ABSTRACT
Objectives: The aim of the study was to evaluate the activity of xanthine oxidase (XO) in the blood of men and women during the first hour following a single anaerobic exercise (AN-EX), and after 24 hours of recovery, and to determine whether the changes in XO activity in the blood after AN-EX are dependent on anaerobic performance.
Methods: Ten men and ten women performed a single AN-EX. Blood was collected before and five times after completion of the AN-EX. The activity of XO was determined.
Results: In both groups, a significant (P < 0.05) increase in blood XO activity was found only 24 hours after the AN-EX. The increased activity of XO in men was significantly lower than in women (P < 0.05). Negative correlations were found between the increase in XO activity in the blood plasma 24 hours after the AN-EX and anaerobic power, the total work performed during the AN-EX and the power decrease.
Discussion: In the first hour after the single AN-EX, XO activity in the blood of women and men did not change, but after 24 hours of recovery, it was significantly higher compared to baseline levels in both sexes. Single AN-EX causes a smaller increase in XO activity in people with higher anaerobic performance.
Introduction
Increased production of reactive oxygen species (ROS) during physical exercise, with insufficient antioxidant defense, intensifies the reaction of oxidation of lipids, proteins and nucleic acids, and can induce systemic oxidative stress [Citation1,Citation2]. Oxidative stress may be the result of aerobic and anaerobic efforts [Citation2,Citation3]. At the same time, it is demonstrated that anaerobic exercise, which increases the production of ROS, stimulates the expression of proteins essential in mitochondria biogenesis [Citation4], and used in the high-intensity interval training model, it causes up-regulation of systemic antioxidant activity [Citation5]. The findings suggest that similar loads of aerobic exercise and anaerobic exercise induce ROS differently: aerobic exercise seems to initially generate more ROS, whereas anaerobic exercise may induce prolonged ROS generation [Citation6].
There are many sources of ROS formation during and after exercise (including the respiratory chain in the inner mitochondrial membrane, NADPH oxidases, xanthine oxidase – XO) whose participation is dependent on the interaction of aerobic and anaerobic metabolism [Citation1]. According to studies conducted among humans and animal models, the main source of ROS in high-intensity exercises is catalyzed reactions through Nox2 and Nox4 isoforms of NAD(P)H oxidase, located respectively in the sarcolemma and sarcoplasmic reticulum of myocytes, as well as the NAD(P)H oxidase of microvascular endothelial cells, muscle cells and phagocytes [Citation1,Citation7–11]. In these reactions, the following develop: superoxide anion () and hydrogen peroxide (H2O2), belonging to ROS [Citation1].
Increased levels of ROS concentration after anaerobic exercise are also the consequence of hypoxia of shrinking muscle cells, with subsequent re-oxidation, and it occurs similarly as in the mechanism of ischemia/reperfusion by activation of XO [Citation12]. The consequence of power generation by the skeletal muscle during anaerobic exercise is metabolic acidosis and an increase in the rate of purine metabolism leading to an increase in the concentration of hypoxanthine in muscle cells and the blood [Citation13,Citation14]. The reaction of XO catalyzed by conversion of hypoxanthine to xanthine and then to uric acid (UA) is coupled with the formation of ROS [Citation13]. Under physiological conditions (O2 concentration 21%, pH 7.4), this reaction generates H2O2 and in a ratio of about 3:1 [Citation15]. The relative amount of H2O2 formed by the reaction of XO is further increased when the O2 concentration is decreased [Citation15].
Changes in UA concentration in the blood have been used in previous studies as an indicator of oxidative stress occurring after anaerobic exercise [Citation3,Citation6,Citation16–19]. The results of the previous studies are not conclusive because UA is a product of purine metabolism and, at the same time, non-enzymatic antioxidant that affects the total antioxidant capacity of the blood [Citation20]. In humans, UA is converted to allantoin by non-enzymatic reaction with [Citation13]. UA concentration in the blood is a result of the reaction in which
(XO reaction) emerges and the reaction in which
is consumed. The UA concentration in the blood does not reflect changes in H2O2 concentration as the primary oxidant formed in the XO reaction. Thus, UA changes cannot be a clear indication of change in the activity of XO after anaerobic exercise.
The conclusive determination of post-exercise changes in XO activity of the blood is necessary for the proper usage of anaerobic exercise in modeling physical training. In the research by Vina et al. [Citation21], it was shown in animals and humans that inhibition of XO activity prevents exercise-induced oxidation of reduced glutathione (GSH). However, Wiecek et al. [Citation3] found that there was an increase in the concentration of oxidized glutathione (GSSG) and a decrease of the GSH/GSSG ratio in both sexes an hour after anaerobic exercise. These changes were also found 24 hours after anaerobic exercise [Citation3], which can indirectly specify the increased activation of XO in the blood at that time. Therefore, the aim of our study was the direct assessment of XO activity in the blood of women and men during the first hour after completion of a single anaerobic exercise and after 24 hours of recovery.
It was also found that higher maximal anaerobic power is correlated with low levels of resting UA in the blood [Citation16], which may indicate lower activity of XO in persons with better anaerobic performance. Accordingly, the objective of our research was to determine whether the gender differences in anaerobic performance [Citation22] influence changes in XO activity in the blood after anaerobic exercise.
Since XO activity increases with age [Citation23] and in various disease states in adults and in children [Citation24–27], in order to eliminate these factors, our study involved young healthy subjects at a similar age. According to our knowledge, our study is the first on this subject.
