ABSTRACT
Objectives: To investigate the effects of multiple cryotherapy applications after muscle injury on markers of oxidative stress.
Methods: Following cryolesion-induced skeletal muscle injury in rats, ice was applied at the injured site for 30 minutes, three times per day, on the day of injury, and for 2 days after injury. To determine the effect of the cryotherapy treatment on markers of oxidative stress, biochemical analyses were performed 3, 7, and 14 days after injury.
Results: Compared with non-treated animals, cryotherapy reduced dichlorofluorescein at 7 and 14 days post-injury and thiobarbituric acid reactive substances levels at 3 and 7 days post-injury (P < 0.05). Additionally, cryotherapy maintained methyl thiazol tetrazolium reduction levels compared to the control group at all analyzed time points (P > 0.05), whereas non-treated groups demonstrated lower levels than the control group (P < 0.05). Superoxide dismutase activity at 7 and 14 days post-injury and catalase activity at 3 days post-injury were lower in cryotherapy groups compared with non-treated groups (P < 0.05). Cryotherapy prevented the reduction of non-protein thiol levels and maintained within control group level, at 3 days post-injury (P = 0.92).
Discussion: Cryotherapy reduced the production of reactive oxygen species after muscle injury, resulting in an attenuated response of the antioxidant system. These findings suggest that using multiple cryotherapy applications is efficient to reduce oxidative stress.
Introduction
Skeletal muscle constitutes the largest tissue mass in the body and is highly susceptible to injury, especially in sporting activities [Citation1]. Thereby, the medical care and indirect costs related to skeletal muscle injury result in large public health costs [Citation2]. Regardless of the cause of the injury, muscle regeneration is an orchestrated process, which is distinguished in three stages: the destruction phase (lasts 0–72 hours); repair phase (lasts 3–4 weeks); and remodeling phase (lasts 3–6 months) [Citation3]. During the destruction phase, the proliferation of inflammatory cells to injured tissue may amplify the injury by releasing reactive oxygen species (ROS) [Citation4]. Furthermore, as a consequence of simultaneous vascular tissue damage, additional ROS production occurs after the restoration of perfusion and oxygen supply to the previously ischemic area [Citation5,Citation6]. ROS may modify lipids, proteins, and DNA, altering their function within the tissue. These molecular modifications are called oxidative damage and can affect cell homeostasis and survival [Citation7,Citation8]. Fortunately, there is a complex antioxidant system to combat the actions of ROS [Citation9], and oxidative stress will occur when the imbalance between ROS and antioxidants favors ROS [Citation10].
Rehabilitation and sport medicine specialists have proposed using cryotherapy during muscle regeneration to minimize muscle injury [Citation11–14], since a greater quantity of injured tissue requires a longer recovery time [Citation5]. Many studies, although not all [Citation15], have highlighted that cryotherapy may be beneficial to recovery, as it reduces inflammation within the affected muscles [Citation16–20]. However, the mechanisms involved in the ability of cryotherapy to reduce oxidative damage are not completely understood. To date, only a few studies [Citation6,Citation9,Citation21,Citation22] have assessed the effects of cryotherapy after acute muscle injury with oxidative stress being a main variable of interest. Moreover, to the best of our knowledge, the effects of multiple cryotherapy applications performed within the destruction phase, as recommended in clinical practice [Citation23,Citation24], have not been assessed oxidative stress in a controlled experimental model. Thus, the aim of the present study was to examine the effect of multiple cryotherapy applications on oxidative stress during the 14 days following cryolesion of the tibialis anterior (TA) of rats. Our hypothesis was that the cryotherapy protocol would minimize the oxidative stress within injured muscles.
Materials and methods
Animals
Three-month old male Wistar rats (n = 35; 325.1 ± 31.1 g) were used in this study. The animals were housed in plastic cages with a controlled temperature, 12-hour light–dark cycle, and free access to water and standard chow throughout the experiment. This study was conducted with University approval for the care and use of animal resources (number 059/2010) and in accordance with the Guide for the Care and Use of Laboratory Animals [Citation25]. For the skeletal muscle injury and the cryotherapy treatment, the rats were anesthetized with an intraperitoneal injection of xylazine (12 mg/kg) and ketamine (95 mg/kg); and, at each group’s final measurement time point, the animals were euthanized by anesthetic overdose.
