopurinol prevents skeletal muscle atrophy: role of p38 MAPKinase and E3 ubiquitin ligases. PLoS One. 2012;7(10):e46668. doi: 10.1371/journal.pone.0046668
- Servais S, Letexier D, Favier R, Duchamp C, Desplanches D. Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis?. Free Radic Biol Med. 2007;42(5):627–635. doi: 10.1016/j.freeradbiomed.2006.12.001
- Westerblad H, Allen DG. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxid Redox Signal. 2011;15(9):2487–2499. doi: 10.1089/ars.2011.3909
- Hardin BJ, Campbell KS, Smith JD, et al. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol. (1985) 2008;104(3):694–699. doi: 10.1152/japplphysiol.00898.2007
- Li X, Moody MR, Engel D, et al. Cardiac-specific overexpression f tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation. 2000;102(14):1690–1696. doi: 10.1161/01.CIR.102.14.1690
- Maes K, Stamiris A, Thomas D, et al. Effects of controlled mechanical ventilation on sepsis-induced diaphragm dysfunction in rats. Crit Care Med. 2014;42(12):e772–e782. doi: 10.1097/CCM.0000000000000685
- Peruchi BB, Petronilho F, Rojas HA, et al. Skeletal muscle electron transport chain dysfunction after sepsis in rats. J Surg Res. 2011;167(2):e333–e338. doi: 10.1016/j.jss.2010.11.893
- Fredriksson K, Hammarqvist F, Strigard K, et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291(5):E1044–E1050. doi: 10.1152/ajpendo.00218.2006
- Chacon-Cabrera A, Rojas Y, Martinez-Caro L, et al. Influence of mechanical ventilation and sepsis on redox balance in diaphragm, myocardium, limb muscles, and lungs. Transl Res. 2014;164(6):477–495. doi: 10.1016/j.trsl.2014.07.003
- Remels AH, Gosker HR, Bakker J, Guttridge DC, Schols AMWJ, Langen RCJ. Regulation of skeletal muscle oxidative phenotype by classical NF-kappaB signalling. Biochim Biophys Acta. 2013;1832(8):1313–1325. doi: 10.1016/j.bbadis.2013.03.018
- Rossignol B, Gueret G, Pennec JP, et al. Effects of chronic sepsis on the voltage-gated sodium channel in isolated rat muscle fibers. Crit Care Med. 2007;35(2):351–357. doi: 10.1097/01.CCM.0000254335.88023.0E
- Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119(10):2868–2878. doi: 10.1172/JCI39421
- Salvemini D, Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic Biol Med. 2002;33(9):1173–1185. doi: 10.1016/S0891-5849(02)00961-9
- Winterbourn CC, Buss IH, Chan TP, Plank LD, Clark MA, Windsor JA. Protein carbonyl measurements show evidence of early oxidative stress in critically ill patients. Crit Care Med. 2000;28(1):143–149. doi: 10.1097/00003246-200001000-00024
- Macarthur H, Westfall TC, Riley DP, Misko TP, Salvemini D. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc Natl Acad Sci USA. 2000;97(17):9753–9758. doi: 10.1073/pnas.97.17.9753
- Andrades ME, Ritter C, Dal-Pizzol F. The role of free radicals in sepsis development. Front Biosci. (Elite Ed). 2009;1(1):277–287.
