ABSTRACT
Objectives: Parkinson disease (PD) is a neurodegenerative disorder affecting mainly the motor system, as a result of death of dopaminergic neurons in the substantia nigra pars compacta. The present scenario of research in PD is directed to identify novel molecules that can be administered individually or co-administered with L-Dopa to prevent the L-Dopa-Induced Dyskinesia (LID) like states that arise during chronic L-Dopa administration. Hence, in this study, we investigated whether Morinda citrifolia has therapeutic effects in rotenone-induced Parkinson’s disease (PD) with special reference to mitochondrial dysfunction mediated intrinsic apoptosis.
Methods: Male Sprague-Dawley rats were stereotaxically infused with rotenone (3 µg in both SNPc and VTA) and co-treated with the ethyl acetate extract of Morinda citrifolia and levodopa.
Results: The results revealed that rotenone-induced cell death was reduced by MCE treatment as measured by decline in the levels of pro-apoptotic proteins. Moreover, MCE treatment significantly augmented the levels of anti-apoptotic Bcl2 and blocks the release of cytochrome c, thereby alleviating the rotenone-induced dopaminergic neuronal loss, as evidenced by tyrosine hydroxylase (TH) immunostaining in the striatum.
Discussion: Taken together, the results suggest that Morinda citrifolia may be beneficial for the treatment of neurodegenerative diseases like PD.
Introduction
Parkinson disease (PD) is a neurodegenerative disorder affecting mainly the motor system, as a result of death of dopaminergic neurons in the substantia nigra pars compacta [Citation1]. Eventhough, the precise etiology of PD still remains elusive, but at the cellular level, excess production of reactive oxygen species (ROS), mitochondrial dysfunction, neuroinflammation, and exposure to environmental toxins are considered to be the key determinants of dopaminergic neuronal susceptibility in PD [Citation2]. Evidences suggest a major role for mitochondrial dysfunction in the pathogenesis of PD, as complex I inhibitors like 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or rotenone show preferential cytotoxicity to the dopaminergic neurons [Citation3]. Therefore, neurochemically, PD is marked by mitochondrial complex I dysfunction and increased indices of oxidative stress which in turn leads to protein misfolding and aggregation, inflammatory responses, excitotoxicity, and finally neuronal cell death i.e. apoptosis [Citation4].
The currently available therapy for PD includes Levodopa, which can replenish the dopamine levels, entacapone, a catechol-O-methyl-transferase (COMT) inhibitor, and selegiline, a monoamine oxidase inhibitor, but none of them prevent the degeneration of the dopaminergic neurons. Therefore, rationalization of therapeutic measures might bring in the radical improvement in preventing progression and deterioration of PD. As antioxidants are well established to maintain redox balance and there are arrays of antioxidants that can attenuate the effects of oxidative stress through multiple mechanisms [Citation5], studies are being carried out to determine the therapeutic effects of these natural antioxidants against various degenerative diseases like PD.
Morinda citrifolia L. (Rubiaceae), commonly known as Noni, has been used extensively in Polynesian folk medicine for over 2000 years. As a popular herb, the fruit juice of Morinda citrifolia has been used as an alternative medicine for different kinds of illnesses such as arthritis, diabetes, high blood pressure, menstrual difficulties, heart disease, cancers, gastric ulcers, mental depression, and atherosclerosis [Citation6]. Further, the plant contains a number of phytochemicals that have been studied for anxiolytic and sedative effects [Citation7]. Moreover, the fruit juice of Morinda citrifolia has been reported to prevent neuronal damage induced by focal ischemia. It is also reported that it can protect against scopolamine, ß-amyloid, and streptozotocin-induced memory impairment in animals [Citation8–11]. Previously in our laboratory we have identified the protective effects of the ethyl acetate extract of Morinda citrifolia fruit against rotenone-induced skeletal muscle apoptosis and striatal protein aggregation in experimental Parkinsonian rats [Citation14]. In the present study, we elucidated the neuroprotective effect of Morinda citrifolia against rotenone-induced neuronal apoptosis in the striatum of PD rats.
Materials and methods
Reagents
Scopoletin, quercetin, rutin, rotenone, and bovine serum albumin were procured from Sigma-Aldrich, USA. All other chemicals used were of analytical grade obtained from Merck Chemical Supplies (Darmstadt, Germany), Hi-Media, and Sisco Research Laboratories (SRL), India. The fruits of Morinda citrifolia (Noni) were collected from NCRN farm, Shalavakkam, Tamilnadu. Authentication of plant material was done by Prof. P. Jayaraman of Plant Anatomy Research Centre, Tambaram, Chennai.
Quantitation of phytoconstituents in MCE
Scopoletin, quercetin, and rutin in MCE was quantitated by high-performance liquid chromatography using a C-18 column [Citation12]. MCE (150 mg) was dissolved in methanol (2 ml). The internal standard solution (scopoletin, quercetin, and rutin were dissolved in methanol at the concentration of 1 mg/ml) was prepared and both the MCE and standard solutions (20 μl) was subjected for HPLC analysis. The mobile phase consisted of 0.1% phosphoric acid (A) and methanol (B), and a gradient elution was programed as follows: A/B: 60/40 (0–10 min) and 30/70 (30–40 min). The flow rate was 1.0 ml/min and detection wavelength was set at 340 nm.
Animals
Healthy male Sprague-Dawley rats about 12–15 months old and weighing about 300–350 g were used throughout the study. The animals were procured from Central Animal House Facility, Dr ALM PGIBMS, University of Madras, Taramani Campus, Chennai – 600 113. All experiments were performed with animals approved by the Institutional Animal Ethical Committee (IAEC No. 01/09/12, 01/09/12 Extension) in compliance with the relevant laws and institutional guidelines. The animals were housed under conditions of controlled temperature (25 ± 2°C) with 12/12 h light/dark cycle and were given food and water ad libitum.