Experimental groups
The effect of cryotherapy on markers of oxidative stress was analyzed at three experimental time points: 3, 7, and 14 days after muscle injury. The rats were randomly distributed into seven experimental groups with five animals each: control, animals with no interventions; TA injured, no cryotherapy treatment, and analyzed 3 days post-cryolesion (L3); TA injured, treated with cryotherapy, and analyzed 3 days post-cryolesion (L3 + C); TA injured, no cryotherapy treatment, and analyzed 7 days post-cryolesion (L7); TA injured, treated with cryotherapy, and analyzed 7 days post-cryolesion (L7 + C); TA injured, no cryotherapy treatment, and analyzed 14 days post-cryolesion (L14); and TA injured, treated with cryotherapy, and analyzed 14 days post-cryolesion (L14 + C). The control group was analyzed at the same time point as L3 and L3 + C ().
Cryolesion
The TA was chosen because of its superficial position. Before inducing skeletal muscle injury, the animals were anesthetized and the skin of the right hind limb around the TA was trichotomized and cleaned with alcoholic iodine solution. A transverse incision (approximately 1 cm) over the muscle belly was made, exposing the TA. Muscle injury was induced on the non-treated and treated groups by cryolesion. This injury model has been shown to be a reproducible method of inducing muscle injury among animals [Citation20,Citation26–28]. Thereby, a rectangular iron bar (40 × 20 mm) was cooled using liquid nitrogen and applied for 10 seconds on the widest part of TA belly. The freezing exposure was repeated twice more at the same site, with an interval of 30 seconds between them. After the final cryolesion application, the skin was sutured.
Cryotherapy
Under anesthesia, the animals were maintained in a horizontal position on a plastic table and the ankle of the right hind limb was fixed by tape. During cryotherapy applications, a plastic pack filled with crushed ice was fixed directly to the skin of the right TA using tape. Each cryotherapy treatment day comprised of three separate applications per day, each lasting for 30 minutes, with an interval of 2 hours between applications [Citation26]. The cryotherapy treatment was performed immediately, 24, and 48 hours after injury, taking into account the destruction phase during muscle regeneration, in line with previous clinical recommendations [Citation23,Citation24] ().
Muscle sample collection
At the time point that defined each experimental group, the respective animals were euthanized by anesthetic overdose and weighed. Then, the right TA was removed and weighed. For biochemical analyses, the belly of the TA was isolated and immediately frozen in isopentane, pre-cooled in liquid nitrogen, and stored in a freezer (Forma Scientific, Marietta, Ohio, U.S.A) at −80°C until analysis.
Biochemical analyses
Biochemical analyses were performed at three time points: 3, 7, and 14 days after TA injury. The TA fragment was homogenized in NaCl (150 mN) and centrifuged at 4000g for 10 minutes at 4°C to obtain a low speed supernatant fraction. The supernatant fraction was used to determine dichlorofluorescein reactive substances (DCF-RS) levels; thiobarbituric acid reactive substances (TBARS) levels; methyl thiazol tetrazolium (MTT) reduction levels; enzymatic activity of catalase (CAT) and superoxide dismutase (SOD); and non-protein thiol (–SH) levels.
DCF-RS levels
DCF-RS levels were determined as an index of peroxide production by cellular components [Citation29], according to the method described by Carvalho et al. [Citation9] The fluorescence analysis was performed with excitation at 488 nm and emission at 525 nm, and both slit widths used were 5 nm. DCF-RS levels were determined using a standard curve of oxidized dichlorofluorescein (DCF) [Citation30]. The results were normalized by the protein content, which was determined according to Lowry et al. [Citation31] using bovine serum albumin (BSA) as the standard.
TBARS levels
TBARS levels were determined in skeletal muscle supernatant fraction samples as a measure of lipid peroxidation, according to the method described by Ohkawa et al. [Citation32] TBARS levels were measured at 532 nm using a standard curve of malondialdehyde and the results were normalized by protein content.
MTT reduction levels
MTT reduction levels were determined as an index of cell viability [Citation33], according to the method described by Mosmann [Citation34]. The formed formazan levels were determined spectrophotometrically at 570 nm [Citation34] and the results were normalized by protein content.