We put forward two hypotheses:
In young healthy subjects, anaerobic exercise induces time delayed activation of XO in the blood.
The increase in XO activity is smaller in individuals with greater anaerobic performance.
Material and methods
Participants
Ten men and 10 women aged 19–24 years participated in the study. Somatic characteristics of the group are presented in . The participants were healthy, physically active, non-trained in competitive sports, and had not performed any laboratory exercise tests before.
Table 1. Characteristics of study participants (mean ± SE).
The relative maximal oxygen uptake (VO2max·BM−1) and maximal heart rate (HR) were, respectively, for the men: 55.61 ± 1.78 mL kg−1 min−1, 201 ± 3 b min−1, and for the women: 44.75 ± 1.14 mL kg−1 min−1, 196 ± 3 b min−1.
All participants were non-smokers, not taking any dietary supplements or any medication permanently, with excluded chronic diseases (such as diabetes, hypertension, asthma), and no contraindications to perform physical exercise of maximum intensity or anaerobic efforts. The results of the medical qualifications are presented in . An additional condition for qualification into the group of women was a proper menstrual cycle course, confirmed during an interview with a physician. The woman had not taken any hormonal treatment for at least 3 months before the exercise test.
Table 2. Results of study participants’ medical qualifications (mean ± SE).
Study design
The research methods were approved by the Bioethics Commission at the Regional Medical Chamber. All test procedures were conducted in accordance with the principles adopted in the Declaration of Helsinki. The participants, after reviewing the study protocol, gave written informed consent to participate in the study. Participation in the study was voluntary. Each of the participants went to the laboratory three times.
During the first visit at the laboratory, anthropometric measurements were taken and the participants were familiarized with the apparatus and course of the test. Each participant performed the 4-minute treadmill effort at a speed of 7 km h−1. In order to avoid the practice effect [Citation28], all the participants performed a 10-second cycling sprint with the same load as in the exercise test.
After two days, the participants performed a graded test on a treadmill (h/p/Cosmos Saturn COS 10198, Germany) in order to measure VO2max.
During the third visit to the lab, participants performed the anaerobic exercise (AN-EX), which was a 20-second maximal cycling sprint test (ergometer 824E Monark, Vansbro, Sweden). The ergometer was connected to a computer and equipped with an electromagnetic timer that measured the duration of each revolution (with 0.001 second accuracy).
The exercise tests were carried out under constant medical supervision. Women performed the graded test and AN-EX between the 6th and 9th day of their follicular phase in consecutive menstrual cycles. Men performed the AN-EX two weeks after the graded test. All the exercise tests were carried out in the morning in thermo-neutral conditions. Seven days before the AN-EX, the participants did not perform any intense physical efforts, and followed a unified, balanced diet established by a dietician taking into account the age and physical activity guidelines (2700 kcal/day: carbohydrates 55%, protein 15%, fats 30%) [Citation29], and did not consume alcohol, caffeine or other stimulants.
Procedures
Somatic measurements
Body height was measured with a Martin type anthropometer (U.S.A.), with a 0.01 cm accuracy. Body mass (BM) and body composition: fat mass, percentage of body fat (%F) and lean body mass (LBM), were determined using the Jawon IOI-353 Body Composition Analyzer (Korea). Body mass index (BMI) was calculated for each participant ().
Graded test
After a 4-minute warm-up performed at a speed of 7.0 km h−1 by men and 6.0 km h−1 by women, the running speed was increased every 2 minutes by 1.2 km h−1 for men and 1.0 km h−1 for women until volitional exhaustion. The inclination angle of the treadmill during the entire graded test was 0° [Citation30].
The criteria applied for VO2max determination were as follows:
a plateau in oxygen uptake (VO2),
a respiratory-exchange-ratio of >1.1,
attainment of a HR within 10 beats per minute of the age-predicted maximum.
However, in situations where no plateau was observed but the rest of the criteria were met, VO2peak was taken as the VO2max [Citation31]. The VO2max·BM−1 was calculated. Measurements of VO2 and respiratory-exchange-ratio were performed using an ergospirometer (Medikro 919, Finland) and HR was measured using a pulsometer (S-610i, Polar Elektro, Kempele, Finland).
Anaerobic exercise
The participants performed a 4-minute warm-up with a load of 90 and 60 W, respectively, for men and women. Pedaling cadence was 60 rpm. At the end of the 2nd and 4th minute of the warm-up, subjects performed 5-second maximal pedaling cadence acceleration. After 4 minutes of resting, the 20-second maximal cycling sprint test was performed (AN-EX). During the AN-EX, participants had to obtain the maximal pedaling cadence as fast as possible and maintain it for as long as it possible. The braking force amounted to 0.075·BM for men and 0.065·BM for women. The participants began the test when hearing the ‘3-2-1-GO!’ command. During the whole test, the subjects remained in a seated position (seat height was selected individually) and were vigorously motivated. After completion of the AN-EX, the participants maintained the cadence of 60 rpm without braking force for 3 minutes.
Peak power (PP), mean power (MP), total work (TW) and power decrease, time to attain PP and time of maintaining PP were automatically calculated using appropriate software (Staniak JBA MCE, Warsaw, Poland). Results were expressed as absolute values and relative to BM and LBM.