Intra-Nigral rotenone infusion and treatment
The animals were divided into five groups (n = 10). Group I rats served as control, while Group II to Group V rats were subjected to stereotaxic surgery. Groups III, IV and Group V rats were stereotaxically infused with rotenone to induce Parkinsonism. Briefly, rats were anesthetized with ketamine hydrochloride and xylazine (80 mg/kg and 10 mg/kg; i.p.) and placed on a small animal stereotaxic frame (Stoelting, IL, USA). Rotenone dissolved in DMSO (1 µg/1 µl) was infused into the right Ventral Tegmental Area (VTA, Anterior-Posterior (AP): 5.0 mm; Laterally (L): 1.0 mm; Dorso-Ventral (DV): 7.8 mm) and into the right Substantia Nigra Pars compacta (SNPc, AP: 5.0 mm; L: 2.0 mm; DV: 8.0 mm) each 3 μl at a flow rate of 0.2 µl/min using a Hamilton 26 gauge needle [Citation13,Citation14]. The infusion needle was left in place for additional five minutes for complete diffusion of the drug. In sham operated controls (Group II), the rats received 6 µl of the vehicle (DMSO and PEG in the ratio of 1:1) instead of rotenone during stereotaxic surgery. After post-operative recovery i.e. after two weeks, Group IV rats were treated orally with Levodopa [LD, 100 mg/kg with 25 mg/kg benserazide] for the next 30 days [Citation15]. While Group V rats were pre-treated orally with the ethyl acetate extract of Morinda citrifolia fruit (MCE, 150 mg/kg body weight) for 30 days prior to stereotaxic surgery and the treatment was continued after recovery for a further period of 30 days [Citation14].
Behavioral assessment
All the behavioral parameters were performed at room temperature in a calm room without any outside interference and were performed between 10am to 5pm.
Spontaneous rotation behavior
After recovery from anesthesia rats were intensively observed for any abnormal activity during the first 72 hours. One of the striking features following recovery from anesthesia was spontaneous circling. The animals were kept in a transparent cage following surgery, and the spontaneous rotations in the cage were counted over a period of one hour.
Rota rod test
The rotarod test is an approved test for evaluation of neuro-logical impairments in rodents and it can be applied repeatedly to the individual rat in order to determine muscle strength, fore and hind limb motor coordination and balance of rats [Citation16,Citation17]. Rotarod test was performed at baseline (before rotenone infusion on first day) and every week from infusion (days 7, 14, 21, and 30). All rats underwent a 5-day training program in order to reach a stable performance. The rotation speed was 15 rpm at first day and reached 20 rpm at the end of the training. The cut off time was 180s and each rat performed three separate trials after a 5 min gap. The average fall of time was recorded and expressed as seconds per 3 min [Citation18].
Catalepsy
The development of Parkinsonism was detected by the occurrence of tremors. Observation of bradykinesia and rigidity in rats were further quantified by a ‘Catalepsy test’ [Citation19]. In the bar test, the rats were placed with both front paws on a horizontal bar which was 9 cm above and parallel from the base. The rats were placed with both front paws on the bar in a half rearing position; here they were timed with the stopwatch. When the animals removed one paw from the bar the stopwatch was stopped and the time noted. The maximum cut off for bar test was fixed at 180 s.
Histopathology and immunohistochemistry
For histopathological studies, the animals were anesthetized with Ketamine (22 mg i/p), perfused intracardially initially with 0.1 M phosphate-buffered saline (PBS) and followed by 4% para-formaldehyde (PFA) in 0.1 M PBS, pH 7.4. Brains were removed and fixed in 4% PFA and allowed to impregnate in 30% sucrose solution in PBS. Coronal sections passing through striatum and SNpc were cut on a microtome (7 μm thickness for IHC and histological staining), collected on to gelatin subbed slides using PBS.
The sections were incubated with the primary antibody (rabbit monoclonal tyrosine hydroxylase primary antibody, 1:1000, Pierce antibodies) in TBS, pH 7.4, containing 2% NGS and 0.2% Triton X-100 for 24 h at 4°C on a platform shaker. After rinsing in TBS, sections were incubated with secondary anti-rabbit IgG-conjugated horseradish peroxidase antibody (1:1000) in TBS, pH 7.4, containing 2% NGS for 45 min at room temperature. Visualization was performed by incubation in 3,3-diaminobenzidine for 5 min after which the sections were examined under a light microscope (Nikon Eclipse Ti series). The average optical density of the immunohistochemical staining for TH in striatal region per unit area from sections each at comparable levels from animals was done in a blinded fashion by different observers using Densitometry protocol through ImageJ computed that showed a significant decrease in the staining intensity in the infused side of striatum as compared to the other side.
Assessment of mitochondrial function
Mitochondria from rat striatum were isolated by the method of Iglesias-Gonzalez et al. [Citation20]. Complex I activity was measured following the decrease in absorbance due to the oxidation of NADH at 340 nm with reference set at 425 nm [Citation21]. Complex IV activity was measured following the oxidation of cytochrome c Fe2+ [Citation22].
Gene expression studies by reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was isolated from the striatum using total RNA isolation (TRI) reagent. cDNA is made from mRNA template using dNTPs and reverse transcriptase (Superscript-III Reverse Transcriptase, Bio-Rad, USA). Oligonucleotide primer sequences of the selected genes for reverse transcription–polymerase chain reaction (RT–PCR) were procured from Sigma–Aldrich (St. Louis, MO, USA). The amplified products were separated by electrophoresis on 2% agarose gel and identified by ethidium bromide staining. Specificity was confirmed by the size of the amplified products with reference of 100 bp DNA ladder (Bio vision, USA) and the band intensities were quantified by Quantity One Software (Bio-Rad, USA) ().
Table 1. PCR Primer sequences and conditions used*.
