ABSTRACT
Objectives: Oxidative stress is known to participate in the progression of sepsis. Definite data regarding the behavior of oxidative stress biomarkers in pediatric sepsis is still lacking. This study hypothesized that oxidative stress occurs in pediatric sepsis and that the magnitude of the redox derangement is associated with worse clinical progression.
Methods: Forty-two previously healthy pediatric patients with sepsis and a group of control subjects were included. Oxidative stress and inflammatory activity biomarkers were determined in blood samples. Patients were prospectively followed until their discharge or death.
Results: Patients with non-severe and severe sepsis showed higher levels of plasmatic antioxidant capacity, lower erythrocyte thiol index, lower superoxide dismutase and catalase activities, higher glutathione peroxidase activity, and higher plasmatic F2-isoprostanes concentration than controls. Patients with severe sepsis had higher NF-kappaB activation than those with non-severe sepsis. Although we observed changes in some biomarkers in patients with worse clinical evolution, the explored biomarkers did not correlate with clinical estimators of outcome.
Discussion: Oxidative stress occurs in pediatric sepsis, resulting in oxidative damage. The explored biomarkers are not useful as outcome predictors in the studied population. The behavior of these biomarkers still needs to be addressed in broader groups of pediatric patients with sepsis.
Introduction
According to the estimations of the World Health Organization, pediatric sepsis, and septic shock are still important causes of morbidity and mortality worldwide. More than 42 000 cases of sepsis per year are estimated to occur only in the United States, with an associated mortality rate of 10% and high hospitalization costs [Citation1]. Despite decades of improving antibiotic use and a global initiative to optimize timely and appropriate supportive care, mortality caused by sepsis in children is still a major concern, even in developed countries.
Oxidative stress is a phenomenon that participates in the pathogenesis of several diseases. Many pathological processes can induce the production of reactive oxygen species (ROS) and reactive nitrogen species. These compounds can damage cellular structures by oxidizing proteins, lipids, and DNA. There are antioxidant defense mechanisms that can prevent oxidative damage, including enzymatic defense mechanisms such as superoxide dismutase (SOD), catalase and glutathione peroxidase (GSHPx), and non-enzymatic defense mechanisms such as ascorbic acid, alpha-tocopherol, and reduced glutathione (GSH). The imbalance between pro-oxidant and antioxidant mechanisms, in favor of the former, is known as oxidative stress.
Severe sepsis has been classically understood as an uncontrolled inflammatory process, which is triggered by an infectious insult. Over-activation of nuclear factor-kappa B (NF-κB) has been hypothesized to play a cardinal role in the unrestrained inflammatory response that is seen in this condition [Citation2]. Progressive involvement of different tissues can lead to multiple organ dysfunction syndrome (MODS). Available evidence in the adult population supports a major role of ROS production in the development of the systemic response occurring in sepsis and septic shock as well as its contribution to the progression toward MODS [Citation3,Citation4]. There are few studies regarding the occurrence of oxidative stress in pediatric sepsis after the neonatal period, which show conflicting results [Citation5–7]. Overall, the behavior of oxidative stress biomarkers in pediatric sepsis pathophysiology and the role of oxidative stress as a potential therapeutic target have not been properly addressed. The purpose of this study was to assess the occurrence of oxidative stress in pediatric patients with sepsis. Several biomarkers of oxidative stress are evaluated in patients with sepsis, including enzymatic and non-enzymatic antioxidant defense mechanisms and lipid oxidative damage. Additionally, the relationship between biomarkers of oxidative stress and clinical disease severity indicators is explored.