- Barichello T, Fortunato JJ, Vitali AM, et al. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med. 2006;34(3):886–889. doi: 10.1097/01.CCM.0000201880.50116.12
- Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JCF, Dal-Pizzol F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med. 2004;32(2):342–349. doi: 10.1097/01.CCM.0000109454.13145.CA
- Brealey D, Karyampudi S, Jacques TS, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R491–R497. doi: 10.1152/ajpregu.00432.2003
- Crouser ED, Julian MW, Blaho DV, Pfeiffer DR. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit Care Med. 2002;30(2):276–284. doi: 10.1097/00003246-200202000-00002
- Javeshghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165(3):412–418. doi: 10.1164/ajrccm.165.3.2103028
- Luchtemberg MN, Petronilho F, Constantino L, et al. Xanthine oxidase activity in patients with sepsis. Clin Biochem. 2008;41(14-15):1186–1190. doi: 10.1016/j.clinbiochem.2008.07.015
- Zhou G, Kamenos G, Pendem S, Wilson JX, Wu F. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2012;302(4):R409–R416. doi: 10.1152/ajpregu.00153.2011
- Ritter C, Andrades M, Frota Junior ML, et al. Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med. 2003;29(10):1782–1789. doi: 10.1007/s00134-003-1789-9
- Groen A, Lemeer S, van der Wijk T, et al. Differential oxidation of protein-tyrosine phosphatases. J Biol Chem. 2005;280(11):10298–10304. doi: 10.1074/jbc.M412424200
- Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003;14(8 Suppl 3):S211–S2115. doi: 10.1097/01.ASN.0000077404.45564.7E
- Rhee SG, Kang SW, Jeong W, Chang T-S, Yang K-S, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17(2):183–189. doi: 10.1016/j.ceb.2005.02.004
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599–622. doi: 10.1210/er.2001-0039
- Pierre N, Appriou Z, Gratas-Delamarche A, Derbre F. From physical inactivity to immobilization: dissecting the role of oxidative stress in skeletal muscle insulin resistance and atrophy. Free Radic Biol Med. 2015;S0891-5849(15):01181–01188.
- Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol. 2003;285(4):C806–C812. doi: 10.1152/ajpcell.00129.2003
- Barreiro E, Gea J, Di Falco M, Kriazhev L, James S, Hussain SNA. Protein carbonyl formation in the diaphragm. Am J Respir Cell Mol Biol. 2005;32(1):9–17. doi: 10.1165/rcmb.2004-0021OC
- Barreiro E, Sanchez D, Galdiz JB, Hussain SN, Gea J. N-acetylcysteine increases manganese superoxide dismutase activity in septic rat diaphragms. Eur Respir J. 2005;26(6):1032–1039. doi: 10.1183/09031936.05.00003705
- Hussain SN, Matar G, Barreiro E, Florian M, Divangahi M, Vassilakopoulos T. Modifications of proteins by 4-hydroxy-2-nonenal in the ventilatory muscles of rats. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L996–L1003. doi: 10.1152/ajplung.00337.2005
- Caillaud C, Py G, Eydoux N, Legros P, Prefaut C, Mercier J. Antioxidants and mitochondrial respiration in lung, diaphragm, and locomotor muscles: effect of exercise. Free Radic Biol Med. 1999;26(9–10):1292–1299. doi: 10.1016/S0891-5849(98)00342-6
- Hollander J, Bejma J, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mech Ageing Dev. 2000;116(1):33–45. doi: 10.1016/S0047-6374(00)00130-5
- Supinski G, Nethery D, Stofan D, Hirschfield W, DiMarco A. Diaphragmatic lipid peroxidation in chronically loaded rats. J Appl Physiol. (1985). 1999;86(2):651–658.
- Kelly AM, Rosser BW, Hoffman R, et al. Metabolic and contractile protein expression in developing rat diaphragm muscle. J Neurosci. 1991;11(5):1231–1242.
- Lyons CN, Mathieu-Costello O, Moyes CD. Regulation of skeletal muscle mitochondrial content during aging. J Gerontol A Biol Sci Med Sci. 2006;61(1):3–13. doi: 10.1093/gerona/61.1.3
- Yu Z, Li P, Zhang M, Hannink M, Stamler JS, Yan Z. Fiber type-specific nitric oxide protects oxidative myofibers against cachectic stimuli. PLoS One. 2008;3(5):e2086. doi: 10.1371/journal.pone.0002086
- Capel F, Buffiere C, Patureau Mirand P, Mosoni L. Differential variation of mitochondrial H2O2 release during aging in oxidative and glycolytic muscles in rats. Mech Ageing Dev. 2004;125(5):367–373. doi: 10.1016/j.mad.2004.02.005
- Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol Cell Physiol. 2006;290(3):C844–C851. doi: 10.1152/ajpcell.00402.2005