The AN-EX lasting 20 seconds offers a reliable and valid test of anaerobic power and could replace the classic 30-second Wingate test [Citation32]. At the same time, shortening the test time increases the share of the anaerobic resynthesis path of ATP [Citation13].
Biochemical assays
Five minutes before beginning the warm-up and 3 minutes, 15 minutes, 30 minutes, 60 minutes and 24 hours after the AN-EX completion, the activity of XO, creatine kinase (CK), lactate dehydrogenase (LDH) and concentration of acid–base balance indicators (the concentration of hydrogen ions (H+), the concentration of bicarbonate ions (HCO3−), the excess/deficiency of base buffers (BE), anion gap – AG = (Na+ + K+)–(Cl− + HCO3−), lactate concentration (Lac), hemoglobin (Hb) concentrations and hematocrit (Hct) were assessed. Venous blood was collected into tubes (Vacutainer BD, Franklin Lakes, New Jersey, U.S.A.) containing:
K2EDTA for the determination of Hb and Hct (whole blood) and XO activity in the blood plasma,
a clotting activator for the assay of CK and LDH activity.
Cannula was introduced into the venous vessels in the inside area of the elbow, which were flushed with saline solution (1 mL 0.9% NaCl) immediately after insertion, and before and after each blood collection to prevent clotting. The first milliliter of the obtained blood was discarded. After blood was collected for biochemical analysis, the cannula was closed with a catheter.
The blood intended for plasma obtainment was stored on ice and centrifuged as soon as possible. The blood intended for serum obtainment was centrifuged after 20 minutes of clotting at room temperature. Venous blood was centrifuged for 15 minutes at 4°C, RCF 1000 × g (MPW 351R, Poland). The plasma and serum samples were stored until analysis at −70°C (ULF 390 Arctiko, Denmark).
XO activity was determined using the Xanthine Oxidase Assay Kit 10010895 (Cayman Chemical Company, Ann Arbor, Michigan, U.S.A.). This assay is based on a multistep enzymatic reaction in which XO first produces H2O2 during oxidation of hypoxanthine. In the presence of horseradish peroxidase, the H2O2 reacts with ADHP (10-acetyl-3,7-dihydroxyphenoxazine) in a 1:1 stoichiometry to produce the highly fluorescent resorufin compound. Resorufin fluorescence was analyzed with an excitation wave-length of 520–550 nm and an emission wave-length of 585–595 nm (Infinite M200 PRO, TECAN, Grödig, Austria). Detection range was up to 100 mU L−1. Intra-assay coefficient of variation was 1.9%, coefficient of variation inter-assay was 3.9%.
CK and LDH activity were determined using the following tests, respectively: CK BioMaxima (Lublin, Poland) and LDH BioMaxima (Lublin, Poland) performing spectrophotometric kinetic measurements. Linearity of measurements was up to 1000 U L−1 for CK and up to 2400 U L−1 for LDH. In the CK assays, the reaction of the phosphate group transfer from creatine phosphate to ADP with simultaneous ATP formation was conducted and catalyzed by CK contained in the sample. Subsequently, with the usage of ATP and in the presence of hexokinase, the phosphorylation reaction of glucose to glucose-6-phosphate took place. Then, catalyzed by dehydrogenase glucose-6-phosphate, the reaction of the glucose-6-phosphate oxidation with concomitant reduction of NADP+ to NADPH occurred. LDH was determined at 37°C (TPS-1500W Sealed Peltier Thermostat, Thermo Scientific, Milwaukee, Wisconsin, U.S.A.), using the reduction reaction of pyruvate by NADH to lactate and NAD+ with LDH in the sample. The rate of absorbance change over time, measured at a wave-length of 340 nm (UV/Vis Evolution 201 Thermo Scientific Spectrofotometer, Milwaukee, Wisconsin, U.S.A.), was proportional to the assayed enzyme activity.
Hb concentration was determined by spectrophotometry with the cyanide-methemoglobin method using Drabkin's reagent (Poland). Hematocrit was determined using the micro-hematocrit method in triplicate and the mean results were calculated.
To determine the Lac concentration, 300 μL of arterialized blood was collected from the finger-tip into tubes containing K2EDTA as an anticoagulant and sodium fluoride as a glycolysis inhibitor. The blood samples were stored on ice for no longer than 20 minutes, and centrifuged for 3 minutes at RCF 14,300 × g (MPW 55, Poland). The Lac concentration of the 10 µl of plasma was measured immediately after centrifugation using the L-Lactate Randox enzyme test (UK). Test sensitivity was 0.165 mM, and linearity to 19.7 mM. The absorbance was measured using the UV/Vis Evolution 201 Thermo Scientific spectrophotometer (U.S.A.) at 540 and 550 nm, respectively, determining the concentration of Hb and Lac.
Acid–base balance was determined using the RapidLab 348 Siemens (Germany) analyzer, directly after collecting 80 μL of arterialized blood from the fingertip, using lithium heparin as an anticoagulant.
Percentage changes in plasma volume (%ΔPV) were calculated on the basis of Hb concentration and Hct value according to the equation by Dill and Costill, modified by Harrison et al. [Citation33,Citation34]. Owing to the decrease in plasma volume occurring after short exercise at very high intensity [Citation35], which may affect the apparent increase in the concentration/enzymatic activity of proteins, the activity of XO, CK and LDH were adjusted according to the formula by Kraemer and Brown [Citation36].