Materials and methods
Study design and patient selection
This study was conducted in a university-affiliated, tertiary care, medical and surgical pediatric intensive care unit (PICU), at the city of Santiago, Chile, between January 2011 and September 2014. Approval was obtained from the University of Chile Faculty of Medicine ethics committee. Patients were prospectively included if admitted to the PICU with the diagnosis of sepsis, according to international consensus definitions [Citation8]. Informed consent was obtained at admission or as soon as parents or legal guardians were available. Study exclusion criteria were: age under 1 month or over 7 years, chronic illness, systemic inflammatory response syndrome (SIRS) from noninfectious cause (burns, trauma, major surgery, pancreatitis), malnutrition, chronic use of corticosteroid (over 1 month), and hospital length of stay of more than 48 h. Clinical management was conducted by board-certified pediatric intensivists, according to international, goal-directed, sepsis management guidelines [Citation9]. Standard therapy included vasoactive drug support and mechanical ventilation when needed. Antibiotics were chosen according to the suspected infection site and adjusted with bacterial confirmation when available. Use of stress-dose corticosteroids (hydrocortisone 100 mg/m2/day) was decided by the attending physician on a case-by-case basis, without plasma cortisol level determination. Hemofiltration was used when needed because of acute renal failure or volume overload, and no high volume hemofiltration was performed. The included patients were prospectively followed until their discharge from the hospital and clinical data was recorded.
Control subjects were otherwise healthy children who were admitted to the hospital for elective surgery (phimosis, umbilical or inguinal hernia, hydrocele, undescended testicle, and benign skin tumors). Absence of acute or any other chronic disease was determined by medical history and physical examination. Informed consent was obtained prior to the admittance to the operating room. Although these are not truly healthy subjects, a genuinely healthy population could not be recruited because of ethical limitations. A total of 49 control subjects were included, in which reference values of the studied oxidative stress biomarkers were determined. From this group, we selected 42 subjects for comparison with the study group, paired by age and gender in a 1:1 fashion.
A blood sample was drawn from control subjects and from patients with sepsis at admission and at day 2 of PICU hospitalization. Plasmatic antioxidant capacity was measured as the ferric reducing ability of plasma (FRAP). Erythrocyte antioxidant mechanisms were measured as the thiol index [GSH to oxidized glutathione (GSSG) ratio; GSH/GSSG], and as SOD, catalase, and GSHPx antioxidant enzyme activity. Lipid oxidative damage in plasma was measured by F2-isoprostanes concentration. Inflammatory activity was estimated by NF-κB activation in lympho-monocytes at day 2. Detailed laboratory methodology and statistical analysis are provided as supplemental material (Supplemental material 1).
Results
Oxidative stress biomarkers in control subjects
A total of 49 control subjects were recruited. Biomarkers of enzymatic defense mechanisms, non-enzymatic defense mechanisms, and lipid oxidative damage were determined. No difference was observed when comparing biomarkers values by gender. A weak positive correlation of SOD (r = 0.49; P < 0.001) and a negative correlation of FRAP (r = −0.44; P < 0.01) with age was observed (). Demographic characteristics of this group and measured oxidative stress biomarker values are shown in .
Figure 1. Oxidative stress biomarkers in relation to age in control subjects. (a) Correlation between age and SOD activity; (b) Correlation between age and FRAP. SOD, superoxide dismutase; FRAP, ferric reducing ability of plasma.
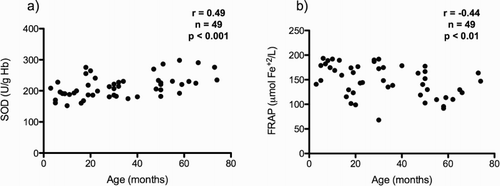
Table 1. Demographic characteristics and oxidative stress biomarkers in control subjects.