CAT and SOD activities
The CAT and SOD enzyme activities were analyzed as an index of the enzymatic antioxidant mechanisms. CAT enzyme activity was kinetically determined according to the method proposed by Aebi [Citation35] and SOD enzyme activity was kinetically determined according to the method proposed by Misra and Fridovich [Citation36].
Non-protein -SH levels
The non-protein –SH levels were determined as a non-enzymatic antioxidant mechanism, according to the method proposed by Ellman [Citation37] with some modifications. Non-protein –SH levels were measured spectrophotometrically at 412 nm [Citation37]. Results were calculated in relation to a standard curve constructed with glutathione (GSH) [Citation37], and normalized by protein content.
Statistical analysis
Descriptive statistics (means and standard deviations) were used to report all data. The MTT reduction levels were calculated as a percentage of the control group. The Shapiro–Wilk and Levene tests showed that all data were normally and homogeneously distributed. Two-way analyses of variance (ANOVA) were used to analyze time/intervention interactions for each variable. In the case of a significant difference, Tukey’s post-hoc test was performed. For all analyses, a P value equal or less than 0.05 was accepted as statistically significant. Findings were analyzed with Statistical Package for Social Sciences (version 21.0).
Results
Oxidative markers and cell viability index
Compared to the control group, DCF-RS levels were greater in the non-treated groups at 7 days post-injury (L7: 7.5 fold, P < 0.001; L14: 9.0 fold, P < 0.001; (a)). However, cryotherapy reduced the increase of DCF-RS levels at 7 and 14 days post-injury compared with non-treated groups (L7 + C: 0.1 fold, P < 0.001; L14 + C: 0.3 fold, P < 0.001; (a)).
Figure 2. Effects of multiple cryotherapy applications performed during the destruction phase following cryolesion in the tibialis anterior (TA) muscle of rats on markers of reactive species production, oxidative damage, and cell viability, respectively: (a) dichlorofluorescein reactive substances (DCF-RS) levels; (b) thiobarbituric acid reactive substances (TBARS) levels; (c) methyl thiazol tetrazolium (MTT) reduction levels. Data are expressed as mean ± SD. *Differences when compared to control group (P < 0.05); #differences between the treated and non-treated groups at the same time point (P < 0.05); †different from the value at 3 days after muscle injury (P < 0.05).
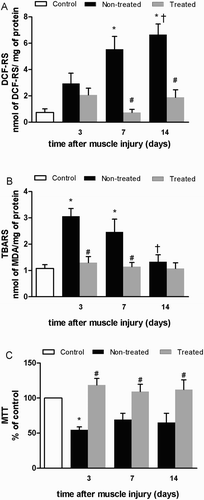
Compared to the control group, TBARS levels increased in non-treated groups at 3 and 7 days post-injury (L3: 2.8 fold, P < 0.001; L7: 2.3 fold, P = 0.022; (b)), and returned to control levels by 14 days (P = 0.94; (b)). Interestingly, compared to the non-treated groups, the cryotherapy groups presented a reduced increase of TBARS levels, both at 3 and 7 days post-injury (L3 + C: 0.4 fold, P = 0.002; L7 + C: 0.5 fold, P = 0.032; (b)).
The non-treated L3, presented a decrease in MTT reduction levels (L3: 0.5 fold, P = 0.023; (c)), compared with control group. There were no differences between cryotherapy groups and control at any analyzed time points (P > 0.05). However, there were differences between treated and non-treated groups at all-time points (L3 + C: 2.2 fold, P = 0.002; L7 + C: 1.7 fold, P = 0.044; L14 + C: 1.7 fold, P = 0.035; (c)).
Enzymatic and non-enzymatic antioxidant mechanisms
Compared to the control group, SOD enzyme activity increased in the non-treated groups at all analyzed time points (L3: 2.2 fold, P = 0.005; L7: 2.8 fold, P < 0.001; L14: 2.8 fold, P < 0.001; (a)). Nevertheless, at 7 and 14 days post-injury, cryotherapy reduced the increase of SOD enzyme activity in the treated groups compared to the non-treated groups (L7 + C: 0.5 fold, P = 0.001; L14 + C: 0.6 fold, P = 0.008; (a)).