Western blotting
Striatal tissue lysates (50 µg protein) were separated by SDS-PAGE on 10–12% polyacrylamide gels and transferred to polyvinylidene difuoride membranes. The membranes were incubated with specific primary antibodies and the antibodies used were Bax, Bcl2 (Abcam 1:1000 dilution), Caspase-3, Caspase-9, (Cell Signaling Technology, 1:1000 dilution). To verify the uniformity of protein load and transfer efficiency across the test samples membranes were re-probed with β-actin and COX-IV (for cytochrome c). Immuno-reactive bands were developed with Immobilon Western-Chemiluminescent HRP substrate (Millipore Corporation, Billerica, USA) and visualized by using an enhanced chemiluminescence system (Chemi-Doc, Bio-Rad, USA).
Statistical analysis
Data are presented as mean ± standard error of mean (SEM) of the results obtained from the average of at least three to six independent experiments. Results were analyzed by one-way analysis of variance (ANOVA) using the SPSS software package for Windows (Version 20.0; SPSS Inc., Chicago, IL, USA) and p values were determined using the Student–Newman–Keuls and least significant difference (LSD) post hoc test. Differences among means were considered statistically significant when the P value was less than 0.05. The statistical comparisons are made between control/sham and rotenone which is designated as ‘a’ and between rotenone and LD/MCE treated groups which is designated as ‘b’.
Results
Characterization of MCE
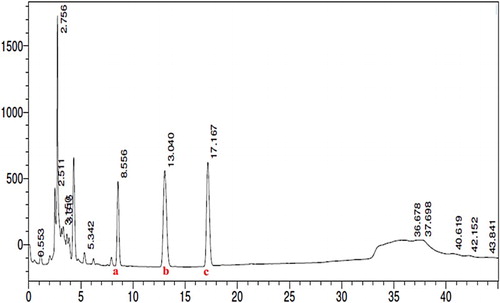
shows the HPLC chromatogram of the ethyl acetate extract of Morinda citrifolia fruit. Rutin (2.6 μg) was found to be in higher concentration followed by quercetin (1.4 μg) and scopoletin (0.6 μg) in 1 mg of MCE. Therefore, a dose of MCE given to rats (150 mg/kg body weight) contains 392 μg of rutin, 210 μg of quercetin and 90 μg of scopoletin.
Figure 1. HPLC chromatogram of the ethyl acetate extract of Morinda citrifolia fruit. Chromatographic peaks depicting the presence of scopoletin (a), quercetin (b), and rutin (c). Rutin (2.6 μg) was found to be in higher concentration followed by quercetin (1.4 μg) and scopoletin (0.6 μg) in 1 mg of MCE (n = 3).
Behavioral analysis
Spontaneous rotation behavior after recovery from anesthesia
After recovery from anesthesia, spontaneous rotations to the opposite side of the infusion site were observed in all the rats receiving infusions of rotenone into the VTA and SNPc. The frequency of spontaneous rotations was 226 ± 17 times per hour during the first day after surgery. The intensity of the behavior persisted for about 24 hours, gradually declined thereafter, and vanished after 48–72 hours.
Rotarod and catalepsy
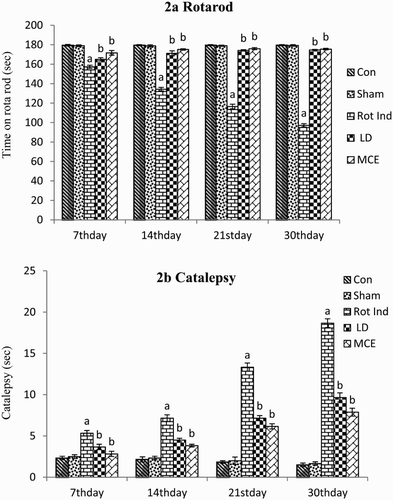
Motor coordination and grip strength of the animals were tested by Rotarod test. The rigidity or inability to correct an externally induced posture of the limb was measured by catalepsy bar test. During the 4-week acquisition trials in the catalepsy test and Rotarod motor coordination test, all the rats stereotaxically infused with rotenone displayed progressive increases in cataleptic behavior and decreases in motor coordination and muscle activity when compared with the Group I control rats ((a) and (b)). Prominent progression was observed on the 30th day. Notably, both the MCE/LD prevented the progressive increases in catalepsy and enhanced the motor co-ordination performance when compared with the untreated rotenone-induced animals in a time-dependent manner, based on the rat’s performance at the 4-week time point.
Figure 2. Behavioral analysis in rotenone-infused Parkinsonian rats treated with or without MCE/LD in different time periods. (a) depicts the rotarod activity, (b) depicts the cataleptic activity. Values are expressed as mean ± SEM (n = 10). Values are statistically significant at the level of P < 0.05 where ‘a’ represents Control, Sham Vs other groups, ‘b’ represents Rot Ind vs LD, MCE.
Histopathological and IHC of TH-positive neurons
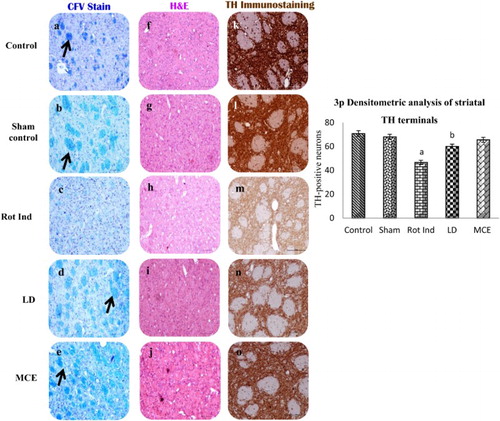
represents the histopathological analysis and immunohistochemical localization of TH-positive neurons in the striatum of various experimental animals. Images a–e represent Kluver Barrera staining; Black arrow shows compact white matter fascicles and prominent Nissl staining which was absent in rotenone-infused PD rats. Further, rotenone-induced rats demonstrated lesser degree staining intensity. Similar findings were observed in H&E images (f–j); however, it is important to observe that there is a remarkable restoration seen in LD and MCE administered rats. In this juncture, histochemical investigation makes it convenient to understand efficacy of the MCE treatment, as the TH expression (k–o) is very meager in Group III rotenone-infused PD rats signifying the degeneration and impairment of nigrostriatal pathway, which was induced by the nigral neuron degeneration. On the contrary, Group V MCE treated rats showed a better expression than LD treated rats.