Statistical analysis
All data were presented as mean ± standard error. Data distribution was assessed with the Shapiro–Wilk test. The significance of differences by sex for one-time measurements was assessed with either the t-test for independent samples or the Mann–Whitney U test, depending on the distribution of variables. Sex differences in post-exercise changes of biochemical indicator concentrations were compared using multiple analysis of variance. If a main factor (sex, anaerobic exercise or sex and anaerobic exercise) was found to be significant, the significance of differences between appropriate means was assessed using post hoc analysis (Tukey’s test and planned comparisons).
Correlations between variables were determined using Pearson's test. For all variables, differences were assumed to be statistically significant at P < 0.05.
Statistica 10 (Stat-Soft, Inc., Tulsa, Oklahoma, U.S.A.) software was used to perform calculations and draw charts.
Results
Anaerobic performance
PP and MP, both the absolute value and in relation to BM and LBM, obtained during AN-EX by men were significantly higher in comparison to women (P < 0.01). The absolute PP in men and women was, respectively, 870.6 ± 39.5 and 513.3 ± 19.8 W. PP relative to BM and LBM were, respectively, 11.3 ± 0.3 W kg−1 and 13.8 ± 0.3 W kg LBM−1 for men and 8.6 ± 0.1 W kg−1 and 11.4 ± 0.2 W kg LBM−1 for women. MP in the AN-EX test measured in men was 724.9 ± 29.6 W (9.4 ± 0.2 W kg−1 and 11.5 ± 0.2 W kg LBM−1 relative to BM and LBM). Respectively, the values of MP measured in women were 438.2 ± 18.6 W and 7.3 ± 0.2 W kg−1 and 9.7 ± 0.2 W kg LBM−1 for MP·BM−1 MP·LBM−1.
The men performed a significantly (P < 0.01) higher TW (14.5 ± 0.6 kJ) during the AN-EX than the women (8.8 ± 0.4 kJ). TW performed by the men expressed relative to BM and LBM was, respectively: 188.1 ± 3.9 and 230.5 ± 4.9 J kg−1. These values were higher (P < 0.01) than those obtained by the women (TW·BM−1 146.5 ± 3.1 J kg−1, TW·LBM−1 193.7 ± 3.9 J kg−1).
The time to attain PP was shorter in the men (4.25 ± 0.25 s) than women (5.41 ± 0.17 s) (P < 0.01). The power decrease was higher (0.33 ± 0.02 W kg−1 s−1, P < 0.01), and the time of maintaining PP shorter (3.02 ± 0.15 s, P = 0.03) in the group of men compared to women (0.22 ± 0.01 W kg−1 s−1, 3.60 ± 0.21 s).
Biochemical indicators
Acid–base balance
Changes in Lac concentration and parameters of acid–base balance in the blood after the AN-EX were statistically significant in both groups, and the largest change was noted 3 minutes after completion of exercise ().
Table 3. Lactate concentration changes and acid–base balance indicators in the blood after anaerobic exercise (mean ± SE).
In the men, in the 60th minute of recovery, Lac concentration was still higher in comparison to the baseline level (P < 0.05), the concentrations of H+, HCO3−, BE and AG were higher than in the baseline up to the 30th minute following the AN-EX (P < 0.05).
In the women, changes in concentrations of Lac, H+, HCO3− and BE were statistically significant up to the 30th minute of recovery, and the AG was significantly increased in the 3rd and 15th minutes of recovery (P < 0.01).
Significant inter-group differences were only found in the values of the H+ concentration during the 15th minute following AN-EX ().
Muscle damage
No significant post-exercise or inter-group changes in CK and LDH activity in the blood were noted ().
Table 4. Changes of LDH, CK and XO activity, after anaerobic exercise (mean ± SE).
Xanthine oxidase
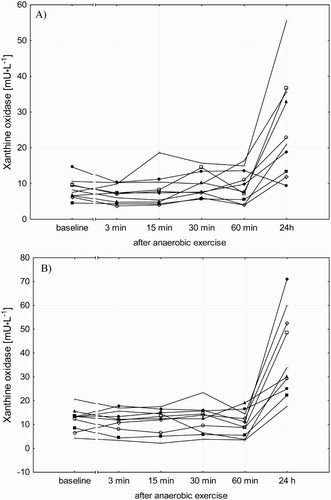
In both groups, XO activity in the blood remained at a level similar to the baseline level for 60 minutes after completion of the AN-EX (). A significant increase in the activity of XO in the blood (P < 0.05) was found in men (3.3 fold) and women (3.5 fold) 24 hours after AN-EX. At the same time, the increase in XO activity in the blood of men 24 hours after AN-EX was significantly lower than in the blood of women (P < 0.05).
The profile of changes in XO activity was similar for most of the participants; there was no increase in XO activity 24 hours after the AN-EX in only one of the tested man ().
Correlations
There was a significant negative correlation between the increase in the activity of XO in the plasma 24 hours after the AN-EX compared to the baseline level and the anaerobic power (PP, MP), the amount of TW performed during the AN-EX and the power decrease (). There was no significant correlation between the increase in XO activity in the blood 24 hours after the AN-EX and the time to attain and maintain PP. However, there was no correlation between the increase in activity of XO in the blood plasma 24 hours after EX-AN compared to the values before exercise and the indicators of anaerobic performance in the separate analyses for specific genders.
Table 5. Correlations between the increase in XO activity 24 hours after anaerobic exercise compared to the baseline and values characterizing anaerobic performance.