Figure 3. Effects of multiple cryotherapy applications performed within the destruction phase, following cryolesion in the tibialis anterior (TA) muscle of rats on antioxidant enzymatic and non-enzymatic detoxification mechanisms: (a) enzymatic activity of superoxide dismutase (SOD); (b) enzymatic activity of catalase (CAT); (c) non-protein thiol (–SH) levels. Data are expressed as mean ± SD. *Differences when compared to control group (P < 0.05); #differences between the treated and non-treated groups at the same time point (P < 0.05); †different from the value at 3 days after muscle injury (P < 0.05).
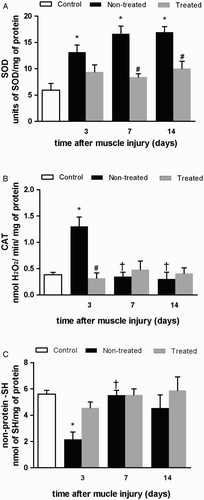
Only at 3 days post-injury, CAT enzyme activity of the non-treated groups was greater compared to control (L3: 3.4 fold, P < 0.001; (b)). Interestingly, cryotherapy reduced the increase of CAT enzyme activity at the same time point compared to the non-treated group (L3 + C: 0.2 fold, P < 0.001; (b)). The non-treated L3 group displayed lesser non-protein –SH levels (L3: 0.5 fold, P = 0.015; (c)) compared with control. On the other hand, at the same time point, cryotherapy maintained non-protein –SH levels compared to control (L3 + C: P = 0.920; (c)), while also not being significantly different from the non-treated group (L3 + C: 2.1 fold, P = 0.174, (c)).
Discussion
This study was conducted in order to evaluate the effects of multiple cryotherapy applications on classical markers of oxidative stress in the TA of rats obtained 3, 7, and 14 days after cryolesion-induced muscle injury. In agreement with our initial hypothesis, multiple cryotherapy applications, performed within the destruction phase after injury, alleviated markers of oxidative stress.
It is well established that the inflammatory response leads to elevated production of ROS, as consequence of an increased number of macrophages and neutrophils [Citation9,Citation14,Citation38]. To combat the production of ROS, evidence has demonstrated that cryotherapy may be effective, as it reduces ROS in injured tissue by reducing the magnitude of inflammatory response [Citation6,Citation9,Citation21]. Additionally, a recent study from our laboratory [Citation20] demonstrated that multiple applications of cryotherapy reduced the quantity of macrophages in the injured area. One potential explanation for the reduction of macrophages in the injured area might be related to the blood-flow reducing effects of cryotherapy [Citation14,Citation17,Citation39] or the capacity of cryotherapy to reduce leukocyte adhesion in the microvasculature [Citation16,Citation40]. The present study showed that multiple cryotherapy applications during the destruction phase of muscle regeneration attenuated the increase of DCF-RS and TBARS levels compared to the levels seen in the non-treated rats. Thus, cryotherapy appeared to influence the production of ROS and reduce the biomolecular impairments associated with ROS [Citation41,Citation42]. Therefore, it is possible that post-injury cryotherapy may reduce the magnitude of a muscle injury that has already occurred [Citation6,Citation9,Citation21]. In addition, as the treated rats displayed greater MTT reduction levels than non-treated rats ((c)), cryotherapy may serve as a potential therapeutic strategy to maintain cell viability after injury. Although the treated groups presented mean MTT reduction values that were slightly greater than control group, these values were not significant and we believe that such slight increases in MTT reduction levels presented by treated groups might be due an elevated cell’ metabolic rate [Citation43] than non-treated groups. Nevertheless, this idea is hypothetical and should be explicitly examined in future research.