Figure 3. Histopathological analysis and immunohistochemical localization of TH-Positive neurons and striatal bundles in the striatum of rotenone-infused Parkinsonian rats treated with or without MCE/LD. Images a–e represents Kluver Barrera staining; Black arrow shows compact white matter fascicles and prominent Nissl staining which was absent in rotenone-infused PD rats. Further, rotenone-induced rats demonstrated lesser degree staining intensity. Similar findings were observed in H&E images (f–j); however, it is important to observe that there is a remarkable restoration seen in LD and MCE administered rats. In this juncture, histochemical investigation makes it convenient to understand efficacy of the MCE treatment, as the TH expression (k–o) is very meager in Group III rotenone-infused PD rats signifying the degeneration and impairment of nigrostriatal pathway (3p).
Mitochondrial electron transport chain (ETC) complexes
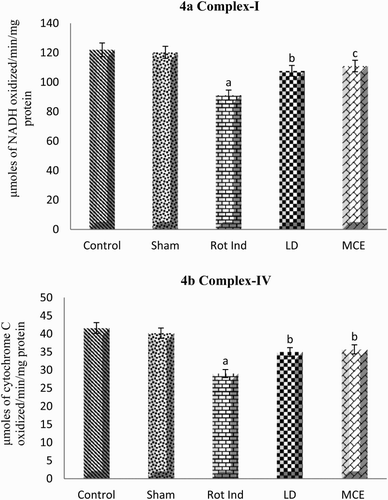
portrays the activities of mitochondrial electron transport chain complexes (I and IV) in the striatal mitochondria of stereotaxically rotenone-infused Parkinsonian rats supplemented with or without MCE/LD. Group III rats showed a significant (P < 0.05) decrease in the activities of complexes I and IV when compared with that of control rats. However MCE supplementation significantly augmented the activity of complex I by 22% and complex IV by 23% compared to Group III rats.
Figure 4. Activity of mitochondrial electron transport chain complexes I (a) and IV (b) in the striatum of rotenone-induced Parkinsonian rats treated with or without MCE/LD. Values are expressed as mean ± SEM (n = 10). Values are statistically significant at the level of P < 0.05 where ‘a’ represents Control, Sham Vs other groups, ‘b’ represents Rot Ind vs LD, MCE.
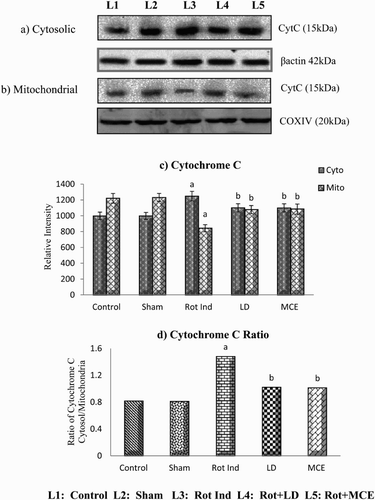
Cytosolic release of cytochrome C
Western blot analysis of cytochrome C in the striatal mitochondria and cytosol of rotenone-infused Parkinsonian rats treated with or without MCE/LD is depicted in . An increase of about 25% in the cytosolic cytochrome c levels and a concomitant decrease of mitochondrial cytochrome c (31%) was observed in rats administered with rotenone (Group III) when compared with that of control (Group I) rats. Both MCE and LD treatment reduces the cytochrome c levels in the cytosol by 12% (as the levels of mitochondrial cytochrome c levels were increased on treatment with MCE/LD by 28%) when compared with rotenone alone infused Group III rats.
Figure 5. Immunoblot analysis of cytosolic (a) and mitochondrial (b) cytochrome c in the striatum of experimental animals followed by densitometric analysis of respective protein levels (c). (d) depicts the ratio of cytosolic and mitochondrial cytochrome c. Values are expressed as mean ± SEM for three experiments in each group. Values are statistically significant at the level of P < 0.05 where ‘a’ represents Control, Sham Vs other groups, ‘b’ represents Rot Ind vs LD, MCE.
mRNA and protein expression of pro-apoptotic and anti-apoptotic proteins
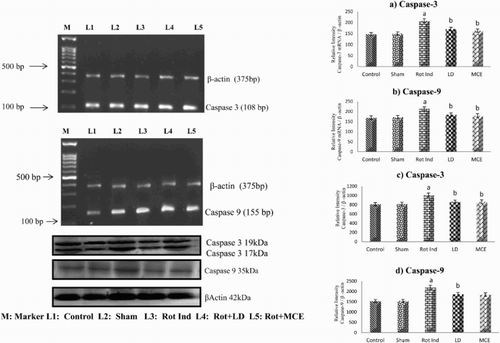
and show the levels of mRNA and protein of pro-apoptotic Bax, caspase-3 and caspase-9 and anti-apoptotic Bcl-2 in the striatum of stereotaxically rotenone-infused Parkinsonian rats treated with or without MCE/LD. Rotenone-infused Parkinsonian rats showed a significant (P < 0.05) increase in both the mRNA and protein levels of Bax by 45 and 37% respectively, caspase-3 by 42 and 25% respectively and caspase-9 by 26 and 19% respectively. On the other hand a significant (P < 0.05) decrease in the mRNA and protein levels of Bcl-2 were observed in these rats when compared to that of the control rats. On treatment with MCE/LD, both the mRNA and protein levels of pro-apoptic Bax, caspase-3 and caspase-9 were significantly receded with the maximum reduction (22%) in the levels of caspase-3 and MCE supplementation significantly augmented the mRNA and protein levels of Bcl-2 by 34 and 23% respectively.