There was no significant correlation between the increase in XO activity in the blood plasma 24 hours after the AN-EX and the changes in post-exercise Lac concentration and acid–base balance parameters.
Discussion
Our study shows that during the first hour after the completion of anaerobic exercise, there were no changes in XO activity in the blood plasma of men or women. However, a significant increase in the activity of this enzyme was found in the blood plasma 24 hours after completing anaerobic exercise. The increase in XO activity in the blood following anaerobic exercise was smaller in men than women.
The results of previous studies, in which researchers determined changes in UA concentration as a product of a reaction catalyzed by XO, are not clear. A significant increase in the blood concentration of UA beginning at various times after the completion of anaerobic exercise could be found (3, 10, 15, 60 minutes) [Citation3,Citation16,Citation18,Citation19]. Peak concentration of UA in the blood occurred 1 hour after the end of anaerobic exercise, with a subsequent decrease in concentration up to 24 hours [Citation3], or the UA concentration decreased immediately after exercise and increased after 24 hours of rest [Citation17]. In other studies, no significant changes in UA concentration in the blood 24 hours after anaerobic exercise were found [Citation6].
The activity of XO may indirectly indicate the intensity of GSH oxidation [Citation20]. In the study by Cuevas et al. [Citation37], in men, there was a significant decrease in GSH and increase in the GSSG/GSH ratio in the blood immediately after a single 30-second cycling sprint, and after 15, 60 and 120 minutes of recovery. When a series of four similar anaerobic exercises was carried out with 60-minute intervals, only after the first three series were the levels of GSH significantly lower, and the GSSG/GSH ratio higher compared to baseline, without any significant changes during recovery [Citation37]. Regardless of the research model – single exercise or a series of anaerobic exercises – after 24 hours of recovery, no significant changes in the GSH or GSSG/GSH concentrations in the blood were found [Citation37].
In our research, we determined XO activity in the blood plasma directly. In contrast to our results, another study found an increase in XO activity immediately after the end of anaerobic exercise, involving a single 30-second cycle sprint [Citation38], and also following a series of twelve 30-m running sprints [Citation39]. A significant increase in XO activity in the blood serum was also found 4 hours after strenuous exercise (running 21 km, average speed 15.5 km h−1) [Citation40].
In these studies [Citation38–40], however, the changes in plasma volume that occur after anaerobic exercise [Citation35] or the activity of XO at a later phase of recovery were not taken into account. In our assays, we included adjustment of the results to the changes in plasma volume [Citation33,Citation34,Citation36]. Not considering the reduction in plasma volume that occurs after anaerobic exercise [Citation35] can cause false results in the form of an apparent increase in the concentrations of biochemical markers including proteins or enzymes.
Differences in the results obtained by us and other researchers [Citation38–40] may also result from the usage of anaerobic exercise which in some cases differed in time, load and performance model (single/series). In our study, we used single 20-second cycling sprints to increase the share of anaerobic glycolysis in ATP resynthesis, compared to the classic 30-second Wingate test [Citation13]. At the same time, our choice of exercise allows to assess anaerobic performance [Citation32].
Our study showed that anaerobic exercise induced similar changes in lactate concentration and acid–base balance indicators in the blood of both sexes. The research by Wiecek et al. [Citation3] showed that similar disruptions to acid–base balance between sexes, 20-second anaerobic exercise causes the same changes in both sexes in the following indicators of oxidative stress: total anti-oxidative capacity, total oxidative status, the oxidative stress index and non-enzymatic antioxidants of low molecular weight.
The results of this study indicate that the increase in the activity of XO in the blood plasma after anaerobic exercise is not dependent on changes in acid–base balance, but is significantly lower in men. At the same time, an exercise-induced increase in the activity of XO was negatively correlated with the TW performed during anaerobic exercise and with the peak and MP, which were significantly higher in men. In our study, changes in the activity of XO after anaerobic exercise, with a significant increase after 24 hours of recovery, were very similar in most of the participants. Twenty-four hours after anaerobic exercise, only one of the men had a decrease in XO activity in the blood plasma, as compared to the baseline measurement. He was the participant who had the highest peak and mean anaerobic power among the participants.
After the anaerobic exercise, there was an increase in hypoxanthine concentration in the blood, which is a substrate in the reaction catalyzed by XO [Citation13,Citation14]. Based on a review of research, it was found that the formation and release of hypoxanthine from myocytes to the blood is slow and takes place mainly during the first 90 minutes of recovery [Citation13], not immediately after anaerobic exercise [Citation41]. However, in the study by Gerber et al. [Citation14], immediately after 30 minutes of exercise, consisting in a series of 20-second cycle sprints at an intensity of 150%VO2max with a 40-second interval between them, an increase in the concentration of hypoxanthine in the blood plasma lasting only up to the 20th minute of recovery was noted. It was also found that sprint training leads to a reduction in the outflow of hypoxanthine from the muscle cells into the blood [Citation42]. In men who completed a 60-minute simulated team sports match, there was a significant decrease in hypoxanthine concentrations in the blood immediately after exercise, and 2 hours after the test there was an increase of XO activity [Citation43]. Therefore, the gender differences in the activation of XO found in our study may result from the smaller outflow of hypoxanthine from the muscles to the blood in the group of men, whose anaerobic performance is greater compared to the women, even though these were untrained individuals.