Similar effects of cryotherapy were observed by Puntel et al. [Citation21], who associated MTT reduction levels with specific indicators of mitochondrial function. From this vantage point, the efficacy of cryotherapy could be explained by a reduction in the rate of chemical reactions and, therefore, the demand for ATP, reducing cellular collapse [Citation19]. However, it is not possible to determine if cryotherapy prevents or delays the death of cells that were primarily injured but not initially destroyed. Therefore, cryotherapy continues to be credited for reducing secondary injury [Citation5]. Similar to previous studies [Citation6,Citation9,Citation21], the present study showed that multiple cryotherapy applications maintained SOD and CAT activity and non-protein –SH levels within control levels. Taking into account the oxidative markers, cell viability, and antioxidant system, these results show that cryotherapy maintained homeostatic mechanisms by reducing ROS production and avoiding an increase in antioxidant system activity after injury.
The post-injury biochemical responses indicate that the cryolesion model adopted in the present study was effective at inducing muscle injury. When collectively comparing the results of control group with the non-treated groups, the results suggest that an early increase of TBARS and a decrease of MTT reduction levels could have been associated with ROS that were not detectable in the DCF-RS assay [Citation28], whereas at later time points, a greater activation of antioxidant mechanisms neutralized a significant generation of hydroperoxides. Interestingly, SOD activity showed a progressive increase over the duration of this study, differing from previous studies showing that SOD activity has not significantly changed after injury [Citation6,Citation9,Citation21,Citation22]. The increase of SOD activity may be related to a greater muscle injury caused by cryolesion compared to other models of injury such as skeletal strain [Citation9], contusion [Citation21], ischemia–reperfusion [Citation6], and exercise-induced muscle damage [Citation22]. Factually, previous studies had associated cryolesion’s low temperature to an alteration in quaternary structures of enzymes [Citation28,Citation44].
Although the present study indicates that cryotherapy may attenuate the oxidative stress following muscle injury, limitations are present. For example, tissue and core body temperature were not measured during cryotherapy applications and biochemical markers of muscular damage were not quantified. Nonetheless, it is also important to emphasize that studies investigating the effects of cryotherapy on oxidative stress are scarce in the literature and show heterogeneity between cryotherapy protocols.
Finally, only one clinical trial has been conducted with the purpose to investigate the effect of cryotherapy on oxidative stress biomarkers after muscle injury [Citation22]. Similar to previous animal trials [Citation9,Citation21], a reduction in TBARS plasma concentration after a submaximal physical exertion was observed after 5 minutes of cold water immersion [Citation22]. Although this study contributes to the elucidation of cryotherapy’s effects on oxidative damage in humans, our study is the first study that has determined the effects of a multi-application cryotherapy protocol on markers of oxidative stress. Nevertheless, further studies should be conducted, especially in humans, in order to address the effects of a cryotherapy protocol on muscle regeneration.
Conclusion
In conclusion, multiple cryotherapy applications, performed within the destruction phase after TA injury of rats, reduced oxidative stress. In terms of practical applications, cryotherapy can play an important role during the destruction phase of the regeneration process by limiting the severity of muscle skeletal injury.
Acknowledgments
The authors acknowledge the Aldrich Chemical Co. (St. Louis, MO) for the supply of thiobarbituric acid, dichloroflouresceine diacetate, methyl thiazol tetrazolium, and Ellman’s reagent. We also wish to thank Writing Unrivaled for the English review.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Angelina Freitas Siqueira PhD student, Physical Education College, University of Brasilia. Current research in Skeletal Muscle Regeneration and Cryotherapy.
Amilton Vieira PhD and Assistant Professor of Exercise Physiology, Department of Physical Education, UDF – University Center. Researcher at Strength Training Group, Physical Education College, University of Brasilia. Current research in Strength Training, Skeletal Muscle Plasticity and Cryotherapy.
Gracielle Vieira Ramos PhD and Assistant Professor of Kynesiology, Department of Physical Therapy, UNIP – Paulista University. Researcher at Muscle-tendinous Plasticity Group, Physical Therapy Division, University of Brasilia. Current research in Skeletal Muscle Plasticity.
Rita de Cássia Marqueti PhD and Assistant Professor of Physiology, Physical Therapy Division, University of Brasilia. Current research in Muscle-tendinous Plasticity and Extracellular Matrix Adaptations.
Tania de Fátima Salvini PhD and Full Professor of Skeletal Muscle Plasticity, Department of Physical Therapy, Federal University of São Carlos. Current research in Skeletal Muscle Plasticity and its implications for the field of Physical Therapy and Rehabilitation.