Figure 6. Immunoblot and RT-PCR analysis of Bax & Bcl2 in the Striatum of experimental rats. mRNA expression of Bax (a) and Bcl2 (b), protein expression of Bax (c) and Bcl2 (d). (e) depicts the ratio of Bax and Bcl2. Values are expressed as mean ± SEM for three experiments in each group. Values are statistically significant at the level of P < 0.05 where ‘a’ represents Control, Sham Vs other groups, ‘b’ represents Rot Ind vs LD, MCE.
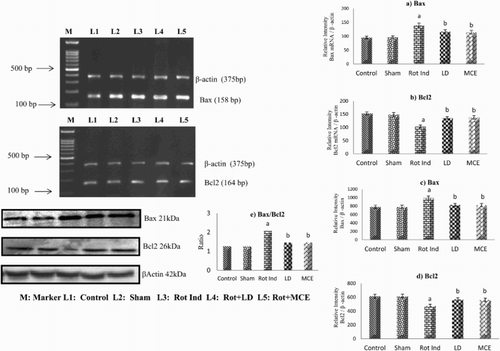
Figure 7. RT-PCR and western blot analysis of Caspase 3 and 9 in the striatum of experimental rats. mRNA expression of Caspase-3 (a) and Caspase-9 (b), protein expression of Caspase-3 (c) and Caspase-9 (d). Values are expressed as mean ± SEM for three experiments in each group. Values are statistically significant at the level of P < 0.05 where ‘a’ represents Control, Sham Vs other groups, ‘b’ represents Rot Ind vs LD, MCE.
Discussion
Epidemiologic studies have suggested that impairment in the mitochondrial complex I activity and an imbalance in the redox status of the nigro-striatal region leads to cell death and progression of Parkinson’s disease (PD). Increasing interest has been focused on phytoconstituents, as the prolonged administration of the standard drugs like L-Dopa does not address this underlying etiology, and further complicated with Levodopa-Induced Dyskinesia (LID). Hence, the present study is undertaken to check in depth the mito-protective and anti-apoptotic activity of the ethyl acetate extract of Morinda citrifolia against rotenone-induced nigral degeneration.
GC-MS analysis of MCE (data not shown) showed the presence of many phytoconstituents along with scopoletin, rutin, and quercetin, which is well supported in the literature [Citation23–25]. Thus, HPLC analysis was carried out to quantitate the levels of these phytoconstituents in the MCE and the results are depicted in . The levels of quercetin were found to be 1.41 µg mg−1 of MCE, which is 2-fold higher than in the ethanolic extract of Morinda citrifolia fruit [Citation24], and the levels of scopoletin and rutin were found to be 0.6 µg and 2.61 µg mg−1 of MCE, which are 10-fold and 30-fold higher, respectively than in the fruit at different stages of ripeness [Citation25].
PD is now being recognized as a complex illness with numerous behavioral symptoms, in addition to the well-recognized motor symptoms such as tremor, rigidity, postural instability, and bradykinesia [Citation26]. Hence, in the present study the behavioral alterations in the rats were studied by performing appropriate experimental protocols. As expected significant changes in the behavioral parameters are observed in rotenone-infused PD rats as these rats displayed progressive increase in cataleptic behavior and decrease in motor coordination, muscle activity and rearing when compared with the control rats. Similar reports were obtained by Khurana and Gajbhiye, who have stated that rotenone-induces cataleptic behavior, postural instability, and decreases rearing behavior [Citation27]. These alterations induced by rotenone is due to degeneration of dopaminergic neurons in SNPc as observed from the immunohistochemical localization of TH-positive neurons; which in turn is corroborated with decreased intensity of TH staining in the striatal region upon rotenone infusion.
However, rotenone-induced behavioral alterations are attenuated to a greater extent on treatment with MCE/LD. Alam et al. have reported that the neurobehavioral changes induced by bilateral rotenone lesion in medial forebrain bundle of rats are reversed upon treatment with LD [Citation28]. Notably, MCE at a dose of 150 mg/kg/day results in a better outcome in alleviating these motor deficits than LD in the grid test and the muscular coordination test. Morinda citrifolia fruit is shown to reduce stress-induced impairment of cognitive function in mice [Citation29]. This improvement in the behavioral changes by MCE/LD might be attributed to the prevention of degeneration of TH-positive neurons which would have restored the catecholamine levels thereby attenuating the rotenone-induced PD-like behavioral symptoms.
Defective mitochondrial respiration, in particular at the level of complex I, has long been associated with the pathogenesis of PD which impairs ATP production and thereby leads to increased production of reactive oxygen species (ROS) that culminate in cellular dysfunction and death [Citation30]. As expected, a decrease in mitochondrial complex I activity is observed in the striatum of rotenone-induced Parkinsonian rats as rotenone specifically blocks the ubiquinone binding site of Complex I, preventing the transport of electrons from Complex I to ubiquinone leading to the release of free radicals into the mitochondrial matrix [Citation31]. Interestingly in our current study, we have observed that LD has augmented the activity of complex I. However, conflicting reports are available in literature regarding the neuroprotective actions of LD irrespective of its well-established symptomatic actions. On the other hand, Dixit et al. [Citation32] have reported that levodopa was found to increase both the MnSOD and complex I activity, when given with MPTP or maneb and paraquat. This is in accordance with the previous reports, which have demonstrated the improvement in motor dysfunctions by levodopa in mice having mitochondrial defects [Citation33]. MCE treatment has also significantly enhanced the activities of complexes I and IV when compared with rotenone-induced Parkinsonian rats. The protection offered by MCE might be due to rich phytoconstituents as reports suggest that quercetin has the ability to prevent the NSAID-induced inhibition of complex-I by mimicking the action of coenzyme Q [Citation34] and rutin was capable of ameliorating HepG2 cells apoptosis by restoring complex-I activity [Citation35].