XO is present in endothelial muscle cells, but it is also present in the cytoplasm of myocytes [Citation1]. Owing to the presence of intracellular XO, we assessed whether anaerobic exercise caused micro-injury to muscle fibers, as in the case of other studies [Citation17,Citation44]. Damage to cell membranes could result in the outflow of cytoplasmic proteins to the extracellular fluid, and among others, may cause an increase in XO activity in the blood which is not the result of the severity of exercise metabolic changes [Citation45]. In the previous study, it was also shown that the inhibition of XO activity prevents the increase in cytosolic enzyme activity in the blood plasma (LDH, CK, aspartate aminotransferase), thereby indicating the role of XO in oxidative damage to cell membranes [Citation21]. In our study, we did not find significant changes in CK or LDH activity in the blood serum – inner-muscle enzymes, which are biochemical indicators of muscle damage [Citation45]. Our results indicate the absence of myocyte cell membrane micro-injury in men and women, 24 hours after anaerobic exercise and activation of mainly endothelial XO. Also in the study by Bloomer et al. [Citation46], 24 hours after anaerobic exercise (six sprints lasting 10 seconds each) there was no muscle damage in anaerobically trained men. There was no desmin destruction (cytoskeletal protein) in m. vastus lateralis cells. Two days after the completion of this effort, however, a significant increase in CK activity was found in the blood without significant changes in protein (protein carbonyls) and lipid (malondialdehyde) oxidation indicators [Citation46].
Hellsten et al. [Citation47] stated, that after the effort activation mainly concerns the endothelial XO in the human muscles and is associated with the secondary inflammation process. They postulated the role of leukocyte infiltration in the activation of XO [Citation47].
It is known that single and repeated anaerobic exercise can cause inflammation and oxidative damage to macromolecules. In the period of up to 4 hours after single anaerobic exercise, there was a significant increase in the total number of leukocytes and an increase in the percentage of granulocytes in the blood [Citation9]. At the same time, the respiratory burst of the granulocyte population was found to be enhanced [Citation9]. In the research by Deminice et al. [Citation48], 1 hour after the repeated sprint exercise (six 35-m sprints with 10-second intervals between them), there was a significant increase in the concentration of tumor necrosis factor alpha and C-reactive protein – indicators of inflammation, accompanied by an increase in LDH activity and indicators of oxidative stress concentration. In the study by Shi et al. [Citation6], all the men performing anaerobic exercise (cycle ergometer test: 2.5 minutes, 120%VO2max) demonstrated oxidative DNA damage – an increase in concentration of 8-hydroxy-2′-deoxyguanosine (8-OHdG) in leukocytes – but not until 24 hours after the exercise. In other studies, both single 30-second cycling sprints and those performed in a series (four times, 30 seconds, every 60 minutes) caused a significant increase in the concentration of 8-OHdG in the leukocytes of men. This increase could also be found 24 hours after completion of the exercise [Citation37].
The release of ROS (, H2O2) during the respiratory burst of phagocytic cells is due to the catalyzed reaction of oxidase through NAD(P)H [Citation10]. However, according to the research by McNally et al. [Citation49], the endothelial activity of XO is modulated by ROS derived from the NAD(P)H oxidase. Therefore, it can be assumed that the delay found in the increase of XO activity in the blood after a single anaerobic exercise is not directly the result of hypoxia of the shrinking myocytes, and is associated with secondary inflammation. Nonetheless, the conclusive determination of the cause of delayed XO activation requires further, detailed research.
Limitation of the study
In our study, we found a significant increase in XO activity in the blood 24 hours after anaerobic exercise, but the results did not allow for unambiguous determination of whether this is the result of anaerobic metabolism intensification in muscle cells or a secondary effect of post-exercise inflammatory processes. In our study, we did not assess changes of hypoxanthine and xanthine concentration in the blood. Furthermore, we did not examine such indicators of oxidative stress as GSSG/GSH, ox-LDL, 8-OHdG and others. Thus, we cannot say whether the increase in the activity of XO in our subjects was associated with oxidative stress. Even though we did not find any significant changes in the activity of CK and LDH in the blood which would be evidence of muscle micro-damage [Citation44], we cannot rule out the presence of inflammatory processes. In our study, we did not assess leukocyte quantitative changes, shifts in the activity of phagocytes (respiratory burst, NAD(P)H oxidase activity) or changes in the concentration of pro-inflammatory interleukins after anaerobic exercise in women and men. In addition, the assays were conducted in the first hour and then 24 hours after completion of the anaerobic exercise. Thus, we cannot clearly state when the increase in XO activity in the blood occurred. Limited conclusions are also due to the small number of participants in the studied groups.
Further studies should include assessment of these indicators, while simultaneously increasing the number of measurement points and study participants.
Conclusions
The obtained results allow to formulate the following conclusions:
In the first hour after a single anaerobic exercise session, XO activity in the blood of women and men did not change, but after 24 hours of resting, it was significantly higher compared to baseline values in both sexes.
Changes in XO activity in the blood of young healthy individuals after anaerobic exercise are dependent on their anaerobic performance. Single anaerobic exercise causes a smaller increase in the activity of XO in people with higher anaerobic performance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Magdalena Wiecek and Marcin Maciejczyk are based in the Institute of Biomedical Sciences, Department of Physiology and Biochemistry, University of Physical Education in Krakow, Poland. Magdalena Wiecek is biochemist, her main studies are focused on oxidative stress. Marcin Maciejczyk is exercise physiologist.