Gustavo Orione Puntel PhD and Assistant Professor of Human Anatomy, Department of Morphology, Federal University of Santa Maria. Current research in Oxidative Stress, Lipid Peroxidation, Natural and Synthetic Antioxidants, Muscle Injuries, Cryotherapy and Physical Exercise.
Joao Luiz Quagliotti Durigan PhD and Assistant Professor of Therapeutics Physical Agents, Physical Therapy Division, University of Brasilia. Current research in Skeletal Muscle Plasticity and Electro-photo-thermal therapies.
ORCID
Angelina Freitas Siqueira http://orcid.org/0000-0001-6795-3445
Amilton Vieira http://orcid.org/0000-0002-6027-4367
Gracielle Vieira Ramos http://orcid.org/0000-0003-4746-0357
Rita de Cássia Marqueti http://orcid.org/0000-0001-9126-3882
Tania de Fátima Salvini http://orcid.org/0000-0002-6353-6393
Gustavo Orione Puntel http://orcid.org/0000-0002-1641-9477
Joao Luiz Quagliotti Durigan http://orcid.org/0000-0002-7511-5289
Additional information
Funding
References
- Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347(3):759–774. doi: 10.1007/s00441-011-1185-7
- Christoffel T, Gallagher SS. The importance of injury problem: magnitude, cost, and preventability. In: Christoffel T, Gallagher SS, editors. Injury prevention and public health: practical knowledge, skills, and strategies. 2nd ed. Sedbury: Jones & Bartlett Learning; 2006. p. 3–24.
- Baoge L, Van Den Steen E, Rimbaut S, et al. Treatment of skeletal muscle injury: a review. ISRN Orthop. 2012. DOI:10.5402/2012/689012.
- Prisk V, Huard J. Muscle injuries and repair: the role of prostaglandins and inflammation. Histol Histopathol. 2003;18(4):1243–1256.
- Merrick MA. Secondary injury after musculoskeletal trauma: a review and update. J Athl Train. 2002;37(2):209–217.
- Puntel GO, Carvalho NR, Dobrachinski F, et al. Cryotherapy reduces skeletal muscle damage after ischemia/reperfusion in rats. J Anat. 2013;222(2):223–230. doi: 10.1111/joa.12009
- Clarkson PM, Thompson HS. Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr. 2000;72(2):637s–646s.
- Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1(1):244–257. doi: 10.1016/j.redox.2013.01.014
- Carvalho N, Puntel G, Correa P, et al. Protective effects of therapeutic cold and heat against the oxidative damage induced by a muscle strain injury in rats. J Sports Sci. 2010;28(9):923–935. doi: 10.1080/02640414.2010.481722
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001
- White GE, Wells GD. Cold-water immersion and other forms of cryotherapy: physiological changes potentially affecting recovery from high-intensity exercise. Extrem Physiol Med. 2013;2(26):1–11.
- Bleakley CM, Davison GW. What is the biochemical and physiological rationale for using cold water immersion in sports recovery? A systematic review. Br J Sports Med. 2010;44:179–187. doi: 10.1136/bjsm.2009.065565
- Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39(1):88–94.
- Ferreira-Junior JB, Bottaro M, Loenneke JP, et al. Could whole-body cryotherapy (below −100°C) improve muscle recovery from muscle damage?. Front Physiol. 2014;5:247. doi: 10.3389/fphys.2014.00247
- Takagi R, Fujita N, Arakawa T, et al. Influence of icing on muscle regeneration after crush injury to skeletal muscles in rats. J Appl Physiol. 2011;110(2):382–388. doi: 10.1152/japplphysiol.01187.2010
- Schaser KD, Disch AC, Stover JF, et al. Prolonged superficial local cryotherapy attenuates microcirculatory impairment, regional inflammation, and muscle necrosis after closed soft tissue injury in rats. Am J Sports Med. 2007;35(1):93–102. doi: 10.1177/0363546506294569
- Pournot H, Bieuzen F, Louis J, et al. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS One. 2011;6(7):e22748. doi: 10.1371/journal.pone.0022748
- Banfi G, Melegati G, Barassi A, et al. Effects of whole-body cryotherapy on serum mediators of inflammation and serum muscle enzymes in athletes. J Therm Biol. 2009;34(2):55–59. doi: 10.1016/j.jtherbio.2008.10.003
- Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999;31(11):1516–1521. doi: 10.1097/00005768-199911000-00004
- Ramos GV, Pinheiro CM, Messa SP, et al. Cryotherapy reduces inflammatory response without altering muscle regeneration process and extracellular matrix remodeling of rat muscle. Sci Rep. 2016;6:18525. doi: 10.1038/srep18525
- Puntel GO, Carvalho NR, Amaral GP, et al. Therapeutic cold: an effective kind to modulate the oxidative damage resulting of a skeletal muscle contusion. Free Radic Res. 2011;45(2):133–146. doi: 10.3109/10715762.2010.517252
- Sutkowy P, Augustyńska B, Woźniak A, et al. Physical exercise combined with whole-body cryotherapy in evaluating the level of lipid peroxidation products and other oxidant stress indicators in kayakers. Oxid Med Cell Longev. 2014. DOI:10.1155/2014/402631.