Oxidative stress biomarkers in sepsis
A total of 42 patients with sepsis were enrolled, along with 42 control subjects, paired by age and gender. Patients with sepsis were classified according to the diagnosis of non-severe sepsis or severe sepsis. Demographic and clinical data of these three groups presented in . Patients with non-severe sepsis and severe sepsis had higher levels of FRAP compared to controls at admission (P < 0.01 for non-severe sepsis and P < 0.05 for severe sepsis), while only patients with severe sepsis showed higher levels than controls at day 2 (P < 0.01). Compared to controls, patients with non-severe and severe sepsis had low erythrocyte thiol index at admission and at day 2 (P < 0.001). In relation to antioxidant enzymes, at both studied days, the SOD activity was lower in both groups of patients with sepsis than in controls (P < 0.001), catalase activity was lower only in patients with severe sepsis than in controls (P < 0.001), and GSHPx activity was higher only in patients with non-severe sepsis than in controls (P < 0.01). Both at admission and at day 2, F2-isoprostanes plasmatic levels were higher in the non-severe (P < 0.01) and severe sepsis groups (P < 0.001) than in controls. Comparing of these biomarkers in terms of clinical severity revealed that catalase activity was lower in the severe sepsis group than in the non-severe sepsis group (P < 0.05), and it was the lone biomarker that showed difference between these two groups. Comparisons of oxidative stress biomarkers between these three groups, at admission and at day 2 are summarized in .
Table 2. Demographic and clinical characteristics of control group and patients with sepsis.
Table 3. Oxidative stress biomarkers in control group and patients with sepsis.
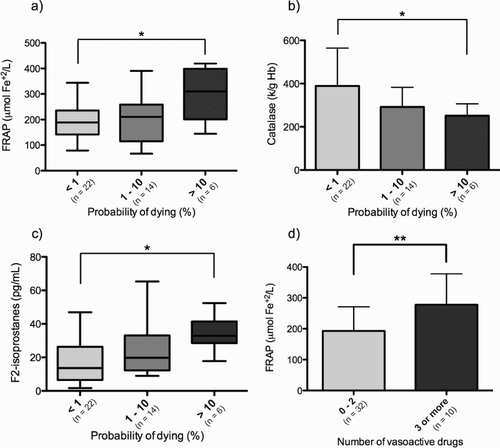
In relation to the probability of death, patients with a probability of dying of more than 10% showed a higher FRAP at day 2 (P < 0.05), lower catalase activity at day 1 (P < 0.05), and higher F2-isoprostanes plasmatic concentration at day 2 (P < 0.05) than patients with a probability of dying of less than 1%. Patients who required the use of three or more vasoactive drugs also showed higher FRAP values at admission and at day 2 (P < 0.01). These results are depicted in . The remaining assessed biomarkers showed no differences in relation to these classifications (data not shown).
Figure 2. Biomarkers of oxidative stress in patients with sepsis according to clinical severity as estimated by the probability of dying and vasoactive drug requirement. (a) FRAP, day 2; (b) Catalase, day 1; (c) F2-isoprostanes, day 2; (d) FRAP, day 2. *P < 0.05; **P < 0.01. FRAP, ferric reducing ability of plasma.
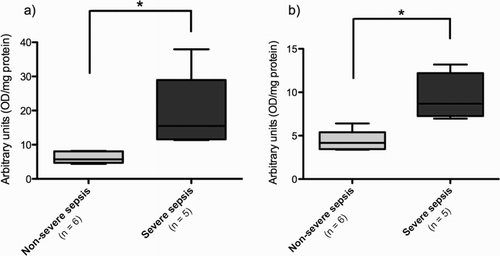
Inflammatory activity
Analysis of NF-κB activation showed no difference between the control group and the sepsis group (data not shown). However, when we classified the patients according to severity, those with the diagnosis of severe sepsis had a higher activation of both p50 and p65 NF-κB subunits at day 2 than patients with non-severe sepsis ().
Oxidative stress biomarkers as predictors of clinical severity
Correlations between oxidative stress biomarkers and length of PICU stay, length of total hospitalization, duration of invasive ventilatory support, and duration of the disease prior to PICU admission were assessed, and no correlation was observed. Using logistic and ordinal regression, we assessed the association between the studied biomarkers at admission and the clinical outcome in terms of the diagnosis (non-severe sepsis or severe sepsis) and in PELOD score estimated mortality rate. In addition, a survival analysis was carried out for the association between these biomarkers and length of PICU stay, length of total hospitalization, and duration of invasive ventilatory support. No association between the studied biomarkers and any of these clinical outcomes was observed.