As redox imbalance is an important factor that modulates intrinsic pathway of apoptosis and contributes to PD pathogenesis i.e. nigral degeneration [Citation36], the effect of MCE on the nigral apoptosis was assessed by the mRNA and protein expression of various pro and anti-apoptotic proteins. Bcl-2-family proteins are important in regulating cell death by either inducing (Bax, Bid) or inhibiting (Bcl-2, Bcl-XL) apoptosis. Bax promotes apoptosis by inducing mitochondrial membrane depolarization and cytochrome c release while Bcl-2 inhibits apoptosis by preventing mitochondrial membrane depolarization [Citation37]. Hence, the ratio of Bax/Bcl-2 is commonly used to indicate mitochondrial permeability and apoptosis in the damaged cells. In the present study, the Bax/Bcl2 ratio was altered and a stipulated increase in the ratio (64%) was observed in the striatum of PD rats which clearly shows that the pro-apoptotic Bax level was significantly higher, whereas the Bcl2 level was significantly down-regulated. Our results are consistent with the previous studies in which they have reported that administration of rotenone significantly decrease the basal level of Bcl-2 and increase the Bax protein expression in rotenone-treated SH-SY5Y cells and adult male Sprague-Dawley rats [Citation37,Citation38]. ROS is capable of inducing apoptosis by regulating the phosphorylation and ubiquitination of Bcl2 family proteins resulting in increased pro-apoptotic protein levels and decreased anti-apoptotic protein expression [Citation39]. Moreover, in the present study increase in both the mRNA and protein levels of caspase-3 and caspase-9 are reported in the present study in the striatum of rats infused with rotenone. Caspase-3 activation may participate in the vulnerability of dopaminergic neurons in Parkinson’s disease [Citation40]. Li et al. have reported that rotenone induces both caspase 9/3-independent and -dependent cell death [Citation41]. On the other hand, co-administration of MCE/LD significantly augmented the levels of Bcl2 and down-regulated the levels of Bax both at the transcriptional and at the translational level. Khalil et al. have reported that administration of L-Dopa to rotenone-induced Parkinson’s mice has significantly reduced the levels of Bax, caspase-3 activity and improved the levels of Bcl2 [Citation42]. Contradictorily literature reports have shown that Morinda citrifolia to be an apoptosis promoting factor in many cancer conditions [Citation43,Citation44]. However, previous report from our laboratory has shown that MCE prevents apoptosis by up-regulating Bcl2 levels in the skeletal muscle of rotenone-infused PD rats [Citation14]. The phytoconstituents quercetin, rutin, and scopoletin identified and quantitated in MCE would have been probably responsible for this anti-apoptotic activity as these constituents have been shown to prevent apoptosis in neuronal precursor cells (NPCs) and experimental head trauma [Citation45,Citation46].
Bax has been shown to induce cytochrome c release and caspase activation both in vivo [Citation47] and in vitro [Citation48]. Bcl-2 is capable of inhibiting Bax-induced apoptosis by blocking spontaneous cytochrome c release in cell-free extracts and in cells treated with apoptosis-inducing agents [Citation49]. As the Bax/Bcl2 ratio is altered in the current scenario; we further investigated the levels of cytochrome c in mitochondria and cytosol. The cytosolic level of cytochrome c is significantly elevated in the striatum of rotenone-infused PD rats, indicating a typical release of cytochrome c from mitochondria to the cytosol when compared with that of the control rats. Our results are coherent with the previous studies carried out by Li et al. who have reported that rotenone administration decreased mitochondrial cytochrome c levels and increased cytosolic cytochrome c levels [Citation50]. Co-treatment with MCE resulted in decreased levels of cytosolic cytochrome c in the striatum when compared with rats infused with rotenone alone. This might be attributed to the mito-protective effect of Morinda citrifolia, which blocks the release of cytochrome c [Citation14]. Moreover, the phytoconstituent quercetin is also shown to prevent H2O2-induced apoptosis via its antioxidant activity and by blocking the release of cytochrome c in RAW 264.7 cells [Citation51]. The phytoconstituents such as quercetin, rutin, and scopoletin, identified and quantitated in MCE, have been shown to exert anti-apoptotic activity and might have been responsible to prevent apoptosis in various degenerative disorders [Citation45].
MCE not only blocks the release of cytochrome c but also subsequently decreased the protein levels of caspase-3 and caspase-9 in the striatum when compared with rats infused with rotenone alone. Moreover, MCE also down-regulated the mRNA levels of both the caspase-3 and caspase-9. This might be attributed to the presence of quercetin, rutin, and scopoletin identified in MCE. There are reports stating that these compounds confer protection against glutamate-induced apoptosis and ethanol-induced neurotoxicity in hippocampal HT22 cells by reducing caspase 3 activity [Citation46,Citation52]. Ola et al. have reported the effectiveness of rutin in ameliorating the levels of neuroprotective factors in streptozotocin-induced diabetic rat retina as it exerts anti-apoptotic activity by decreasing the levels of caspase-3 and increasing the levels of Bcl2 [Citation53].
Conclusion
In essence, administration of rotenone has decreased the number of TH-positive neurons by activating the mitochondrial-dependent apoptotic pathway which augments the levels of Bax, down-regulates the Bcl2 which further leads to cytochrome c release. Increase in the cytosolic cytochrome c activates the caspases 3 and 9 leading to degeneration of striatal neurons. On the other hand treatment with Morinda citrifolia has significantly blocked the release of cytochrome c and augmented the anti-apoptotic Bcl2 thereby increases the number of TH-positive neurons and alleviates the rotenone-induced apoptosis. Hence this preliminary study paves a way to show cast that the anti-apoptotic activity of MCE, which might be due to the presence of scopoletin and other phytoconstituents, and can be exploited to alleviate nigral degeneration in PD.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
S. Narasimhan Kishore Kumar is an M.Sc in Medical Biochemistry, and Ph.D in Medical Biochemistry, with research interests on Neuroscience, Parkinson's Disease, and Cell signalling.