- Knight KL. Cryotherapy in sport injury management. Champaign: Human Life Press; 1995.
- Oakley ET, Pardeiro RB, Powell JW, et al. The effects of multiple daily applications of ice to the hamstrings on biochemical measures, signs, and symptoms associated with exercise-induced muscle damage. J Strength Cond Res. 2013;27(10):2743–2751. doi: 10.1519/JSC.0b013e31828830df
- Garber JC, Barbee RW, Bielitzki JT, et al. National Research Council. Guide for the care and use of laboratory animals. Washington: The National Academy Press; 2011.
- Irintchev A, Zweyer M, Cooper RN, et al. Contractile properties, structure and fiber phenotype of intact and regenerating slow-twitch muscles of mice treated with cyclosporin A. Cell Tissue Res. 2002;308:143–156. doi: 10.1007/s00441-002-0519-x
- Miyabara EH, Aoki MS, Soares AG, et al. Expression of tropism-related genes in regenerating skeletal muscle of rats treated with cyclosporin-A. Cell Tissue Res. 2005;319(3):479–489. doi: 10.1007/s00441-004-1027-y
- Oliveira NMLD, Durigan JLQ, Munin FS, et al. The effect of intermittent cryotherapy on the activities of citrate synthase and lactate dehydrogenase in regenerating skeletal muscle. Braz Arch Biol Technol. 2013;56(1):61–68. doi: 10.1590/S1516-89132013000100008
- Myhre O, Andersen JM, Aarnes H, et al. Evaluation of the probes 2′, 7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003;65(10):1575–1582. doi: 10.1016/S0006-2952(03)00083-2
- Pérez-Severiano F, Rodrígues-Pérez M, Pedraza-Chaverrí J, et al. S-Allylcysteine, a garlic-derived antioxidant, ameliorates quinolinic acid-induced neurotoxicity and oxidative damage in rats. Neurochem Int. 2004;45:1175–1183. doi: 10.1016/j.neuint.2004.06.008
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3
- Bernas T, Dobrucki J. Mitochondrial and nonmitochondrial reduction of MTT: interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry. 2002;47(4):236–242. doi: 10.1002/cyto.10080
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1):55–63. doi: 10.1016/0022-1759(83)90303-4
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105;121–126. doi: 10.1016/S0076-6879(84)05016-3
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1952;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6
- Peake J, Nosaka KK, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev. 2005;11:64–85.
- Khoshnevis S, Craik NK, Diller KR. Cold-induced vasoconstriction may persist long after cooling ends: an evaluation of multiple cryotherapy units. Knee Surg Sports Traumatol. 2015;23(9):2475–2483. doi: 10.1007/s00167-014-2911-y
- Deal DN, Tipton J, Rosencrance E, et al. Ice reduces edema. J Bone Joint Surg. 2002;84(9):1573–1578.
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141(2):312–322. doi: 10.1104/pp.106.077073
- Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7
- Alexandrov K. Effects of inducers and inhibitors of the benzo (a) pyrene hydroxylase of isolated rat liver nuclei and nuclear envelopes on the binding of benzo (a) pyrene to DNA. Eur J Cancer. 1977;13(8):847–853. doi: 10.1016/0014-2964(77)90140-2