Discussion
In this study, we show that the occurrence of sepsis in children is associated with changes in pro-oxidant and antioxidant regulatory mechanisms, which results in extracellular lipid oxidative damage. Changes in some of these biomarkers were observed in relation to clinical severity. However, the studied biomarkers could not predict clinical outcome.
As the control group, we studied patients admitted for elective minor surgery. A truly healthy group could not be recruited because of ethical constraints. However, none of these control subjects had a clinically evident acute or chronic systemic disease at the time of the inclusion; hence, we believe that this group is probably representative of healthy pediatric population. We report for the first time an increase in erythrocyte SOD activity and a decrease in plasmatic antioxidant activity at higher ages within the pediatric population. Although this correlation is weak, we studied only children under 7 years of age; thus, the strength of the correlation could be higher if this tendency is maintained during the rest of the pediatric age range. Previously, only a lower erythrocyte SOD activity has been reported in preterm newborns [Citation10], which could be related to the presence of oxidative stress-related diseases in these patients, such as bronchopulmonary dysplasia. The clinical implications of these findings are yet to be established.
We studied a group of patients with the diagnosis of sepsis and classified them according to severity in non-severe sepsis and severe sepsis groups. Although the sepsis definition is known for having a low specificity (and consequently is being revisited), it is currently the accepted way to recognize patients with a systemic response to infection. Patients with sepsis had higher levels of plasmatic antioxidant capacity, which is apparently in contradiction with the hypothesis of a pro-oxidant status in this condition. This is the first time an increase in plasmatic antioxidant capacity is reported in children with sepsis. However, studies in adult population have shown contradictory results. Both lower [Citation11] and higher [Citation12–14] plasmatic antioxidant capacity have been reported in adult patients with SIRS and sepsis. These contradictory results can be explained by differences in the studied sample (total plasma, deproteinized plasma, or serum) and by the particular laboratory technique, which determines the relative contribution of each plasmatic antioxidant to the total antioxidant capacity [Citation14]. Some authors have attributed the increase in antioxidant capacity to a higher uric acid concentration. However, we corrected the FRAP value with uric acid antioxidant contribution. Moreover, septic patients did not show higher uric acid concentration (data not shown); hence, in our study, this compound was not responsible for the observed differences. Usually, the major components of plasmatic antioxidant capacity, such as ascorbic acid, alpha-tocopherol, and albumin, are diminished in sepsis [Citation15,Citation16]. It is possible that the increase in plasmatic antioxidant potential could be explained by an increase in the relative contribution of bilirubin (which we did not determine) and of ‘unidentified antioxidants’ (referring to compounds with antioxidant capacity which are not usually determined), which have been shown to have a higher relative contribution to total antioxidant capacity in adult non-survivor patients with SIRS [Citation12]. In relation to intracellular antioxidant capacity, we observed a lower thiol index in patients with non-severe and severe sepsis. A previous study did not show differences in erythrocyte GSH and GSSG concentrations between patients with sepsis and controls [Citation6]. However, the GSSG/GSH index (the reciprocal of thiol index) has been shown to be higher in whole blood of pediatric patients with septic shock [Citation17]. It is possible that the expected higher ROS production in sepsis results in GSH consumption, but a lower GSH synthesis has also been observed during pediatric sepsis [Citation5]. In our results, the lower concentration of GSH did not correlate with higher GSHPx activity; hence, this finding is probably secondary to non-enzymatic GSH consumption.