Jayakumar Deepthy is an M.Sc in Medical Biochemistry, an M.Phil. Biochemistry, with research interests on Neuroscience, and Parkinson's Disease.
Uthamaraman Saraswathi is an M.Sc in Medical Biochemistry, with research interest on Neuroscience.
Mohan Thangarajeswari is an M.Sc in Medical Biochemistry, and Ph.D in Medical Biochemistry, with research interests on Neuroscience, and Diabetic nephropathy.
Sathyamoorthy Yogesh Kanna obtained B.Tech in Biotechnology, MS in Neuroscience, and Ph.D in Neuroscience-Anatomy, with research interests on Neuro pathology, and histology.
Pannerselvam Ezhil is an MBBS, with research interest on Neuro pathology. Periandavan Kalaiselvi obtained M.Sc in Medical Biochemistry, and Ph.D in Medical Biochemistry, with research interests on Neurodegenerative diseases, Aging, Diabetes, and Hypercholesterolemia.
ORCID
S Narasimhan Kishore Kumar http://orcid.org/0000-0003-2208-0809
Jayakumar Deepthy http://orcid.org/0000-0001-5270-2651
Mohan Thangarajeswari http://orcid.org/0000-0002-5000-8911
Sathyamoorthy Yogesh Kanna http://orcid.org/0000-0002-5592-4783
Periandavan Kalaiselvi http://orcid.org/0000-0001-5270-2651
Additional information
Funding
References
- Ong WY, Farooqui T, Koh HL, et al. Protective effects of ginseng on neurological disorders. Front Aging Neurosci. 2015;7:129.
- Ryan BJ, Hoek S, Fon EA, et al. Mitochondrial dysfunction and mitophagy in Parkinson's: from familial to sporadic disease. Trends Biochem Sci. 2015;40(4):200–210.
- Blesa J, Przedborski S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front Neuroanat. 2014;8:155.
- Olanow CW. The pathogenesis of cell death in Parkinson's disease–2007. Mov Disord. 2007;22(S17):S335–S342.
- Facecchia K, Fochesato LA, Ray SD, et al. Oxidative toxicity in neurodegenerative diseases: role of mitochondrial dysfunction and therapeutic strategies. J Toxicol. 2011;2011:683728.
- Wang MY, West BJ, Jensen CJ, et al. Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacol Sin. 2002;23(12):1127–1141.
- Deng S, West BJ, Palu AK, et al. Noni as an anxiolytic and sedative: a mechanism involving its gamma-aminobutyric acidergic effects. Phytomedicine. 2007;14(7):517–522.
- Harada S, Hamabe W, Kamiya K, et al. Preventive effect of Morinda citrifolia fruit juice on neuronal damage induced by focal ischemia. Biol Pharm Bull. 2009;32(3):405–409.
- Muralidharan P, Kumar VR, Balamurugan G. Protective effect of Morinda citrifolia fruits on β-amyloid (25–35) induced cognitive dysfunction in mice: an experimental and biochemical study. Phytother Res. 2010;24(2):252–258.
- Pachauri SD, Tota S, Khandelwal K, et al. Protective effect of fruits of Morinda citrifolia L. on scopolamine induced memory impairment in mice: a behavioral, biochemical and cerebral blood flow study. J Ethnopharmacol. 2012;139(1):34–41.
- Pachauri SD, Verma PR, Dwivedi AK, et al. Ameliorative effect of Noni fruit extract on streptozotocin-induced memory impairment in mice. Behav Pharmacol. 2013;24(4):307–319.
- Hsu PW, Shia CS, Wu CT, et al. Noni increased the systemic exposure of methotrexate in rats through inhibition on multi-drug resistance protein 2 (MRP 2) and breast cancer resistance protein (BCRP). J Funct Foods. 2013;5(3):1414–1420.
- Xiong N, Huang J, Zhang Z, et al. Stereotaxical infusion of rotenone: a reliable rodent model for Parkinson's disease. PLoS One. 2009;4(11):e7878.
- Narasimhan KK, Paul L, Sathyamoorthy YK, et al. Amelioration of apoptotic events in the skeletal muscle of intra-nigrally rotenone-infused Parkinsonian rats by Morinda citrifolia–up-regulation of Bcl-2 and blockage of cytochrome c release. Food Funct. 2016;7 922–937.
- Adams CE, Hoffman AF, Hudson JL, et al. Chronic treatment with levodopa and/or selegiline does not affect behavioral recovery induced by fetal ventral mesencephalic grafts in unilaterally 6-hydroxydopamine-lesioned rats. Exp Neurol. 1994;130(2):261–268.
- Rogers DC, Fisher EM, Brown SD, et al. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8(10):711–713.
- Urbach YK, Bode FJ, Nguyen HP, et al. Neurobehavioral tests in rat models of degenerative brain diseases. Methods Mol Biol. 2010;597:333–356.
- Kumar P, Kumar A. Effect of lycopene and epigallocatechin-3-gallate against 3-nitropropionic acid induced cognitive dysfunction and glutathione depletion in rat: a novel nitric oxide mechanism. Food Chem Toxicol. 2009;47(10):2522–2530.
- Chen Y, Zhang DQ, Liao Z, et al. Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease. Mol Neurodegener. 2015;10(1):4.
- Iglesias-González J, Sánchez-Iglesias S, Beiras-Iglesias A, et al. A simple method for isolating rat brain mitochondria with high metabolic activity: effects of EDTA and EGTA. J Neurosci Methods. 2013;213(1):39–42.