Jadwiga Szymura is a physiotherapist and lecturer at University of Physical Education in Krakow, the Department of Clinical Rehabilitation. She is also interested in physiology and biochemistry.
Malgorzata Kantorowicz is a Ph.D. student, Faculty of Physical Education and Sports, University of Physical Education, Krakow, Poland.
Zbigniew Szygula is head of Department of Sports Medicine and Nutrition, University of Physical Education, Krakow, Poland. His main research focus is sports hematology and sports medicine.
ORCiD
Magdalena Wiecek http://orcid.org/0000-0002-5390-3049
Marcin Maciejczyk http://orcid.org/0000-0002-9505-4081
Jadwiga Szymura http://orcid.org/0000-0003-4594-7954
Zbigniew Szygula http://orcid.org/0000-0002-6685-6579
References
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007
- Wiecek M, Maciejczyk M, Szymura J, et al. Effect of maximal-intensity exercise on systemic nitro-oxidative stress in men and women. Redox Rep. 2016;14:1–7. doi: 10.1080/13510002.2016.1169622
- Wiecek M, Maciejczyk M, Szymura J, et al. Changes in non-enzymatic antioxidants in the blood following anaerobic exercise in men and women. PLoS ONE. 2015 [cited 2016 Jun 20]; [16 p.]. doi:10.1371/journal.pone.014349
- Kang C, O’Moore KM, Dickman JR, et al. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med. 2009;47(10):1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007
- Bogdanis GC, Stavrinou P, Fatouros IG, et al. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem Toxicol. 2013;61:171–177. doi: 10.1016/j.fct.2013.05.046
- Shi M, Wang X, Yamanaka T, et al. Effects of anaerobic exercise and aerobic exercise on biomarkers of oxidative stress. Environ Health Prev Med. 2007;12(5):202–208. doi: 10.1265/ehpm.12.202
- Ferreira LF, Laitano O. Regulation of NADPH oxidases in skeletal muscle. Free Radic Biol Med. 2016;98:18–28. doi: 10.1016/j.freeradbiomed.2016.05.011
- Cocks M, Wagenmakers AJ. The effect of different training modes on skeletal muscle microvascular density and endothelial enzymes controlling NO availability. J Physiol. 2016;594(8):2245–2257. doi: 10.1113/JP270329
- Yalcin O, Erman A, Muratli S, et al. Time course of hemorheological alterations after heavy anaerobic exercise in untrained human subjects. J Appl Physiol. 2003;94(3):997–1002. doi: 10.1152/japplphysiol.00368.2002
- Robinson JM. Reactive oxygen species in phagocytic leukocytes. Histochem Cell Biol. 2008;130(2):281–297. doi: 10.1007/s00418-008-0461-4
- Henríquez-Olguín C, Díaz-Vegas A, Utreras-Mendoza Y, et al. NOX2 inhibition impairs early muscle gene expression induced by a single exercise bout. Front Physiol. 2016 [cited 2016 Sep 3];[12 p.]. doi:10.3389/fphys.2016.00282
- Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: a review. Can J Appl Physiol. 2004;29(3):245–263. doi: 10.1139/h04-017
- Morales-Alamo D, Calbet JAL. Free radicals and sprint exercise in humans. Free Radic Res. 2014;48(1):30–42. doi: 10.3109/10715762.2013.825043
- Gerber T, Borg ML, Hayes A, et al. High-intensity intermittent cycling increases purine loss compared with workload-matched continuous moderate intensity cycling. Eur J Appl Physiol. 2014;114(7):1513–1520. doi: 10.1007/s00421-014-2878-x
- Kelley EE, Khoo NK, Hundley NJ, et al. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med. 2010;48(4):493–498. doi: 10.1016/j.freeradbiomed.2009.11.012
- Groussard C, Machefer G, Rannou F, et al. Physical fitness and plasma non-enzymatic antioxidant status at rest and after a Wingate test. Can J Appl Physiol. 2003;28(1):79–72. doi: 10.1139/h03-007
- Baker JS, Bailey DM, Hullin D, et al. Metabolic implications of resistive force selection for oxidative stress and markers of muscle damage during 30 s of high-intensity exercise. Eur J Appl Physiol. 2004;92:321–327. doi: 10.1007/s00421-004-1090-9
- Souza-Junior TP, Lorenço-Lima L, Ganini D, et al. Delayed uric acid accumulation in plasma provides additional antioxidant protection against iron-triggered oxidative stress after a Wingate test. Biol Sport. 2014;31(4):271–276. doi: 10.5604/20831862.1120934
- Hammouda O, Chtourou H, Chaouachi A, et al. Effect of short-term maximal exercise on biochemical markers of muscle damage, total antioxidant status, and homocysteine levels in football players. Asian J Sports Med. 2012;3(4):239–246. doi: 10.5812/asjsm.34544
- Waring WS, Convery A, Mishra V, et al. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci. 2003;105(4):425–430. doi: 10.1042/CS20030149
- Vina J, Gimeno A, Sastre J, et al. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life. 2000;49(6):539–544. doi: 10.1080/15216540050167098
- Wiecek M, Szymura J, Maciejczyk M, et al. Effect of sex and menstrual cycle in women on starting speed, anaerobic endurance and muscle power. Acta Physiol Hung. 2016;103(1):127–132. doi: 10.1556/036.103.2016.1.13
- Aranda R, Doménech E, Rus AD, et al. Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic Res. 2007;41(11):1195–1200. doi: 10.1080/10715760701481461
- Feoli AM, Macagnan FE, Piovesan CH, et al. Xanthine oxidase activity is associated with risk factors for cardiovascular disease and inflammatory and oxidative status markers in metabolic syndrome: effects of a single exercise session. Oxid Med Cell Longev. 2014 [cited 2016 Jun 20]:[8 p.]. doi:10.1155/2014/587083
- George J, Struthers AD. Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vasc Health Risk Manag. 2009;5(1):265–272. doi: 10.2147/VHRM.S4265
- Tam HK, Kelly AS, Metzig AM, et al. Xanthine oxidase and cardiovascular risk in obese children. Child Obes. 2014;10(2):175–180. doi: 10.1089/chi.2013.0098
- Ali OS, Abdelgawad HM, Mohammed MS, et al. Ischemic heart diseases in Egypt: role of xanthine oxidase system and ischemia-modified albumin. Heart Vessels. 2014;29(5):629–637. doi: 10.1007/s00380-013-0413-3
- Barfield JP, Sells PD, Rowe DA, et al. Practice effect of the Wingate anaerobic test. J Strength Cond Res. 2002;16(3):472–473.