In pediatric patients with sepsis, we observed a lower enzymatic activity of SOD and catalase. Previously, a higher tissue SOD activity has been reported in an animal model of sepsis [Citation18], and a higher plasmatic SOD activity was observed in adult patients with sepsis [Citation19]. These results can be explained by a compensatory response to a pro-oxidant status by genetic transcription mechanisms induced by ROS-mediated activation of nuclear factor Nrf2 (nuclear factor erythroid 2 – related factor 2) [Citation20]. In contrast, because of a lack of the nucleus, it would not be possible to observe these compensatory mechanisms in erythrocytes. Additionally, oxidative damage of antioxidant enzymes could contribute to the lower observed enzymatic activities [Citation21–23]. The previously reported increase in plasmatic SOD could reflect a diminished activity of extracellular SOD [Citation19], which would further impair tissue antioxidant defenses. Accordingly, the observed increase in red cell GSHPx activity also cannot be explained by transcriptional mechanisms. The only previously described mechanism of nontranscriptional GSHPx activity induction is related to selenium administration [Citation24], a compound known to be diminished in sepsis [Citation25]. Despite the increment in the measured GSHPx activity, the in vivo enzymatic activity is probably not increased because of the lower levels of erythrocyte GSH and the GSH dependence for GSHPx-mediated hydrogen peroxide (H2O2) removal. Overall, pediatric patients with sepsis show impairment in erythrocyte antioxidant defenses. Erythrocytes are the second most important source of GSH after the liver, accounting for a 10% of total body GSH production [Citation26]. Red cell oxidative damage can alter its deformability and cause premature aging and lower oxygen transport capability [Citation27], which is of vital relevance in the context of septic shock. Additionally, erythrocytes can protect tissues such as the endothelium [Citation28] and lung [Citation29] against oxidative damage. In consequence, weakening of erythrocyte antioxidant defenses could result in tissue oxidative stress. Regarding oxidative damage, our study shows that pediatric patients with sepsis have higher concentrations of plasmatic lipoperoxides than controls. This finding implies that in these patients, the final balance of the previously referred alterations in oxidative defense systems is a pro-oxidant status, which results in oxidative damage.
Catalase was the only oxidative stress biomarker that showed a difference between the non-severe and severe sepsis groups. As previously referred, the lower activity of catalase in severe sepsis could be related to oxidative damage of the enzyme. On the other hand, patients with severe sepsis had higher activation of p50 and p65 subunits of NF-κB. The p50/p65 heterodimer is the most abundant in different tissues [Citation30], and its activation in sepsis is related to the transcription of the inflammatory response genes. This finding is in concordance to previously reported studies of NF-κB activity in pediatric sepsis [Citation31], and supports the theory of an exacerbated inflammatory response that contributes to sepsis progression toward MODS. We did not include C-reactive protein in the analysis, because our measurement technique had an upper detection limit of 270 mg/l and most of the studied patients had a C-reactive protein level of over that limit. According to the probability of death (as estimated by PELOD score), patients with more severe disease had also lower erythrocyte catalase activity and higher plasmatic antioxidant capacity, which was also observed in patients requiring more vasoactive drug support. Patients with a higher probability of dying had higher lipid oxidative damage, which supports the idea of oxidative stress as a part of the pathophysiologic events that lead to the progression of sepsis to MODS. However, the observed changes in these biomarkers were not consistent across the different severity estimators. Moreover, these biomarkers were not capable to predict clinical evolution. We believe that this could be related to the shortage of very severe cases of sepsis, which reduced the statistical power. Additionally, we selected a subset of patients who were admitted to the PICU, which theoretically had a worse clinical evolution that motivated this admission. The ability of the studied biomarkers to predict the need for PICU admission among patients with initially mild cases of sepsis still needs to be addressed. We also think that the behavior of these biomarkers should further be explored in cases of sepsis affecting patients with chronic metabolic or nutritional conditions, who may have a diminished capacity to deal with an additional oxidative injury [Citation32].
Conclusions
In this study, we report changes in oxidative stress biomarkers occurring in pediatric sepsis. Although some of these biomarkers showed changes in relation to clinical severity, they could not predict clinical evolution. In consequence, these findings do not yet support the use of antioxidant reinforcement therapy in this group of patients to prevent clinical progression. Further studies are needed to properly address the behavior of these biomarkers in pediatric sepsis in broader groups of patients, including those with mild cases of sepsis and with associated diseases.