- Shults CW, Haas RH, Passov D, et al. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann Neurol. 1997;42(2):261–264.
- Tzagoloff A, Wharton DC. Studies of the electron transport system Lv. The influence of PH and of protein-depolymerizing reagents on the reaction of cytochrome A with borohydride. J Biol Chem. 1964;239(2):582–585.
- Mahattanadul S, Ridtitid W, Nima S, et al. Effects of Morinda citrifolia aqueous fruit extract and its biomarker scopoletin on reflux esophagitis and gastric ulcer in rats. J Ethnopharmacol. 2011;134(2):243–50.
- Thoo YY, Ho SK, Abas F, et al. Optimal binary solvent extraction system for phenolic antioxidants from mengkudu (Morinda citrifolia) fruit. Molecules. 2013;18(6):7004–7022.
- Lewis Luján LM, Assanga I, Bernard S, et al. Nutritional and Phenolic composition of Morinda citrifolia L.(Noni) fruit at different ripeness stages and seasonal patterns harvested in Nayarit, Mexico. Int J Food Sci Nutr. 2014;3(5):421–429.
- Anderson KE. Behavioral disturbances in Parkinson's disease. Dialogues Clin Neurosci. 2004;6(3):323.
- Khurana N, Gajbhiye A. Ameliorative effect of Sida cordifolia in rotenone induced oxidative stress model of Parkinson's disease. Neurotoxicology. 2013;39:57–64.
- Alam M, Mayerhofer A, Schmidt WJ. The neurobehavioral changes induced by bilateral rotenone lesion in medial forebrain bundle of rats are reversed by L-DOPA. Behav Brain Res. 2004;151(1):117–124.
- Muto J, Hosung L, Uwaya A, et al. Morinda citrifolia fruit reduces stress-induced impairment of cognitive function accompanied by vasculature improvement in mice. Physiol Behav. 2010;101(2):211–217.
- Perier C, Vila M. Mitochondrial biology and Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2(2):a009332.
- Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH: ubiquinone oxidoreductase (complex I). J Biol Chem. 2004;279(38):39414–39420.
- Dixit A, Srivastava G, Verma D, et al. Minocycline, levodopa and MnTMPyP induced changes in the mitochondrial proteome profile of MPTP and maneb and paraquat mice models of Parkinson's disease. Biochim Biophys Acta. 2013;1832(8):1227–1240.
- Pickrell AM, Pinto M, Hida A, et al. Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci. 2011;31(48):17649–17658.
- Sandoval-Acuña C, Lopez-Alarcón C, Aliaga ME, et al. Inhibition of mitochondrial complex I by various non-steroidal anti-inflammatory drugs and its protection by quercetin via a coenzyme Q-like action. Chem Biol Interact. 2012;199(1):18–28.
- Usta J, Kreydiyyeh S, Knio K, et al. Linalool decreases HepG2 viability by inhibiting mitochondrial complexes I and II, increasing reactive oxygen species and decreasing ATP and GSH levels. Chem Biol Interact. 2009;180(1):39–46.
- Tsang AH, Chung KK. Oxidative and nitrosative stress in Parkinson's disease. Biochim Biophys Acta. 2009;1792(7):643–650.
- Erbaş O, Oltulu F, Taşkiran D. Amelioration of rotenone-induced dopaminergic cell death in the striatum by oxytocin treatment. Peptides. 2012;38(2):312–317.
- Hu LF, Lu M, Wu ZY, et al. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol. 2009;75(1):27–34.
- Li D, Ueta E, Kimura T, et al. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004;95(8):644–650.
- Hartmann A, Hunot S, Michel PP, et al. Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci U S A. 2000;97(6):2875–2880.
- Li J, Spletter ML, Johnson DA, et al. Rotenone-induced caspase 9/3-independent and-dependent cell death in undifferentiated and differentiated human neural stem cells. J Neurochem. 2005;92(3):462–76.
- Khalil WK, Assaf N, ElShebiney SA, et al. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem Int. 2015;80:79–86.
- Taşkın Eİ, Akgün-Dar K, Kapucu A, et al. Apoptosis-inducing effects of Morinda citrifolia L. and doxorubicin on the Ehrlich ascites tumor in Balb-c mice. Cell Biochem Funct. 2009;27(8):542–546.
- Kharis Z, AzimahtolHawariah LP, Normah A, et al. Morinda citrifolia extract inhibits proliferation of non-small human lung cancer cell line NCI-H23 via apoptosis by modulating the Bax: Bcl-2 ratio. J Trop Agric Food Sci. 2010;38(2):203–209.
- Sajad M, Zargan J, Zargar MA, et al. Quercetin prevents protein nitration and glycolytic block of proliferation in hydrogen peroxide insulted cultured neuronal precursor cells (NPCs): implications on CNS regeneration. Neurotoxicology. 2013;36:24–33.
- Yang EJ, Kim GS, Kim JA, et al. Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn Mag. 2013;9(36):302.
- Rosse T, Olivier R, Monney L, et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391(6666):496–499.
- Jurgensmeier JM, Xie Z, Deveraux Q, et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95(9):4997–5002.
- Yang XF, Weber GF, Cantor H. A novel Bcl-x isoform connected to the T cell receptor regulates apoptosis in T cells. Immunity. 1997;7(5):629–639.
- Li N, Ragheb K, Lawler G, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278(10):8516–8525.
- Chow JM, Shen SC, Huan SK, et al. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem Pharmacol. 2005;69(12):1839–1851.
- Song K, Na JY, Kim S, et al. Rutin upregulates neurotrophic factors resulting in attenuation of ethanol-induced oxidative stress in HT22 hippocampal neuronal cells. J Sci Food Agric. 2015;95(10):2117–2123.
- Ola MS, Ahmed MM, Ahmad R, et al. Neuroprotective effects of rutin in streptozotocin-induced diabetic rat retina. J Mol Neurosci. 2015;56(2):440–448.