- Jarosz M. Normy zywienia dla populacji polskiej – nowelizacja [Nutrition standards for the Polish population – amendment]. Warsaw: Institute of Food and Nutrition; 2012. Polish.
- Wiecek M, Maciejczyk M, Szymura J, et al. Changes in oxidative stress and acid-base balance in men and women following maximal-intensity physical exercise. Physiol Res. 2015;64(1):93–102.
- Howley ET, Basset DR, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27(9):1292–1301. doi: 10.1249/00005768-199509000-00009
- Attia A, Hachana Y, Chaabène H, et al. Reliability and validity of a 20-s alternative to the Wingate anaerobic test in team sport male athletes. PLoS ONE. 2014 [cited 2016 Jun 20]; [10 p.]. doi:10.1371/journal.pone.0114444
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma and cells in dehydration. J Appl Physiol. 1974;37(2):243–248.
- Harrison MH, Graveney MJ, Cochrane LA. Some sources of error in the calculation of relative change in plasma volume. Eur J Appl Physiol. 1982;50(1):13–21. doi: 10.1007/BF00952240
- Weinstein Y, Bediz C, Dotan R, et al. Reliability of peak-lactate, heart rate, and plasma volume following the Wingate test. Med Sci Sports Exerc. 1998;30(9):1456–1460.
- Kraemer RR, Brown BS. Alterations in plasma-volume-corrected blood components of marathon runners and concomitant relationship to performance. Eur J Appl Physiol. 1986;55(6):579–584. doi: 10.1007/BF00423200
- Cuevas MJ, Almar M, Garcia-Glez JC, et al. Changes in oxidative stress markers and NF-kappaB activation induced by sprint exercise. Free Radic Res. 2005;39(4):431–439. doi: 10.1080/10715760500072149
- Bloomer RJ, Smith WA. Oxidative stress in response to aerobic and anaerobic power testing: influence of exercise training and carnitine supplementation. Res Sports Med. 2009;17(1):1–16. doi: 10.1080/15438620802678289
- Abbey EL, Rankin JW. Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int J Sport Nutr Exerc Metab. 2011;21(2):91–96. doi: 10.1123/ijsnem.21.2.91
- Tong TK, Kong Z, Lin H, et al. Serum oxidant and antioxidant status following an all-out 21-km run in adolescent runners undergoing professional training – a one-year prospective trial. Int J Mol Sci. 2013;14(7):1567–1578. doi: 10.3390/ijms140715167
- Ihara H, Shino Y, Morita Y, et al. Is skeletal muscle damaged by the oxidative stress following anaerobic exercise? J Clin Lab Anal. 2001;15(5):239–243. doi: 10.1002/jcla.1034
- Zielinski J, Kusy K. Training-induced adaptation in purine metabolism in high-level sprinters vs. triathletes. J Appl Physiol. 2012;112(4):542–551. doi: 10.1152/japplphysiol.01292.2011
- Slattery KM, Wallace LK, Bentley DJ, et al. Effect of training load on simulated team sport match performance. Appl Physiol Nutr Metab. 2012;37(2):315–322. doi: 10.1139/h2012-001
- Karamizrak SO, Ergen E, Töre IR, et al. Changes in serum creatine kinase, lactate dehydrogenase and aldolase activities following supramaximal exercise in athletes. J Sport Med Phys Fit. 1994;34(2):141–146.
- Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48(6):757–767. doi: 10.1515/CCLM.2010.179
- Bloomer RJ, Falvo MJ, Fry AC, et al. Oxidative stress response in trained men following repeated squats or sprints. Med Sci Sports Exerc. 2006;38(8):1436–1442. doi: 10.1249/01.mss.0000227408.91474.77
- Hellsten Y, Frandsen U, Orthenblad N, et al. Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. J Physiol. 1997;498(1):239–248. doi: 10.1113/jphysiol.1997.sp021855
- Deminice R, Rosa FT, Franco GS, et al. Effects of creatine supplementation on oxidative stress and inflammatory markers after repeated-sprint exercise in humans. Nutrition. 2013;29(9):1127–1132. doi: 10.1016/j.nut.2013.03.003
- McNally JS, Davis ME, Giddens DP, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285(6):H2290–H2297. doi: 10.1152/ajpheart.00515.2003