YRER_A_1239866_Supplemental_material_1.docx
Download MS Word (20.7 KB)Acknowledgments
The authors would like to thank the Hospital Roberto del Río Transfusion Medicine Unit for their help with sample processing and storage.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Víctor M. Molina, MD, PhD in Medical Sciences, Universidad de Chile. Pediatrics specialist, Universidad de Chile. Pediatric Intensive Care Unit attending staff until 2015, Hospital Roberto del Río, Santiago, Chile. Pediatric Cardiology fellow, Pontificia Universidad Católica de Chile. Scientific articles and book chapters (last 5 years): 6.
Bettina M. von Dessauer, MD, Master of Health Administration, Universidad de los Andes. Professor of Pediatrics and Intensive Care, Universidad de Chile. Member of the board of directors of the Latin American Society of Pediatric Intensive Care (SLACIP) and of the board of the World Federation of Pediatric Critical Care Societies (WFPICCS) until 2016. Past-Director of the Pediatric Intensive Care Unit, Hospital Roberto del Río, Santiago de Chile, Chile. Director of Projects and Teaching, Pediatric Intensive Care Unit, Hospital Roberto del Río. Scientific articles and book chapters (last 5 years): 7.
Ramón Rodrigo, MSc of Medical Sciences, University of Chile. Professor of Pathophysiology, Faculty of Medicine, Universidad de Chile. Head of the Laboratory of Oxidative Stress and Nephrotoxicity, Institute of Biomedical Sciences. Principal researcher of 12 projects. Editor of 5 books and author of about 200 ISI articles. Current research: Prevention of heart reperfusion damage in patients subjected to percutaneous coronary angioplasty following acute myocardial infarction.
Cristian M. Carvajal, MD. Pediatric intensivist, Universidad de Chile. Associate professor of Pediatrics, Pediatrics and Pediatric Surgery Department, Universidad de Chile. Postgraduate diploma in research methodology and evidence-based medicine. Current areas of development: clinical research, biostatistics, and evidence-based medicine. Scientific articles and book chapters (last 5 years): 2.
References
- Watson RS, Carcillo JA, Linde-Zwirble WT, et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC
- Böhrer H, Qiu F, Zimmermann T, et al. Role of NFkappaB in the mortality of sepsis. J Clin Invest. 1997;100:972–985. doi: 10.1172/JCI119648
- Motoyama T, Okamoto K, Kukita I, et al. Possible role of increased oxidant stress in multiple organ failure after systemic inflammatory response syndrome. Crit Care Med. 2003;31:1048–1052. doi: 10.1097/01.CCM.0000055371.27268.36
- Ware LB, Fessel JP, May AK, et al. Plasma biomarkers of oxidant stress and development of organ failure in severe sepsis. Shock. 2011;36:12–17. doi: 10.1097/SHK.0b013e318217025a
- Lyons J, Rauh-Pfeiffer A, Ming-Yu Y, et al. Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Crit Care Med. 2001;29:870–877. doi: 10.1097/00003246-200104000-00036
- Cherian S, Jameson S, Rajarajeswari C, et al. Oxidative stress in sepsis in children. Indian J Med Res. 2007;125:143–148.
- Vázquez-Armenta G, González-Leal N, Vázquez-de la Torre MJ, et al. Short (GT)n microsatellite repeats in the heme oxygenase-1 gene promoter are associated with antioxidant and anti-inflammatory status in Mexican pediatric patients with sepsis. Tohoku J Exp Med. 2013;231:201–209. doi: 10.1620/tjem.231.201
- Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6
- Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–688. doi: 10.1097/CCM.0b013e31819323c6
- Nassi N, Ponziani V, Becatti M, et al. Anti-oxidant enzymes and related elements in term and preterm newborns. Pediatr Int. 2009;51:183–187. doi: 10.1111/j.1442-200X.2008.02662.x
- Cowley HC, Bacon PJ, Goode HF, et al. Plasma antioxidant potential in severe sepsis. Crit Care Med. 1996;24:1179–1183. doi: 10.1097/00003246-199607000-00019
- Tsai K, Hsu T, Kong C, et al. Is the endogenous peroxyl-radical scavenging capacity of plasma protective in systemic inflammatory disorders in humans? Free Radic Biol Med. 2000;28:926–933. doi: 10.1016/S0891-5849(00)00180-5
- Pascual C, Karzai W, Meier-Hellmann A, et al. Total plasma antioxidant capacity is not always decreased in sepsis. Crit Care Med. 1998;26:705–709. doi: 10.1097/00003246-199804000-00019
- Chuang C-C, Shiesh S-C, Chi C-H, et al. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit Care. 2006;10:R36. doi: 10.1186/cc4826
- Doise J-M, Aho LS, Quenot J-P, et al. Plasma antioxidant status in septic critically ill patients: a decrease over time. Fundam Clin Pharmacol. 2008;22:203–209. doi: 10.1111/j.1472-8206.2008.00573.x
- Margarson MP, Soni NC. Effects of albumin supplementation on microvascular permeability in septic patients. J Appl Physiol. 2002;92:2139–2145. doi: 10.1152/japplphysiol.00201.2001
- Németh I, Boda D. Xanthine oxidase activity and blood glutathione redox ratio in infants and children with septic shock syndrome. Intensive Care Med. 2001;27:216–221. doi: 10.1007/s001340000791
- Ritter C, Andrades M, Frota Júnior ML, et al. Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med. 2003;29:1782–1789. doi: 10.1007/s00134-003-1789-9
- Guerreiro MO, Petronilho F, Andrades M, et al. Plasma superoxide dismutase activity and mortality in patients with septic. J Trauma. 2010;69:E102–E106. doi: 10.1097/TA.0b013e3181dbb289
- Zhou S, Sun W, Zhang Z, et al. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid Med Cell Longev. 2014;2014:1–16.
- Salo DC, Pacifici RE, Lin SW, et al. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990;265:11919–11927.
- Kim SY, Kwon OJ, Park JW. Inactivation of catalase and superoxide dismutase by singlet oxygen derived from photoactivated dye. Biochimie. 2001;83:437–444. doi: 10.1016/S0300-9084(01)01258-5
- Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257:5751–5754.
- Baker RD, Baker SS, LaRosa K, et al. Selenium regulation of glutathione peroxidase in human hepatoma cell line Hep3B. Arch Biochem Biophys. 1993;304:53–57. doi: 10.1006/abbi.1993.1320
- Weber SU, Lehmann LE, Schewe J-C, et al. Low serum α-tocopherol and selenium are associated with accelerated apoptosis in severe sepsis. BioFactors. 2008;33:107–119. doi: 10.1002/biof.5520330203
- Lyons J, Rauh-Pfeiffer A, Yu YM, et al. Blood glutathione synthesis rates in healthy adults receiving a sulfur amino acid-free diet. Proc Natl Acad Sci USA. 2000;97:5071–5076. doi: 10.1073/pnas.090083297
- Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol. 2014;5:84. doi: 10.3389/fphys.2014.00084
- Richards RS, Roberts TK, Dunstan RH, et al. Erythrocyte antioxidant systems protect cultured endothelial cells against oxidant damage. Biochem Mol Biol Int 1998;46:857–865.
- Heffner JE, Repine JE. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis 1989;140:531–554. doi: 10.1164/ajrccm/140.2.531
- Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappaB. Annu Rev Cell Biol 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201
- Hotta N, Ichiyama T, Shiraishi M, et al. Nuclear factor-κB activation in peripheral blood mononuclear cells in children with sepsis. Crit Care Med. 2007;35:2395–2401. doi: 10.1097/01.CCM.0000284502.38701.E6
- Khare M, Mohanty C, Das BK, et al. Free radicals and antioxidant status in protein energy malnutrition. Int J Pediatr 2014;2014:1–6. doi: 10.1155/2014/254396